Neutrophils in aging and aging-related pathologies
This article is part of a series of reviews covering Neutrophils and Friends appearing in Volume 314 of Immunological Reviews.
Summary
Over the past millennia, life expectancy has drastically increased. While a mere 25 years during Bronze and Iron ages, life expectancy in many European countries and in Japan is currently above 80 years. Such an increase in life expectancy is a result of improved diet, life style, and medical care. Yet, increased life span and aging also represent the most important non-modifiable risk factors for several pathologies including cardiovascular disease, neurodegenerative diseases, and cancer. In recent years, neutrophils have been implicated in all of these pathologies. Hence, this review provides an overview of how aging impacts neutrophil production and function and conversely how neutrophils drive aging-associated pathologies. Finally, we provide a perspective on how processes of neutrophil-driven pathologies in the context of aging can be targeted therapeutically.
1 IMPACT OF AGING ON NEUTROPHIL BIOLOGY
Organismal aging (distinct from “daily” neutrophil aging) is a complex, multimodal process that influences all organs and tissues, and affects the biology of any cell type. Gradual deterioration of the immune system over time is among the key features of aging. The presence of excessive baseline inflammation, often referred to as “inflammageing,” is a common denominator of immune aging. Studies in both mice and humans have illustrated that the persistence of an unresolved general inflammation inhibits immune responses,1 and that this can be reversed during aging by blocking inflammatory processes in older individuals.2, 3 Collectively termed “immunosenescence,” nearly every component of the immune system including neutrophils, undergoes substantial age-related remodeling including neutrophils. Although generally short-lived, neutrophils are particularly sensitive to changes in host physiologic states, including tissue environment, circadian rhythms, sex, and age. For instance, cytokine signaling, superoxide generation, and the phagocytic activity of neutrophils are all diminished in elderly humans.4-6 These fundamental changes are often correlated with a poor prognosis in the context of infectious diseases and sepsis and failed responses to vaccination,7-9 and are likely to contribute to the higher incidence of infections in the elderly.
1.1 Age-driven alterations in neutrophil production
Globally, aged mouse hematopoietic stem cells (HSCs) have defective regenerative potential, as they fail to efficiently reconstitute myeloablated mice after transplantation. In addition, studies in mice have shown that aging differentially affects early myelopoiesis and lymphopoiesis in the bone marrow. Indeed, changes in the bone marrow of aged mice lead to a clonal skewing of HSCs toward the myeloid lineage at the expense of lymphocyte differentiation.10-13 A similar bias is evidenced in human HSCs from older donors.14 The mechanisms that underlie the accumulation of myeloid-biased HSCs during aging probably involve a combination of both aging-driven cell-intrinsic changes and alterations in the bone marrow environment. For example, RNA sequencing of the bone marrow stromal milieu revealed an age-associated upregulation of Il6 and Il1b expression, cytokines reported to compromise HSC self-renewal and to promote myeloid bias.15 The presence of low levels of cytokines (e.g., interleukin-1β), chemokines (e.g., CC-chemokine ligand 5), or lipopolysaccharide may further shift the balance toward HSCs with myeloid potential.16-18
Despite this shift of aged HSCs to the myeloid cell lineage, the size of neutrophil populations remains stable with age in the circulation of older adults without co-morbid medical conditions.19-21 The proliferative responsiveness of neutrophil precursor cells to granulocyte-colony-stimulating factor (G-CSF) seems to be blunted with aging,19 yet no change in the numbers of neutrophil progenitors in the bone marrow of the healthy elderly has been reported.19 Conversely, responses to granulocyte/macrophage-colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) appear to be unaffected by age.19 This unaltered responsiveness likely contributes to the ability of the healthy elderly to maintain stable neutrophil numbers during aging. However, age-associated increases in the number of neutrophils have also been reported.22-24 Given this, it is notable that increased neutrophil numbers were found to be a risk factor for age-related frailty.25, 26 Moreover, in a multivariate analysis of one cohort of aged adults, individuals showing neutrophilia were at higher risk of death.27 In mice, aging is more consistently associated with increased numbers of neutrophils. In the circulation of mice, neutrophil counts rise with age.18, 28, 29 Similarly, evidence indicates that chronic baseline inflammation in aged mice is associated with an expansion of neutrophils within lymphoid organs—including bone marrow, lymph nodes, and spleen.18, 30 Also, tissue neutrophils are increased in the aging lung and liver of mice.31, 32
1.2 Neutrophil phenotypes driven by aging
In successfully aged adults (i.e., without co-morbid medical conditions), neutrophil development and counts seem to be unaltered by aging,19-21 yet a body of evidence indicates that age-associated changes could remodel neutrophils. For instance, the expansion of circulating CD16bright/CD62Ldim immunosuppressive neutrophils with aging has been reported and was associated with impaired superoxide generation and phagocytic activity.33 The underlying basis of these aging-related neutrophil phenotypes is complex and linked to multiple factors, including cell-intrinsic alterations, changes in neutrophil local microenvironments, and increased circulating pro-inflammatory mediators with age. Because continuously produced in the bone marrow and generally short-lived, neutrophils will be affected mostly by the aging of their milieu or potential defects inherited from their progenitor cells, rather than by accumulating true cell-autonomous, aging-associated defects.
1.2.1 Age-related cell-intrinsic changes
Both in steady-state and after transplantation of aged HSCs into young mice, HSCs are biased toward myeloid lineage specification.10 These observations suggest that the aging-associated myeloid skewing is cell intrinsic. In earlier studies, age-related defects that are intrinsic to old neutrophils have been shown. Chemotaxis (i.e., directional movement) of neutrophils from older humans and mice is reported to be diminished,6, 34-39 and this was linked to enhanced baseline phosphoinositide 3-kinase (PI3K) signaling in old neutrophils.6 In the same study, rejuvenation interventions of old neutrophils with young plasma failed to reverse the aberrant age-associated migratory phenotype. Conversely, exposure of young neutrophils to inflammaging signals (e.g., TNF or plasma from aged hosts) did not mimic the aging-related defect in chemotaxis, and thus, likely reflects cell-intrinsic processes. Studies in humans and mice have illustrated that neutrophils from older adults, compared to those from younger adults, showed impaired phagocytosis of opsonized Streptococcus pneumoniae,7, 8 and diminished capacity to kill phagocytosed microbes.7, 35, 40 In mice, the aging-associated defect in intracellular bacterial killing has been related to reduced production of the neutrophil antimicrobial peptide CRAMP.7 Strikingly, adoptive transfer of neutrophils from young mice reverses the susceptibility of aged mice to pneumococcal infection. This suggests that the age-related defect in antimicrobial activity is neutrophil intrinsic.
Other signaling pathways are reported to be affected in old neutrophils, and this might be reflected by aging-related defects in neutrophil biology. Examples of this include enhanced autophagy responses41 that are essential for neutrophil differentiation and function,42 and blunted antiapoptotic responses to GM-CSF.43, 44 Reduced basal expression of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in old neutrophils likely underlies defective responsiveness to GM-CSF mediated through the PI3K pathway.43 In addition, neutrophils from older adults show an impaired response to triggering receptor expressed on myeloid cells 1 (TREM1),45 an activating receptor that triggers the production of cytokines, chemokines, and reactive oxygen species (ROS). Studies have also illustrated age-related alterations in membrane lipid raft domains of neutrophils. For example, in response to GM-CSF, the negative regulator SH2 domain-containing protein tyrosine phosphatase 1 (SHP1) was shown to be excluded from lipid rafts of neutrophils from young individuals, but retained in rafts of old neutrophils.46 Together, these findings indicate that aging affects several signaling pathways in neutrophils. However, it is not clear which of these defects are intrinsic to old neutrophils.
1.2.2 Changes in the local environment
Most of the events described above are driven by the neutrophils themselves, and not by the local milieu in which they reside. Nonetheless, neutrophils are highly plastic and can readily adapt to their tissue environment.47 Indeed, in vivo, a body of evidence illustrates that age-related alterations in the local tissue environment could remodel neutrophils.32, 41, 48-54 Senescent (p16INK4A-expressing) cells, which no longer proliferate, accumulate in every organ during aging. Senescent nonlymphoid cells secrete inflammatory cytokines, chemokines, growth factors, and matrix metalloproteinases, known as the senescence-associated secretory phenotype (SASP). The secretion of these inflammatory molecules may cause organ dysfunction with aging in humans and in mice. Conversely, the removal of senescent cells in transgenic mice (by inducing cell division or apoptosis) can reverse age-related organ dysfunction. In aged mice, the SASP components CXC-chemokine ligand 1 (CXCL1) and CXCL8 recruit neutrophils to the liver.32 In turn, neutrophils drive telomere dysfunction and induce senescence in bystander non-immune cells via the production of ROS, expanding senescent cell load in the aging liver.32 This provides a striking example of the crosstalk between neutrophils and senescent cells. More recently, CXCL1 was shown to underlie enhanced neutrophil reverse transendothelial migration (rTEM) in inflamed tissues of aged mice.54 Importantly, bone marrow chimeric mice exhibited augmented neutrophil rTEM in mice with aged stroma and young neutrophils, indicating that aging-related changes to the stroma account for neutrophil rTEM. After rTEM back into the circulation, reverse migrated neutrophils are retained in aged lungs and programmed toward and activated phenotype, causing remote organ damage.54
Moreover, aged (CXCR4+CD62Llo) neutrophils may themselves accumulate in aged mice. When senescent, neutrophils are removed from circulation and cleared by bone marrow macrophages through efferocytosis. However, studies in mice have demonstrated that old macrophages are defective in efferocytosis of senescent neutrophils, and that aged mice have an increased load of senescent neutrophils in the circulation and in the bone marrow.18 As senescent neutrophils are a potent source of IL-1β, diminished removal of senescent neutrophils may further contribute to the rise of IL-1β in the bone marrow of aged mice, and remain to promote skewing of HSCs toward myeloid differentiation. Along this line, a recent multi-omics profiling study illustrated that aging-related neutrophil remodeling is evident in bone marrow-residing neutrophils, suggesting a role for the aged bone marrow microenvironment in age-associated alterations of neutrophil biology.41
1.2.3 Systemic changes in inflammatory cytokines
Older healthy individuals (60 years and above) exhibit chronic low-grade sterile inflammation in the absence of pathogens. Indeed, evidence from human studies indicates that older adults have high baseline serum concentrations of pro-inflammatory cytokines, clotting factors, and acute phase reactants in the steady state.55-58 Association studies of human cohorts have shown that elevated levels of cytokines, such as interleukin-6 (IL-6), are profoundly predictive of frailty and premature death in older individuals compared with individuals of the same age group who do not exhibit increased baseline systemic inflammation59, 60 (although not in all studies61). Aging of humans appears to be associated with a shift of circulating neutrophils to an activated phenotype, given that these cells acquire increased levels of CD11b expression and generate higher baseline levels of ROS.24, 62 Elevated CD11b expression correlates with increased levels of tumor necrosis factor α (TNF-α) in the sera of elderly donors. In mice, genetic abrogation of TNF-α has been shown to prevent this age-driven increase in CD11b expression on neutrophils,24 suggesting that systemic inflammaging may contribute to altering the phenotypes of neutrophils in the elderly.
1.3 Influences of aging on neutrophil function
1.3.1 Migration
Neutrophils are the first cells to respond to infection, yet they can turn against host tissues during chronic inflammation. Tissue infiltration of neutrophils is among the hallmarks of immune aging,30-32 and neutrophil migration has been extensively studied in the context of aging. Neutrophil trafficking from the circulation to sites of inflammation is a highly regulated process involving adhesive interactions with vessel walls and local chemotactic cues. Studies of isolated neutrophils from elderly humans have revealed reduced chemotaxis in vitro6, 34-36 (Figure 1A). Similarly, both the speed and directionality of neutrophil movement were found to be blunted in aged mice.38, 39, 52 In addition, studies in mice have linked defective neutrophil chemotaxis to diminished wound healing during aging—an age-associated defect that was linked to reduced expression of intercellular adhesion molecule 1 (ICAM1).63 Conversely, there are also instances of excessive neutrophil recruitment and inflammation found in aging. In vivo, dysregulated neutrophil migration in aged mice is aligned with factors such as aberrant production of systemic or local inflammatory mediators or diminished local anti-inflammatory mechanisms.32, 48-54 For example, senescent cells recruit neutrophils to the aging lungs50 or liver32 through the release of age-related factors, including CXCL1, leading to excessive inflammation and increased mortality from influenza infection. These different findings may reflect tissue context, and such aging-associated environmental and tissue-related factors likely influence the dysregulation of neutrophil infiltration and activation.
1.3.2 Resolution
Dysregulated neutrophil trafficking can lead to not only blunted neutrophil infiltration into the site of inflammation, but also defective neutrophil egress from inflamed tissue. Defective resolution of immune responses in older individuals, due to the impaired neutrophil egress from sites of immune activity, may also lead to sustained inflammation in vivo. Indeed, local neutrophil-driven inflammation was enhanced in aged mice following burn-associated lung injury, and following bacterial or viral infection.49, 52, 64, 65 In the aging lung, an age-related increase in the expression of ICAM1 by pulmonary endothelial cells52 or failed efferocytosis of neutrophils65 contributed to augmented neutrophil retention in the lungs, excessive neutrophil-mediated inflammation, and resultant lung damage.
1.3.3 Antimicrobial activity
The neutrophils of elderly people are less equipped to eradicate pathogens, since they exhibit shorter lifespans66 and decreased capacities for chemotaxis,6, 34-36 phagocytosis,4, 8 neutrophil extracellular trap formation,67 and ROS generation following stimulation35, 67 (although neutrophils from the elderly constitutively produce and secrete higher levels of ROS24, 62). Taken together, these findings indicate that aging influences fundamental neutrophil functions, which may lead to diminished protection from microbial infection.8 Similarly, studies in aged mice have reported defects in microbial killing by neutrophils,7, 9, 40 although in earlier studies phagocytosis and intracellular killing were mostly preserved in aged mice.68 In mice, age-related deficits in NET formation, chemokine production, and recruitment are seen. For example, in a mouse model of methicillin-resistant Staphylococcus aureus infection (MRSA), NET formation decreased with age, and old neutrophils infected in vitro with MRSA showed diminished production of CXCL1 and CXCL2.9
2 NEUTROPHIL–MACROPHAGE INTERACTION AS A HALLMARK OF AGE-RELATED PATHOLOGIES
Continuous removal of apoptotic neutrophils is of critical importance for maintaining blood and tissue homeostasis. Efferocytosis promotes anti-inflammatory cytokine signaling and prevents secondary cellular necrosis.69 Clearance of apoptotic cells is particularly relevant in injured tissues where neutrophil removal is a prerequisite for inflammation resolution. Pioneering work in the eighties provided the concept that macrophages not only phagocytose neutrophils, but that this process closely correlated with neutrophil senescence and features of programmed cell death.70, 71 The macrophage-expressed myeloid-epithelial-reproductive (Mer) proto-oncogene tyrosine kinase (MerTK) receptor plays an important role in recognizing apoptotic cells, that is, through binding of phosphatidylserine, for subsequent efferocytosis.72, 73 Consequently, high level of MerTK expression on macrophages reduces inflammation in rheumatoid diseases,74 while absence of MerTK is associated with systemic inflammation and autoimmunity.75 Clearance of apoptotic neutrophils depends on both expression and activation of MerTK on macrophages.76, 77 The neutrophil secretome thereby stimulates macrophage MerTK expression, and MerTK levels on macrophages are significantly reduced in mice lacking neutrophils.76 Insufficient clearance of apoptotic cells by MertK-deficient macrophages leads to delayed inflammation resolution after MI, adverse remodeling, and decreased cardiac function.78
Together, macrophages clear apoptotic neutrophils, and the liaison of both immune cell types is closely associated with the regulation of efferocytosis. Interestingly, phagocytosis triggers the release of the cell death receptor ligand FasL by macrophages, which in soluble form or through surface expression, promotes apoptosis in adjacent immune cells.79 Thus, uptake of apoptotic neutrophils by macrophages can promote a feedback loop through Fas-mediated apoptosis to clear neutrophils, which could support the resolution of tissue inflammation.
In aging, efficient clearance of apoptotic neutrophils seems to become specifically important. Senescent cells provide a secretome with plenty of inflammatory molecules.80 The repertoire of pro-inflammatory cytokines is reminiscent to that released in tissue (e.g., skin) inflammation and wound healing,81 and is considered to contribute to aging.82 Neutrophils can induce and promote telomere dysfunction and cellular senescence,32 which are hallmarks of aging and age-related pathologies. These processes are particularly sensitive toward oxidative stress, which can be provided by neutrophils transmitting ROS in a paracrine manner.32 Thus, while neutrophils are integral parts of the innate immune defense, they can be harmful to the host by promoting telomere DNA shortening and senescence of neighboring cells. Macrophages balance and counteract neutrophil-mediated effects through their clearance. However, ROS can cleave MerTK and thereby reduce efferocytosis efficiency and inflammation resolution.83 Further, neutrophils display reduced sensitivity to antiapoptotic signals in aging.66 Thus, neutrophil–macrophage interactions are altered in aging, which may contribute to the low-grade inflammatory process termed “inflammaging.”
In tissue injury and infections, resident macrophages represent an important source of inflammatory mediators that attract neutrophils. For example, alveolar macrophages provide leukotriene B4 and interleukin-8 in response to lipopolysaccharide or bacteria mediating pulmonary inflammation.84, 85 Consequently, depletion of tissue macrophages impairs the recruitment of neutrophils to sites of inflammation.86, 87 The guidance of neutrophils by macrophages thereby works in both ways. Whereas macrophages attract neutrophils to areas of significant tissue injury, they can also negatively regulate neutrophil recruitment. This mechanism seems to be important in minor injuries where the presence of macrophages and their “cloaking” of lesions, a term coined by the Germain laboratory, controls excess inflammation.88 The interactions of macrophages and neutrophils are expected to change with age, as a consequence of immune cell alterations and following modifications in tissue structure and environment.89 While resident macrophages are self-renewing long-lived cells, tissue composition of macrophage subsets changes in later life. This leads to alterations in macrophage polarization and functions with impact on tissue homoeostasis.90-93 In vitro assays, however, have not provided a clear picture of the cytokines secreted by activated macrophages in aging.94, 95 In in vivo setting, multiple aspects including tissue environment-, context- and sex-dependent effects will have to be considered in future work. Along this line, causal relationships of age-dependent changes and immune cell interactions need to be established. For example, age-dependent contraction of the splenic marginal zone and reduced tissue macrophage residence in this compartment is associated with accumulation of neutrophils.30, 96
Besides their important role in innate defense against pathogen infection, neutrophils are also involved in other forms of immune responses. In type 2 immunity, which refers to responses to helminth infection as its hallmark condition, but also includes atopic reactions of the body, the role of neutrophils is being increasingly recognized.97 It was shown that IL-4 signaling triggered aging of neutrophils, which were then eliminated at higher rates through macrophage phagocytosis. Accelerated aging and efferocytosis of neutrophil in type 2 inflammation led to increased susceptibility to bacterial infections.98
Together, immune cells undergo various phenotypic and functional changes in aging. Age-dependent activation of neutrophils promotes senescence of neighboring cells and specifically impacts on the efferocytosis capacity of macrophages. Future work will have to identify the molecular cues and signaling pathways, including diurnal activation patterns,99 inherent to age-associated changes in neutrophils, which could provide a basis for the development of novel therapeutic strategies. Maintenance of physiologic macrophage functions, such as MerTK-dependent efferocytosis, seems likewise important.
3 NEUTROPHILS, ATHEROSCLEROSIS, AND AGING
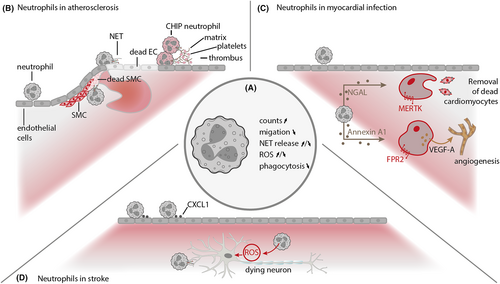
Chronological age outranks all other risk factors as a predictor of the clinical events of cardiovascular diseases (CVDs), including coronary artery disease and stroke,100, 101 and the incidence of atherosclerotic disease increases profoundly with age.102 Atherosclerosis, the primary underlying basis of CVDs, originates from a lipid-driven chronic inflammation of the vessel wall.103, 104 Hyperlipidemia can damage endothelial cells thereby promoting lipid deposition and plaque formation and represents the initial spark in atherosclerosis; however, chronic inflammation fuels progression of the disease. In an instant, the plaque erodes or ruptures, the artery thromboses, the vessel occludes, and the clinical events of atherosclerosis manifest themselves—that is, acute myocardial infarction, stroke, and peripheral artery occlusive disease. Neutrophils have been implicated in all phases of atherosclerotic disease, including initiation, progression, and clinical manifestation (Figure 1B). Circulating neutrophil counts are directly related to atherosclerosis burden,105, 106 and depletion of neutrophils reduces atherosclerosis in animal models.106 Yet, it is not clear how aging adds to the impact of neutrophils on the disease, and the exact nature of these complex relationships lacks conclusive mechanistic explanations.
In fact, it is also poorly understood how aging influences atherosclerosis and its complications, but atherogenic inflammation may relate to inflammaging. Indeed, increased basal inflammation is present in older individuals,55-58, 107 which is markedly predictive of death and chronic diseases.59, 60 In addition, clinical studies have shown that inflammation causally links to atherosclerotic disease,108 laying down a robust clinical and epidemiological basis for the relationship between inflammaging and the increased cardiovascular risk. For example, aging is associated with increased levels of circulating IL-1β in elderly individuals,109 and anti-inflammatory therapy with a monoclonal antibody targeting IL-1β results in lower rates of recurrent cardiovascular events.108 Nonetheless, direct evidence from studies in mice was provided only recently, and, strikingly, clonal hematopoiesis was suggested to link aging, neutrophils, atherosclerotic disease, and thrombosis.110, 111
Age-related changes in HSCs can be reflected as somatic mutations in hematopoietic cells, collectively described as clonal hematopoiesis of indeterminate potential (CHIP). Indeed, in the general population, more than 10% of people older than 70 years of age have clones of blood cells bearing primarily loss-of-function mutations in epigenetic modifiers TET2, ASXL1, and DNMT3A. With a twofold to threefold increase in the risk of atherosclerotic CVD, CHIP was identified as a major risk factor for CVD in the elderly.112, 113 Moreover, the relationship between CHIP and coronary heart disease appears to be a causal one, with an increase in coronary events in relation to clone size and a dose–response relationship between clone size and atherosclerosis.113 Alternatively, CHIP-associated mutations may provide a proliferative advantage to hematopoietic progenitor cells that result in an expansion of circulating neutrophils. Notably, neutrophil counts have been associated with an increased risk of coronary heart disease in the general population.114 However, prospectively studied subjects with CHIP caused by the epigenetic modifier variants,112, 115 as well as mice that lack hematopoietic Tet2, do not have abnormal blood neutrophil counts at baseline.113, 116, 117 Nonetheless, with aging, development of a myeloproliferative state over several years seems likely in some patients,118 and a role for neutrophilia cannot be fully ruled out.
Conversely, individuals with the age-related somatic JAK2V617F (JAK2VF) mutation are at increased risk of atherothrombotic events (including cardiac ischemic events and thrombotic stroke), and the increased risk for venous and coronary thrombosis is accordant to leukocyte counts.119-121 Although less common than the epigenetic modifier variants, the JAK2VF variant increases the risk of CVD by 12-fold. The JAK2VF mutation activates JAK2 (Janus kinase 2) signaling,122, 123 leading to proliferation of HSCs and progenitors. Patients who carry the JAK2VF mutation present with myeloproliferative neoplasms (including essential thrombocytosis, polycythemia vera, and primary myelofibrosis),122, 124-126 and the JAK2VF variant is linked to the formation of neutrophil extracellular traps (NETs) in myeloproliferative neoplasms.110 Studies in mice bearing JAK2VF have confirmed the enhanced propensity for NET formation and thrombosis.110 By contrast, preventing the formation of NETs with ruxolitinib—a clinically available drug that targets JAK2 signaling—diminished thrombosis in a mouse model.110 In addition, in a population study of more than 10 000 individuals without known myeloid disorder, presence of a JAK2VF-positive clonal population of blood cells correlated with an increased incidence of thrombosis.110 More recently, it has been shown that hematopoietic JAK2VF expression in hypercholesterolemic mice relates to pronounced neutrophilia, together with enhanced neutrophil adhesion to the vessel wall and infiltration into early atherosclerotic lesions, correlating with lesion size.111 Thus, the JAK2VF variant promotes accelerated early lesion formation, likely contributing to the atherothrombotic risk in individuals with JAK2VF-positive clonal hematopoiesis or myeloproliferative neoplasms. However, none of the studies with mice that carry the JAK2VF mutation were reported in aged animals, and it remains unclear how these findings compare to those in old mice where neutrophil behavior may be influenced by other age-related environmental and tissue-associated factors.
Beyond neutrophilia and neutrophil activation, interaction of hypercholesterolemia with the JAK2VF mutation enhances several other atherogenic propensities, including baseline platelet P-selectin exposure and platelet-neutrophil aggregates.111 Interactions with activated platelets commit neutrophils to undergo NET formation.127, 128 This can be propagated through interactions of neutrophil P-selectin glycoprotein ligand-1 with P-selectin.129 Vice versa, the adhesion of platelets to NETs via von Willebrand factor leads to platelet aggregation,130, 131 an important step in the formation of a platelet-fibrin clot.130, 132-134 NETs participate in pathological thrombosis, including deep vein thrombosis131, 135 and atherothrombosis,136, 137 and they have been suggested to participate in thrombus growth and stabilization by providing a scaffold for fibrin formation and platelet aggregation.130, 138, 139 Multiple factors can cause thrombin cleavage and fibrin formation on NETs. For example, NETs-associated neutrophil serine proteases, such as neutrophil elastase, locally degrade tissue factor pathway inhibitor (TFPI), impairing the anticoagulant function of TFPI to increase blood coagulation.127 Also, NETs can stimulate the coagulation cascade directly through exposure of tissue factor or by binding and activating factor XII.131 Local accumulation of tissue factor-covered NETs occurs at sites of coronary thrombosis,140 and neutrophils release NETs bearing tissue factor within thrombi of infarcted regions.136 Studies with mice that lack factor XII suggest that NETs contribute to the propagation of intravascular blood coagulation by promoting factor XII activation.131 Along this line, studies in humans have shown that older age is associated with higher levels of clotting factors and acute phase reactants, such as prothrombin and fibrinogen.57-59 Yet, it remains unknown how the hypercoagulability state in older persons incites propagation of the atherothrombotic complications after NET formation, and further studies are greatly needed to explore these possibilities.
Several experimental mouse models of atherogenesis have demonstrated the presence of neutrophils in arterial plaques,141, 142 and that neutrophil-derived factors are able to modulate murine plaque size and composition.143 Neutrophils may contribute to plaque formation through promoting inflammatory monocyte recruitment, and may also participate in lesion evolution and complication. Within atherosclerotic plaques, cathepsin G and cathelicidins exhibit monocyte-chemotactic activity.144 The neutrophil granular antimicrobial peptide CRAMP affects the recruitment and activation of other immune cells, including monocytes and dendritic cells.145 In atherosclerotic vessels, CRAMP is deposited on the inflamed endothelial surface leading to the attachment of monocytes to the vessel wall, and Apoe−/− mice that lack CRAMP develop smaller plaques suggesting that CRAMP is involved in plaque formation.143 An abundant primary granule enzyme in neutrophils is MPO. Beyond its deposition in NETs, MPO is released upon neutrophil activation and degranulation as means to support antimicrobial host defense. In patients, elevated levels of MPO are associated with increased risk of cardiovascular events.146 In plaques, the presence of MPO has been linked to endothelial apoptosis, plaque erosion, and rupture.147 As a consequence of such findings, MPO has emerged as a target for imaging148, 149 and treatment of atherosclerosis.150, 151 Moreover, neutrophil MPO triggers macrophages to release reactive oxygen species and other pro-inflammatory cytokines. Oxidative stress is present in aging and human atherosclerotic vascular disease.152 Recent findings underscore a relationship between mitochondrial oxidative stress and the formation of NETs in animal models for atherosclerosis.153 Irradiation of recipient animals to ablate endogenous hematopoietic tissues, followed by reconstitution of aged atheroprone Ldlr−/− mice with mitochondrial catalase transgenic bone marrow cells suppresses oxidative stress and protects against atherosclerosis development. The higher oxidative burden in these old mice correlates with enhanced NET formation. In vitro, exposure of neutrophils to 7-ketocholesterol led to the formation of NETs, and suppression of mitochondrial oxidative stress reduced NET formation in response to 7-ketocholesterol.153 Notably, treatment with resveratrol—a naturally occurring antioxidant—has been shown to extend lifespan in various experimental models. Yet, how oxidative stress and neutrophils contribute to the instigation of clinically high-risk vulnerable plaques with aging remains to be determined.
Indeed, it is poorly understood how an atherosclerotic plaque becomes rupture-prone with aging. Smooth muscle cells (SMCs) harvested from the aortas of aged mice were found to have pro-atherogenic features, including increased production of pro-inflammatory cytokines (such as IL-6) and chemokines (such as CCL2). The expression of TLR4 was also increased in aged SMCs, and increased production of IL-6 was dependent on TLR4 and myeloid differentiation primary-response protein 88.154 SMCs from aged non-human primates also showed a higher production of pro-inflammatory cytokines, such as IL-1β, TNF-α, and CCL2.155 In that study, the increased inflammatory response correlated with increased mitochondrial oxidative stress. Strikingly, studies in mice have causally linked neutrophils and NETs with SMC death, necrotic core size, and thin fibrous caps.156 Indeed, activated SMCs promote the release of NETs by lesional neutrophils. In turn, NETs induce the death of SMCs, leading to reduced plaque stability. However, it is not clear how the aging-related inflammatory phenotype of old SMCs influences lesional neutrophils, contributing to the pathogenesis of atherosclerosis. Similarly, neutrophils can also cast damage in the arterial wall toward endothelial cells.157-163 Activation of endothelial cells (e.g., through engagement of TLR2) propagates the recruitment of neutrophils and the formation of NETs 158,160,162. Neutrophils localized near the inflamed intimal surface degranulate and generate reactive oxidants, leading to endothelial cell death and detachment. Superficial plaque erosion exposes prothrombotic factors, and activated platelets trigger neutrophils to form NETs and the release of tissue factor.158 Through complement, NETs induce continued endothelial erosion.162, 163 As discussed before, all these components undergo age-associated changes, and it remains unclear to what extent aging affects endothelial cell death, detachment, and thrombosis. Finally, studies in mice have illustrated that aged mice have an increased load of circulating senescent neutrophils as a result of defective efferocytosis by old macrophages.18 Senescent neutrophils are more prone to release NETs, and their population expansion with age may reflect the increased risk of atherothrombotic events in the elderly, but this remains an area of active investigation.
In sum, all phases of atherosclerotic disease involve cells of the immune system, components of the coagulation and acute phase system and stromal cells, and the exact aging-associated nature of these complex interactions is poorly understood, and the role of old neutrophils herein remains to be described.
4 NEUTROPHILS IN MYOCARDIAL INFARCTION
Neutrophils are first-line responders of the innate immune system. In acute myocardial infarction (MI), they are recruited to the ischemic heart within minutes, being attracted by danger signals and inflammatory mediators. At the scene, they release a broad spectrum of cytokines, proteinases, ROS, and NET-forming nucleosomes that contribute to the local inflammatory reaction. Inhibition of neutrophil mediator (e.g., MPO) release has proven effective in reducing infarct size and/or benefit left ventricular remodeling in animal models of MI.164 Consequently, neutrophils have for a long time been considered as being solely detrimental in this condition. It should be kept in mind, however, that a basic principle of immunity is to protect the host. In addition, neutrophils do so very efficiently when we are infected by pathogens. Recently, it was shown that neutrophil depletion disturbs the resolution of inflammation post-MI, resulting in increased fibrosis and reduced functional outcome76 (Figure 1C). The underlying mechanisms have in part been established and relate to the process of neutrophil clearance.165 Neutrophils that are being phagocytosed by macrophages induce an anti-inflammatory and pro-resolving immune response in these cells. Further, neutrophils promote expression of the phagocytosis receptor MerTK on macrophages, which allows the detection of apoptotic cells.76 Loss of macrophage MerTK expression by cleavage impairs cardiac remodeling after MI.166 Thus, neutrophils promote the clearance of apoptotic cells in the infarcted heart and thereby positively affect cardiac healing. In addition, neutrophils can express receptors that scavenge cytokines in the tissue, so-called decoy receptors, which dampen the local injury response and promote inflammation resolution.167 In another study, neutrophil-borne Annexin A1 has been identified as important regulator of myocardial healing.168 Mice lacking annexin A1 exhibited impaired healing after myocardial infarction, an observation linked to failed release of angiogenic growth factors from macrophages. Delivery of a short annexin A1 fragment or its overexpression in pigs significantly improved angiogenesis and myocardial function after infarction. The principal role of neutrophils in MI has been summarized in prestigious overview articles.169-171 Therefore, we will here focus on novel aspects of neutrophil biology in this condition.
One emerging topic relates to neutrophil diversity and the dynamics of neutrophil phenotypes in MI. Two recent studies analyzed neutrophil transcriptomes in the course of MI. Dependent on clustering analysis and marker genes used, they displayed 5–6 major subsets of cardiac neutrophils. Notably, a population of heart-infiltrating neutrophils adopted a SiglecFhigh signature, representing the largest cluster of neutrophils from Day 4 post-MI.172, 173 These immune cells are considered late-stage neutrophils and may therefore impact the process of post-MI remodeling providing a distinct repertoire of inflammatory genes. Unsupervised clustering in infarcted hearts also revealed a cardiac neutrophil subset characterized by interferon (IFN)-stimulated genes.174 MI-associated IFN responses are induced in myeloid cells already in the BM compartment, and ISG expression further increases in BM-egressed circulating neutrophils.174 Thus, recruited neutrophils maturate on their way to the injured heart and develop inflammatory properties en route. This will allow to analyze neutrophil responses directly in blood and may open up novel therapeutic strategies to modulate the function of neutrophils that are being recruited to the injured heart. Generally, senescent neutrophils upregulate Cxcr4 while losing CD62L surface expression. This phenotypic modulation of circulating neutrophils is, however, not only influenced by cardiac inflammation but can be modulated by various factors including the microbiome.175 Senescent neutrophils provide greater ROS production and NET formation, which can contribute to tissue injury in MI.
While neutrophils are being recognized as important modulators of cardiac injury in MI, their therapeutic targeting has only recently gathered momentum. Interestingly, antagonists of the β1-adrenergic receptor (ADRB1) such as metoprolol reduce the migration and activation of neutrophils.176 As metoprolol has been shown to reduce infarct size and improve cardiac function after AMI in a large-scale clinical trial,177 the recent findings link ADRB1-inhibition with innate immunity. They also provide further support for the concept that cardioprotection in MI by metoprolol is more effective when being applied before arterial reperfusion, owing to the deleterious effects of neutrophils in ischemia and reperfusion injury.178 Since on-demand inhibition of neutrophil migration could be desirable, ADRB1 blockade may extend to other inflammatory conditions. Interestingly, metoprolol application also reduces PSGL-1-dependent neutrophil-platelet interactions that cause microvascular obstructions.176
Platelets and neutrophils share a close relationship in the pathophysiology of thrombosis and promote mutual activation. Platelet-derived serotonin triggers neutrophil degranulation (e.g., myeloperoxidase release) and promotes neutrophil-dependent thromboinflammation in MI.179 Platelet granules also store and secrete neutrophil attractant chemokines such as CCL5 and CXCL4 which promote their recruitment and release of NETs.180 Consequently, antibody blockade of CCL5 reduced infarct size and adverse remodeling after AMI in mice.181 Recently evolving concepts have targeted the heterophilic interaction of CCL5 and CXCL4 with designed compounds, and successfully reduced myocardial inflammation and infarct size after ischemia/reperfusion injury.182 Such concepts seem particularly interesting because they allow specifically designed interventions of chemokine interactomes,183 but do not interfere with systemic immune responses.184 Other therapeutic strategies include the development of small polyanions to interfere with neutrophil-derived nucleosomes that are cationic in nature.185 Owing to their pro-inflammatory and prothrombotic properties, NETs represent interesting targets in both acute and chronic cardiovascular conditions.186 In MI, NET structures as well as citrullinated histone H3 (CitH3)-positive, that is, NETosis-primed, neutrophils are abundant in intracoronary thrombi and injured myocardium.138, 187 NET content in intracoronary thrombi is associated with adverse cardiac outcome in human patients with MI,188-190 and levels of circulating dsDNA are associated with microvascular obstruction in MI patients undergoing percutaneous coronary intervention.191 Blockers of NET formation (peptidyl arginine deiminase (PAD)4 inhibitors) or enzymes (e.g., DNAse) disrupting histones have become available for research purposes192, 193 and reduced infarct size in mice.194 Novel therapeutic strategies are aiming at more specific targeting of histones (e.g., H2a) and disruption of their properties (e.g., charge-dependent effects),195 thus focussing the action of tentative drugs on local pathomechanisms such as immune cell recruitment.
Together, neutrophils have a broad range of biological functions in MI. Early inhibition of their activation and NET formation seems important for circulating and recruited neutrophils to decrease thrombus burden and reduce reperfusion injury. Blocking the release of pro-inflammatory mediators such as MPO contributes to limiting infarct size in the early stages of MI. In later stages, however, neutrophils orchestrate the clearance of apoptotic cells and capture cytokines, thereby promoting the resolution of inflammation. Thus, timed interventions against distinct neutrophil functions seem warranted to efficiently target and make optimal use of these versatile immune cells in MI. Addressing specific neutrophil subsets could aid to increase efficiency of future therapeutic strategies. Of note, data on the role of neutrophils during MI in aged hosts (human or mouse) are rare, and hence, future studies are warranted to address this important issue.
5 NEUTROPHILS IN STROKE
The local post-ischemic sterile inflammatory response following cerebral ischemia has been identified as a key element within stroke pathology and one of the main causes for secondary brain damage.196 Ischemic stroke results in a profound activation of brain resident immune cells and infiltration of hematogenous myeloid cells.197, 198 Neutrophils are among the first leukocyte subsets to enter the ischemic tissue, and increasing evidence suggests a profound detrimental role for neutrophils in secondary infarct growth.199, 200 Particularly, the release of reactive oxygen species and proteolytic enzymes by neutrophils contributes to further blood–brain barrier damage potentially resulting in additional brain oedema and hemorrhagic transformation.201-203 Depletion of neutrophils from circulation reduces infarct size, blood–brain barrier damage, improves functional recovery, and increases neovascularization.199 In particular, neutrophil depletion works more effectively in older than in younger mice suggesting a potential correlation between age and neutrophil-mediated damage progression.204 Peripheral neutrophils in stroke patients are characterized by increased CD11b, neutrophil elastase, and elevated ROS production when compared to healthy controls. Further, following stroke neutrophils show increased overactive senescent (CXCR4bright/CD62Ldim) and reverse transendothelial migrating (CD54high/CXCR1low) neutrophil subsets and the extent of ischemia-induced neutrophil activation correlates with NIH stroke scale.205 As of today, clinical trials targeting neutrophil-related pathomechanisms have failed to show benefit in stroke patients.198 The use of mainly young animals without any co-morbidity has been identified as potential cause for this unsuccessful clinical translation since stroke particularly affects the elderly.206, 207 Therefore, a better understanding of the connection between neutrophil activation and advancing age is of great interest to potentially counteract these obstacles.
As aging adversely impacts the immune systems efficacy and neutrophil function in particular, an altered immune and secondary damage response in older patients is likely and could be the link between age and disease severity.203, 204 Research, however, has not yet come to a final conclusion regarding potential age-related effects in stroke. While several preclinical studies confirm increased susceptibility to hemorrhagic transformation and worsened functional outcome in older animals, infarct volume analyses show mixed results.203, 208 Nevertheless, emerging evidence indicates that increased mortality, poor recovery, and increased susceptibility to secondary infections in older patients might be mediated at least in part by aged immune cells.203, 208, 209 Age itself is known to be substantial factor for increased stroke burden as younger stroke patients show a lower morbidity and mortality when compared to older patients.204 Further, older patients show increased intra-cerebral neutrophils, extracellular matrix-degrading matrix metalloproteinase-9 (MMP-9) expression, and elevated hemorrhagic transformation when compared to younger stroke patients.203 Preclinical studies confirm aging as a substantial determinant of stroke outcome.210 Indeed, young, middle-aged, and aged mice subjected to experimental stroke show worsened functional outcome with aging accompanied by increased immigration of neutrophils, macrophages, dendritic cells, and increased activation of resident microglia.211 Particularly, the composition of infiltrating leukocytes is altered in aged mice in favor of neutrophils, representing the dominant hematogenous immune cell population infiltrating into the ischemic brain.203 Young heterochronic chimeric mice reconstituted with aged bone marrow show increased neutrophil influx into the ischemic brain, develop increased hemorrhagic transformation, and worsened functional recovery when compared to old mice which received bone marrow of young animals. Rejuvenation of the hematopoietic system in old mice results in the reversal of this age-dependent effect which is particularly attributed to neutrophil granulocytes.203 Neutrophils in aged mice are characterized by increased ROS and proteolytic enzyme levels consequently resulting in increased hemorrhagic transformation.203 Interestingly, naive bone marrow neutrophils from young and aged mice show no difference in granularity, intracellular reactive oxygen species, cytokine, and chemokine production after PMA challenge. Once released into circulation, however, neutrophils of older mice develop increased granularity and intracellular reactive species upon stimulation. It is hypothesized that neutrophils, after leaving the bone marrow, exhibit elevated oxidative function in the environment of increased pro-inflammatory cytokines within the circulation of older mice.204 Aged mice further exhibit reduced CXCR4, CD44, and CXCL12 expression which are needed for neutrophil bone marrow homing and clearance. This in turn results in deteriorated cell disposal and an increased number of peripheral neutrophils with exceeded expiration date in older mice. Additionally, following experimental stroke, neutrophil-activating interleukin-6 (IL-6) and neutrophil chemoattractant CXCL1 are elevated in aged mice when compared to younger animals204 (Figure 1D). Following experimental permanent stroke, old mice develop increased infarct volume in combination with increased interleukin-6 (IL-6), interleukin-1 β (IL-1β), and matrix metalloproteinase-9 (MMP-9) concentration. Consequently, these mice develop an increased blood–brain barrier degradation when compared to young animals.202 Most importantly, IL-6 and the human CXCL1 homologue interleukin-8 (IL-8) are elevated in patient serum samples and higher age correlates with increased levels of serum IL-6 and IL-8, further suggesting aged neutrophils as a mediator of increased morbidity in the elderly.204
In summary, neutrophils have a central role within the pathophysiology of stroke. Condition and age of the immune system seem to be a crucial determinant for neutrophil release, their activation, and clearance during brain damage progression after ischemic stroke. An altered leukocyte composition and increased infiltration of maladjusted neutrophils indeed could be a driving force behind the aggravated secondary brain damage in older patients. Therefore, it is suggested that future immunomodulatory therapies need to account even more for leukocyte heterogeneity in general and neutrophil heterogeneity in particular.
6 NEUTROPHILS IN NEURODEGENERATIVE DISEASES
Neuronal death and loss of axonal connectivity are main features of several CNS pathologies. Central nervous system resident and infiltrating immune cells are known for their contribution to CNS damage in degenerative disorders like Alzheimer's disease (AD) or Parkinson's disease (PD). These pathologies have already been shown to be exacerbated by infiltrating leukocytes like neutrophils but our understanding of potential connections between immune system aging and neutrophil-mediated pathophysiology is still poor.212, 213 However, signatures of neutrophil numbers and altered neutrophil activation in various neurodegenerative diseases suggest a potential causal connection between aging, disease progression, and severity. Growing evidence further points to the potential existence of alternatively activated leukocytes and neutrophils that contribute to neuroprotection and restoration in several CNS disorders.214, 215 Therefore, modulation of neutrophil maturation, release, activation, and infiltration could be a promising target combating neurodegeneration or even to support neuronal regeneration.214, 216
6.1 Alzheimer's disease
One of the main causative factors within Alzheimer's pathology, the beta-amyloid, activates brain resident microglia and attracts monocytes from the periphery.217 Nonetheless, several studies also demonstrate that neutrophils, attracted by activated micro- and astroglia, cross the blood–brain barrier, and adhere to small vessels.218-220 In fact, AD shares comparable signatures of infiltrating cells with several other neurodegenerative disorders characterized by the presence of neutrophils within the respective lesions.212, 215, 221, 222 In a mouse model of AD, neutrophils infiltrate the brain as early as after six months of age, coinciding with onset of experimental AD symptoms. This suggests a role for neutrophils not only during disease progression but also at the time of its induction, and indeed, Ly6G-antibody-mediated depletion of mature neutrophils mitigates disease progression further undermining a role for mature neutrophils in AD pathophysiology.223 Various pathomechanisms of how neutrophils contribute to neuronal and axonal damage are currently being discussed. These include unterminated inflammation, the release of cytotoxic products or altered neutrophil activation and migration,212, 224, 225 and indeed, circulating neutrophils of AD patients produce higher amounts of ROS and tend to be more activated when compared to healthy individuals. Fast-decliner patients further show increased amounts of hyperactivated neutrophils and a shifted ratio of aged CXCR4hi/CD62Llow and suppressive CD16bright/CD62Ldim in favor of the pro-inflammatory phenotype when compared to slow-decliner patients.224 This could be a hint for a potentially altered granulopoiesis or neutrophil release from the bone marrow as contributing factor in AD. Alzheimer patients further show higher neutrophil CD11b expression when compared to healthy individuals confirming an increased neutrophil activation in AD.226 In summary, there is strong evidence that neutrophils and their state of activation have a substantial role within the pathophysiology of AD. However, no causative correlation between neutrophil activation and increasing age could be determined yet but should be an important question to be addressed.
6.2 Parkinson's disease
Parkinson's disease (PD) is a neurodegenerative disorder pathophysiologically characterized mainly by the demise of dopaminergic neurons within the substantia nigra of the midbrain. Comparable to AD and MS, the chronic inflammation characterized by brain resident glial activation and recruitment of hematogenous immune cells is at least a cofactor for disease progression,227 and indeed, myeloperoxidase particularly expressed by infiltrating monocytes and neutrophils is upregulated within the affected midbrain areas and MPO+-cells accumulate within neurodegenerative brain regions of PD patients.228 Further, dopaminergic neurons are more resistant to experimental PD in MPO-deficient mice undermining a potential role for neutrophils in PD pathophysiology.228 Most interestingly, it has been shown that the immune system of PD patients shows signs of accelerated aging and is actually epigenetically older than in age-matched controls. Moreover, PD patients carry significantly increased neutrophils within their circulation and the disease burden strongly correlated with peripheral neutrophil counts.213 As with AD, we also see in PD first indications rise that point toward a potential connection between increasing immune cell age, disease progression, and severity. As of today, however, a wide variety of questions regarding neutrophil activation and aging in neurodegenerative disorders still remain unanswered and should be addressed. Further research for the development of suitable neutrophil modulatory immune therapies is certainly worthwhile.
7 NEUTROPHILS BRIDGE AGING AND CANCER
7.1 Aging and cancer: two sides of the same coin
Aging results in an augmented risk of developing cancer. Indeed, cancer incidence sharply increases with age, with 25 cases per 100 000 under 20 years of age and more than 1000 cases per 100 000 from 60 years old (Age and Cancer Risk, National Cancer Institute, Risk Factors: Age—NCI cancer.gov accessed on 23/06/2022). In people aged 65–74, the majority of new cases are diagnosed and the highest percent of death is recorded (https://seer.cancer.gov/statfacts/html/all.html accessed on June 23, 2022). Aging and cancer are not just temporally correlated: Accumulation of cellular and tissue damage is a key feature in both phenomena, making an aging tissue at higher risk to harbor cancerous cells.229 Interestingly, it has been suggested that this common feature affects cellular fitness in opposite manner: The accumulation of mutations decreases cellular fitness in aging, while increases cellular fitness in cancer.82 A decrease in cellular fitness in aging is characterized by genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.82 On the contrary, an increase in cellular fitness in cancer is characterized by evading growth suppression, avoiding immune destruction, enabling replicative immortality, initiating tumor-promoting inflammation, activating invasion and metastasis, inducing angiogenesis, genomic instability and mutation, resisting cell death, deregulating cellular energetics, and sustaining proliferation.230 Among these hallmarks, acute cellular senescence is a well-known anti-cancer mechanism. Senescence is characterized by irreversible proliferation arrest and a characteristic inflammatory phenotype associated with a specific secretome defined as SASP (senescence-associated secretory phenotype).231 The SASP is characterized by a transcriptional program mediated by NFkB, triggered by the DNA damage response (DDR), resulting in the production of pro-inflammatory cytokines.232 The SASP can modulate the immune response in a beneficial fashion, by triggering the recruitment of immune cells to eliminate senescent cells and proliferating cancer cells, or in a detrimental, tumor-promoting manner, by attracting immune cells with pro-angiogenic functions as well as inducing immune evasion.233 Senescent cells can further drive cancer progression by promoting epithelial-to-mesenchymal transition (EMT), eliciting stemness,234 and enhancing tumor cell proliferation by producing extracellular vesicles.235 In addition to cellular components, the extracellular matrix also displays changes in aging that are tumor permissive, especially in lung, liver, and bone, which represent the most common site of metastasis for different cancer types.236 While the matrix becomes stiffer allowing better motility of tumor cells, similar changes also affect the infiltration of immune cells in aged tissues.237
In cancer, tumor cells and stromal cells in the tumor microenvironment can acquire a senescent phenotype. The senescent microenvironment modulates tumor progression: In particular, senescent fibroblasts stimulate the proliferation of pre-malignant epithelial cells,238 lose the ability to counteract genomic instability caused by oxidative stress, and promote susceptibility to DNA damage and activation of oncogenic pathways.239 During aging, tissue architecture is altered and some organs may become better fertile soils for metastases. Indeed, differently from the aged skin, where melanoma proliferation is inhibited, aged lungs awake metastatic melanoma cells, driving overt metastases.240 On the contrary, senescent cancer cells themselves trigger strong anti-tumor protection mediated by antigen-presenting cells and CD8 T cells,241 therefore harboring adjuvanticity. However, senescent cancer cells have so far suggested to be tumor-promoting. For instance, tumor cells that escape from senescence maintain epigenetic changes that result in a stem-like state with enhanced proliferative potential.242, 243 Chemotherapy-induced senescent cancer cells display phagocytic cell signature, engulf neighboring cells, and have a survival advantage.244 Finally, senescent tumor cells educate immune cells in the microenvironment by polarizing macrophages and promoting the formation of neutrophil extracellular traps (NETs).245
7.2 Neutrophils, cellular senescence, and cancer
Neutrophils have recently emerged as key players in cancer and triggers of cellular senescence in healthy tissues. Pro-inflammatory mediators in the SASP act as neutrophil attractants,246 amplifiers of senescence247 and strengtheners of tumor aggressive phenotypes.248 In response to inflammation and infection, neutrophils release reactive oxygen species (ROS) and reactive nitrogen species (RNS). Indeed, in a model of acute injury, neutrophils have been found to induce senescence in the liver by causing oxidative damage in telomeres of non-immune cells.32 In the same model, senescent cells promote the recruitment of neutrophils, which, in turn, augment senescence in the tissue, therefore creating a senescence-driven inflammation cycle.32 In chronic inflammation, this mechanism may be even amplified, with neutrophils sustaining accelerated cellular senescence, organ aging, and ultimately cancer. In cancer, ROS-producing neutrophils harbor pro-tumoral activity by inducing genotoxic stress,249 increasing mutational load, leading to epithelial mutagenesis,250 and amplifying DNA damage upon carcinogen exposure.251 Whether pro-tumorigenic neutrophils infiltrating tumor lesions may simultaneously cause senescence in neighboring healthy cells, sustaining tumor-supporting inflammation should be considered. Nevertheless, neutrophil effector functions such as the release of NETs, a feature of tumor-supporting neutrophils, are defective in aged individuals.67 Therefore, a decline in neutrophil functions with age may impact on their pro-tumorigenic activity. To address these questions, the use of preclinical in vivo models of aging will be pivotal. In addition to tissue-infiltrating neutrophils, circulating neutrophils have been described to support circulating tumor cells (CTCs). In fact, CTCs acquire specific mutations when clustering with neutrophils in the bloodstream. In the primary tumor, these mutations promote neutrophil infiltration and increase the likelihood of disseminated neutrophil-CTCs clusters.252 Whether ROS production by neutrophils is responsible for the acquisition of new mutations in CTCs remains to be established. Taking into account, that CTC abundance and neutrophil release from the bone marrow, circulation time in peripheral blood and infiltration into tissues are diurnally regulated,253, 254 disturbance of the circadian rhythm in aging255 may impact on CTC-neutrophil clustering, finally affecting cancer metastasis.
Anti-cancer treatments have been reported to cause cellular senescence, leading to adverse therapy effects and tumor relapse256 as well as initiating the immune response and neutrophil recruitment to tumor lesions upon induction of cell death.257, 258 Therapy (i.e., Doxorubicin and Paclitaxel) induced senescent (TIS) cells persists, cause inflammation and contribute to short- and long-term side effects associated with treatment (bone marrow suppression, cardiac dysfunction, cancer recurrence, impact on physical strength).256 Radiation exposure induces senescence in healthy lungs and prepares the pre-metastatic niche by initiating tissue-repairing functions in neutrophils.259 Cellular senescence promotes the recruitment of neutrophils which may either scavenge pre-malignant cells or sustain inflammation, promoting tissue aging and cancer. This suggests that neutrophils may be able to either suppress or promote cancer, by antagonizing or promoting cellular senescence, respectively, ultimately confirming their controversial function in cancer. However, this may be context-dependent and reflect the tissue specificity of neutrophil dynamics and heterogeneity.47 Neutrophil states characterized by different phenotypes, maturation stages, and functions co-exist in cancer.260 Considering that a myeloid bias occurs in aging, it remains to be established whether senescent cells in the tumor milieu regulate phenotype and function of cancer-associated neutrophils. Targeting senescence has gained recent interest as a therapeutic strategy to tackle recurrence and reduce multi-morbidities associated with anti-cancer treatments.261 Therapies aimed at targeting senescence may ultimately affect neutrophil states. In turn, the identification of specific senescence-associated neutrophil subsets may open a new avenue of treatments that limit senescence and cancer by targeting neutrophils, without affecting neutrophilic response against pathogens.
8 THERAPEUTIC IMPLICATIONS AND PERSPECTIVES
In recent years, several strategies have emerged aimed at targeting neutrophil-driven pathologies including cardiovascular inflammation or tumor progression. Such strategies span from inhibition of neutrophil adhesion and recruitment to dampening the release and the activity of NETs as well as interference with neutrophil cell death pathways.262-264 Yet, most of these therapeutic approaches have not been tested in the context of aging. Given changes in the neutrophil count, phenotype and function as well as of alterations of drug metabolism pathways with aging, one may assume that observations form such therapeutic strategies made in young hosts cannot simply be transferred to aged individuals.
To alleviate senescence-induced pathologies, a number of therapeutic strategies have been suggested including dietary intervention such as caloric restriction, or the intake of senolytics and of rapamycin. As discussed in this review, aged individuals tend to have high counts of circulating neutrophils and recent reports indicate that this increase is alleviated by caloric restriction in humans265 and in rats (Ma et al., 2020). Importantly, such reduction in neutrophil numbers was not just observed in blood, but also in tissues typically affected in aged hosts including adipose tissues, liver, and kidney.266 Cellular senescence is a hallmark of aging, and therapeutics have been developed to remove senescent cells (senolytics) or to remove SASP (senomorphics).267 Senolytics include a broad range of drugs that upregulate antiapoptotic pathways. Such include inhibitors of the antiapoptotic BCL-2 family proteins, HSP90 inhibitors, USP7 inhibitors, and p53 modulators. Based on their described activity, one should assume that these drugs directly interfere with neutrophil counts, phenotype, and function. Indeed, there are scattered reports indicating for example that the pan-BCL2 inhibitor ABT263 induces neutropenia; yet, a systematic analysis on how senolytics impact on neutrophils is currently not available. On the contrary, SASP factors secreted from senescent cells or expressed on their surface regulate neutrophil recruitment and activity. Their controlled removal by regulating neutrophil protease or phagocytic capacity could be an important field or research in upcoming years. Finally, the mTOR inhibitor rapamycin has for long been discussed as an anti-aging drug. In fact, when administered late in life, rapamycin can increase the lifespan by up to 15%.268 Despite such encouraging findings, rapamycin has initially been launched as an immunosuppressant and in fact it alters neutrophil function tremendously (thus limiting the enthusiasm for its broad application).
Taken together, aging strongly impacts of neutrophil counts, phenotype and function and neutrophils hold central roles in aging-associated pathologies. However, currently data connecting these two dimensions are rare, and future studies will need to decipher how neutrophils from aged hosts impact on pathology. Such data will be instrumental for the development of neutrophil-targeted therapies in aged individuals.
ACKNOWLEDGMENT
K.V.A. receives funding from the IMF at the Münster Medical Faculty; C.T. is supported by the Humboldt Foundation and the Marie Curie actions; J.M. is supported by the Interdisciplinary Center for Clinical Research (IZKF) Münster and by the Deutsche Forschungsgemeinschaft (MI 1547/3-1, MI 1547/4–1, FOR 2879/1, CRC TRR332 TP B2); C.S. receives support from German Center for Cardiovascular Research (DZHK) and the BMBF (project 81Z060020) and the Deutsche Forschungsgemeinschaft (CRC914 TP A10, CRC1123 TP A7, CRC TRR332 TP A6, SCHU 2297/1-1); O.S. receives funding from the Deutsche Forschungsgemeinschaft (CRC TRR332 TP A2 & Z1, CRC914 TP B8, CRC1009 TP A13, CRC1123 TP A6, OR465/1-1), the Else Kröner Fresenius Stiftung, the IZKF at the Münster Medical Faculty, Novo Nordisk, and the Leducq Foundation. The authors would like to thank Nina Knubel for generating the display items. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
O.S. holds patents on neutralization of histones and interference with chemokines in inflammation; O.S. receives funding from Novo Nordisk to study the role of histones in inflammation and to study chronopharmacological intervention strategies in atherosclerosis.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




