Arid1a promotes thymocyte development through β-selection-dependent and β-selection-independent mechanisms
Xiaofeng Yang, Xin Wang, and Lei Lei contributed equally to this work.
Funding information
This work was supported by grants from Major International (Regional) Joint Research Project (81820108017, B.Z.), Natural Science Foundation of China (81771673, B.Z.; 82101826, X.Y.), Young Talent Program of Xi'an Jiaotong University (YX1J005, B.Z.), Natural Science Foundation of Shaanxi Province (2020JM-065, X.Z.; 2020JQ-098, L.L.; 2021JQ-025, X.Y.), Project funded by China Postdoctoral Science Foundation (2019M653673, X.Y.) and Openning Project of Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi'an Jiaotong University (2019LHM-KFKT003).
Abstract
Early T-cell development from CD4− CD8− double-negative (DN) stage to CD4+ CD8+ double-positive (DP) stage in the thymus is regulated through multiple steps involving a batch of sequentially expressed factors. Our preliminary data and a recent report showed that AT-rich interaction domain 1A (Arid1a) is required for the transition from DN to DP stages, but the mechanism is not fully understood. In this study, we consolidated that conditional deletion of Arid1a in T-cell lineage intrinsically caused developmental blocks from DN3 to DN4 stages, as well as from DN4 to DP stages using both in vivo adoptive T-cell transfer model and in vitro culture system. The expression of intracellular TCRβ is significantly decreased in Arid1a-deficient DN4 cells compared with WT cells. OT1 transgenic TCR can rescue the defect in the transition from DN3 to DN4 stages, but not from DN to DP stages. Furthermore, we observed a comparable or stronger proliferation capacity accompanied by a significant increase in cell death in Arid1a−/− DP cells compared with that in WT controls. RNA-Seq analysis shows a significant enrichment of apoptotic pathway within differentially expressed genes between Arid1a−/− and WT DP cells, including the upregulation of Bim, Casp3 and Trp53 and the downregulation of Rorc, Bcl-XL and Mcl1. Therefore, our study reveals a novel mechanism that Arid1a controls early T-cell development by maintaining intracellular TCRβ expression-mediated β-selection and activating parallel cell survival pathways.
INTRODUCTION
T cells, as a key component of adaptive immunity, develop and mature in the thymus. Thymocyte development from early progenitors to matured T cells undergoes double-negative (DN), double-positive (DP) and single-positive (SP) stages based on surface CD4 and CD8 expression [1. DN stage is further divided into DN1, DN2, DN3 and DN4 sub-stages according to CD44 and CD25 expression. DN1 cells are capable to give rise to a small population of natural killer (NK) cells and dendritic cells (DCs), while the rest enter into DN2 stage. With a small subset of DN2 cells giving rise to γδ T cells, the majority of DN2 cells enter into DN3 stage with αβ lineage potential [2. The subsequent developmental events include T-cell receptor beta (TCRβ) rearrangement, β-selection and TCRα rearrangement to ensure thymocyte development from DN to DP cells [3. DP cells undergo positive and negative selection to give rise to either CD4 or CD8 single-positive cells [4, which migrate to peripheral organs after maturation.
Of note, cell survival is essential throughout thymic development in addition to lineage differentiation and proliferation [5. IL-7 and Notch signals are required for progenitor cell survival and differentiation at DN1-DN3 stages before β-selection by mediating Bcl-2 expression [6, 7. Subsequently, the rearranged TCRβ genes are expressed intracellularly, forming pre-TCR with pre-Tα and CD3 to ensure the transition from DN3/DN4 to DP stages [8. Pre-TCR signal tightly controls proliferation and survival through proper expression of Notch and E-proteins [5, as well as suppression of Bim and Bid through the inactivation of p53-mediated DNA damage [9, 10. Although prone to apoptosis, DP thymocytes are capable to survive and accumulate through the upregulation of Bcl-XL or Rorc [11-13. Additionally, NFAT, SPIB, Bcl11b and TCF-1 are involved in the regulation of thymocyte survival [14. However, there is still a lack of connection between pre-TCR signal and DP population size.
The AT-rich interaction domain 1A (Arid1a, also known as BAF250a) interacts with 9–11 subunits to form a SWI/SNF chromatin remodelling complex containing either brahma (Brm) or Brm-related gene 1 (Brg1) as the catalytic subunit [15-17. This remodelling system is critical for gene transcription and DNA replication and repair. Homozygous germline deletion of Brg1 produces early embryonic lethality, while the mutation in Brm results in minimal defects [18, 19. BRG1 deletion in T cells leads to a robust reduction of thymocyte number as well as a nearly complete block in the transition from DN4 to DP cell stages [20-22. In contrast, the core component of the murine SWI/SNF complex, SWI3-related gene (SRG3), is required for DP thymocyte development by maintaining positive selection [23-25. When our manuscript is during submission process, Astori et al. [26 report that Arid1a deficiency can result in a prominent developmental arrest at DN3 and DP stages. However, the underlying mechanism remains to be further investigated.
In this study, we have utilized an in vivo adoptive transfer model and an in vitro culture system to demonstrate that Arid1a deletion intrinsically blocks the transition of DN3-DN4 and DN4-DP stages in the thymus, as well as nearly a complete absence of peripheral T cells. Mechanistically, we found a significant defect in β-selection with a decrease in intracellular TCRβ expression and pre-Tα transcription in DN4 cells. Overexpression of OT1-TCR was able to rescue the developmental defect in the transition from DN3 to DN4. Furthermore, Arid1a-deficient DP cells exhibited an increase in apoptosis caused by the elevated expression of apoptotic genes (Casp3, Bim, Bid, Trp53, etc.) and a reduced expression of survival genes (Rorc, Bcl-XL, Mcl1, etc.). Therefore, our study reveals that Arid1a is required for the transition from DN3 to DN4, which is dependent on β-selection, as well as DP population size by controlling cell survival pathway.
MATERIALS AND METHODS
Mice
The Arid1afl//fl, LckCre and OT1TG mouse strains were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Arid1afl/fl mice generated as previously described [38 and backcrossed to B6 background for over 10 generations. Arid1a genotyping was performed using the forward (5′-TGG GCA GGA AAG AGT AAT GG-3′) and reverse primers (5′-AAC ACC ACT TTC CCA TAG G-3′) to generate a 116 bp wild-type (Arid1a+/+) product and a 170 bp Arid1afl/fl product. LckCre mice were crossed with Arid1afl/fl mice to generate Arid1afl/fl-LckCre (KO) mice and littermate control Arid1afl/fl (WT) mice. The LckCre transgene in KO mice was identified using the forward (5′-GCA GGA AGT GGG TAA CTA GAC TAA C-3′) and reverse primers (5′-TCT CCC GTC AGT ACG TGA GAT ATC-3′). The presence of the LckCre recombinase was indicated by an 800 bp product. Mice aged 6–8 weeks were used for analysis in the study. All mice were housed in specific pathogen-free conditions by the Xi'an Jiaotong University Division of Laboratory Animal Research. All animal procedures were approved by the Institutional Animal Care and Use Committee of Xi'an Jiaotong University.
Antibodies and reagents
The following antibodies and kits were purchased from BioLegend (San Diego, CA, USA): APC/Cy7 anti-CD4 (clone GK1·5), PE anti-CD8α (clone 53–6·7), PE/Cy7 anti-TCRβ (clone H57-597), FITC anti-TCRγδ (clone GL3), PE/Cy7 anti-CD44 (clone IM7), PE/Cy5 anti-CD25 (clone 3C7), FITC anti-NK1·1 (clone PK136), FITC anti-CD19 (clone 6D5), FITC anti-CD11b (clone M1/70), FITC anti-CD11c (clone N418), FITC anti-TER-119/Erythroid Cells (TER-119), APC/Cy7 anti-CD45·1 (clone A20), PE anti-CD45·2 (clone 104), Pacific Blue™ anti-Annexin V (Cat # 640918) and the Fixation/Permeabilization Solution Kit (Cat # 554722). PE anti-Ki67 monoclonal antibody (clone SolA15) was purchased from eBioscience (San Diego, CA, USA). Quick-RNA Microprep Kit (Cat # R1051) was obtained from Zymo Research (Irvine, CA, USA).
FACS and sorting
Lymphocytes from Arid1afl/fl, LckCre and Arid1afl/fl-LckCre mice were isolated. For intracellular and surface staining, cells were prepared into single-cell suspensions, and a total of 1 × 106 cells were stained in the dark at 4° for 30 min with indicated antibodies. The analysis was performed on a CytoFLEX flow cytometer (Beckman Coulter; Brea, CA, USA). DN3 (Lin− CD25+ CD44−) and DN4 (Lin− CD25− CD44−) cells were collected by BD FACSAria™ II cell sorter (BD Biosciences, San Jose, CA, USA). FACS data were recorded and analysed using CytExpert software (Version 2.3.0.84; Beckman Coulter; Indianapolis, IN, USA).
Intracellular Ki67 staining
DN and DP thymocytes were phenotyped using a combination of surface antibodies against lineage markers (CD4, CD8α, TCRβ, TCRγδ, NK1.1, CD19, CD11b, CD11c and Ter119), together with CD44 and CD25 and intracellular staining using anti-Ki67 antibody as previously described [39. All assays were performed on single-cell suspensions from freshly isolated thymic tissue. The Ki67 labelled cells were analysed on a CytoFLEX flow cytometer (Beckman Coulter).
Quantitative RT-PCR
Cell lysis was performed with RNA extraction and cDNA synthesis using Quick-RNA Microprep Kit (Zymo Research) and ReverTra Ace qPCR RT Master Mix Kit (TOYOBO), respectively. The qPCRs were carried out using StepOnePlus™ Real-Time PCR Systems (ABI) with SYBR mixture (Genstar) to determine relative gene expression.
RNA sequencing and data analysis
Total RNA was extracted from DP thymocytes using the Quick-RNA Microprep Kit (Zymo Research) according to the manufacturer's protocol. RNA quality was assessed using the Agilent RNA 6000 Nano Kit with the Agilent 2100 Bio analyser (Agilent Technologies).
Library construction and RNA sequencing on the BGISEQ-500 platform were conducted. The raw sequencing reads were filtered before downstream analyses by removing low-quality reads, adaptor-polluted reads and reads with more than 10% of unknown bases. Hierarchical indexing for spliced alignment of transcripts (HISAT) was used to map reads to the mm9 reference genome. The DEseq2 algorithms were used to detect differentially expressed genes (DEGs) between groups. Only genes with a fold change ≥2 and adjusted P-value ≤0·05 were considered as statistically significant DEGs. DEGs were performed GO functional enrichment using gene set enrichment analysis.
Bone marrow transplantation
Lineage-negative BM cells from Arid1afl/fl mice (CD45.1+) and Arid1afl/fl-LckCre mice (CD45.2+) were mixed at a 1:1 ratio and co-transferred into lethally irradiated recipient mice (CD45.1+ CD45.2+). Five weeks after bone marrow transplantation (BMT), thymocytes from recipient mice were harvested for FACS analysis.
In vitro OP9-DL1 cell co-culture system
Both Lin− CD44− CD25+ DN3 cells and Lin− CD44− CD25− DN4 cells were sorted from the thymi of Arid1afl/fl and Arid1afl/fl-LckCre mice and co-cultured with OP9-DL1 feeder cells in α-MEM medium in the presence of IL-7 (1 ng/ml, PeproTech) and Flt3-L (5 ng/ml, PeproTech). At Day 3 and Day 5, total cells were collected for FACS analysis.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was analysed using the GraphPad Prism 7.0 statistical program (GraphPad Software, La Jolla, CA, USA). All comparisons between two experimental groups were performed with an unpaired two-tailed Student's t-test. The levels of significance are indicated as follows: *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001.
RESULTS
Arid1a is essential for thymocyte differentiation from DN to DP stages
Since the mouse ENCODE project showed Arid1a is highly expressed in the thymus (Figure S1), we analysed Arid1a transcription at different stages of thymocyte development by qPCR. We observed that Arid1a is predominantly expressed in DP cells (Figure 1a), suggesting a potential requirement of Arid1a in DP thymocytes. To investigate whether Arid1a mediates thymocyte development, we generated T cell-specific Arid1a knock-out mice by crossing Arid1a-flox mice with LckCre transgenic strain. As LckCre can be toxic to thymocytes, particularly at the DP stage of thymocyte development [27, both Arid1afl/fl mice and hemizygous LckCre-positive (LckCre) mice were used as the control groups. Consistently, we observed a significant reduction in thymus size, abnormal structure of cortex and medulla (Figure 1b), along with the marked decreases in DP and CD4− CD8+ cells accompanied by the increases in DN and CD4+ CD8− cells compared with both Arid1afl/fl mice and LckCre mice (Figure 1c,e). While the total numbers of DN, DP, CD4+ CD8− and CD4− CD8+ cells were all dramatically reduced in Arid1a-deficient (Arid1afl/fl-LckCre, KO) mice (Figure 1f and Figure S2). Interestingly, most of CD4+ CD8− cells and CD4− CD8+ cells were lack of TCRβ expression (Figure 1d–f and Figure S2). Arid1a deletion caused an increase in percentage but not absolute cell number of thymic γδ T cells (Figure S3). Accordingly, there was a significant reduction of CD4+ and CD8+ T-cell populations but not γδ T cells in the spleen (Figure S4A,B) and lymph nodes (Figure S4C,D) of Arid1a-deficient mice.
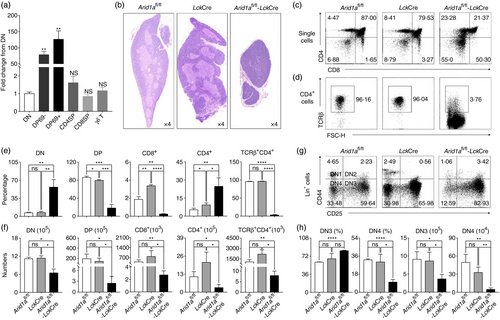
To further examine whether Arid1a is also required for the transition within DN stages, we analysed cell populations based on CD44 and CD25 expression in lineage negative cells (CD4− CD8− TCRβ− TCRγδ− CD19− CD11b− CD11c− NK1.1− Ter119−). Arid1a-deficient DN cells exhibited a significantly higher percentage of DN3 cells and lower percentage of DN4 cells in comparison with two control cells harvested from Arid1afl/fl mice and LckCre mice. (Figure 1g,h). Taken together, we definitively demonstrated an essential role of Arid1a in the transition of DN to DP stages. The LckCre strains exhibited a mild decrease in DP cells compared with Arid1afl/fl mice (85·91 ± 2·74 vs. 79·62 ± 1·45; *P < 0·05), which could not explicate the severe loss of DP thymocytes seen in Arid1a-deficient (Arid1afl/fl-LckCre) mice. Therefore, the Arid1afl/fl mice will be used as the WT group in the following experiments.
Arid1a deletion impaired the transition from DN3 to DN4 stages during thymocyte development
To demonstrate the intrinsic role of Arid1a in DP population, the OP9-DL1 cell co-culture system was used. DN3 cells co-cultured with OP9-DL1 feeder cells resulted in a significant decrease in DP population in Arid1a-deficient group compared with WT controls at Day 3 (Figure 2a,b) and Day 5 (Figure S5A,B). To further examine, we observed a marked increase in DN3 population and a significant decrease in DN4 population in Arid1a-deficient cells, as well as decreased ratios of DN4 to DN3 populations at Day 3 and Day 5 (Figure 2c,d). To further confirm whether the reduced DP population is thymocyte-intrinsic, we adoptively transferred a mixture of bone marrow cells from WT (CD45.1+) and Arid1a-deficient mice (CD45.2+) at a 1:1 ratio into lethally irradiated (7·5 Gy) WT recipient mice (CD45.1+ CD45.2+). Five weeks after transfer, thymocytes were harvested from recipients. Clearly, Arid1a-deficient cells were much lower than WT controls in the mixed chimeric mice (Figure S6). Arid1a-deficient donor cells exhibited the phenotype of DP cell reduction in recipient mice (Figure 2e,f). Moreover, as shown in Figure 2g,h and Figure S7A–D, Arid1a-deficient donor cells in competitive transfer model recapitulated the phenotype in KO mice. Therefore, we definitively demonstrated an intrinsic role of Arid1a in the transition from DN3 to DN4 stages.
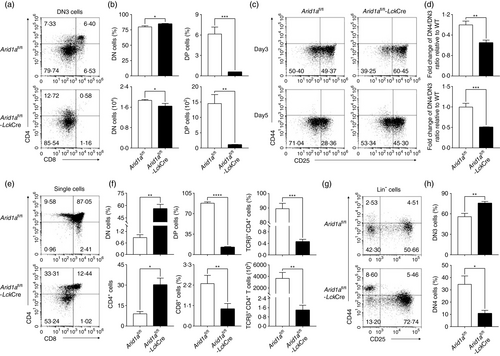
Arid1a deletion perturbed the differentiation from DN3 to DN4 stages through pre-TCR signals
It is well known that pre-TCR signals are responsible for progression from DN3 to DP stages [28. We examined TCRβ and pre-Tα expression in DN3 and DN4 cell populations. Flow cytometry analysis showed that the proportion of intracellular TCRβ (iTCRβ)-positive cells was significantly reduced in DN4 cells from Arid1a-deficient thymi compared with WT counterparts (Figure 3a,b). In addition, both bone marrow chimera mouse model (Figure 3c,d) and OP9-DL1 cell co-culture system (Figure 3e–h) exhibited less iTCRβ expression in Arid1a-deficient DN3 and DN4 cells compared with those in WT counterparts. Furthermore, the mean fluorescence intensity (MFI) of iTCRβ expression was also significantly decreased among these DN4 cell populations in Arid1a-deficient group (Figure S8A–D). In addition, Arid1a-deficient DN4 cells displayed significantly decreased Ptcra, Trbc1 and Trbc2 transcription compared with WT DN4 cells (Figure S9A–C). We also found a remarkable reduction of both CD5+ and CD28+ DN4 cells in KO mice compared with WT controls, indicating an impaired pre-TCR signalling in Arid1a-deficient mice (Figure 3i–l). To address whether Arid1a deficiency affects TCRβ rearrangement, we utilized PCR approach to assess the levels of Vβ5 and Vβ8. Arid1a-deficient DN3 and DN4 cells exhibited similar levels of Vβ5 and Vβ8 products to WT cells (Figure S10). Therefore, the defect in pre-TCR signal in Arid1a-deficient DN cells is most likely due to a decreased transcription of TCR genes including Ptcra, Trbc1 and Trbc2.
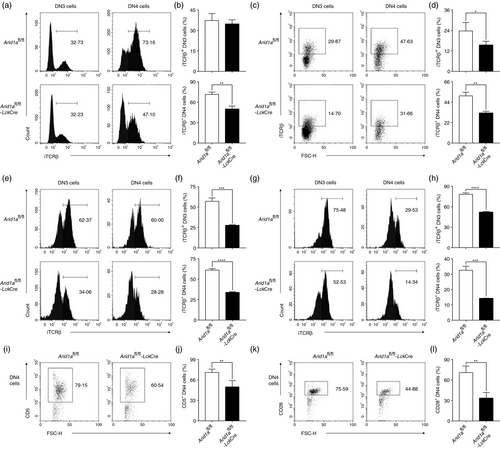
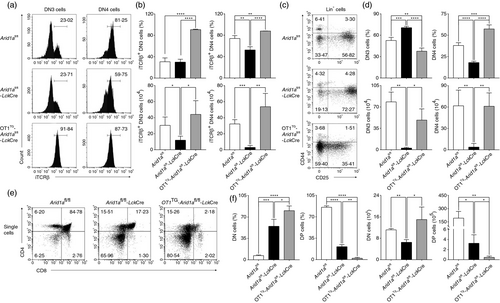
Next, we expressed a fixed TCR in Arid1a-deficient cells by crossing OT1 transgenic (OT1TG) mice to Arid1afl/fl-LckCre mice. Both DN3 and DN4 cells expressed high levels of iTCRβ despite Arid1a deletion (Figure 4a,b). Overexpressed OT1-TCR fully rescued both percentage and absolute number of DN4 cells (Figure 4c,d), but not DP cells (Figure 4e,f), in Arid1a-deficient mice. Therefore, Arid1a controls the differentiation from DN3 to DN4 stages dependent on pre-TCR signal and may affect DP cell development by as-yet-undiscovered mechanism.
Arid1a-deficient DP thymocytes are prone to apoptosis
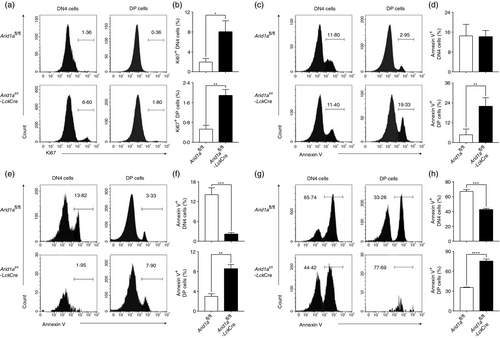
To determine whether the loss of DP cells was due to decreased proliferation or increased apoptosis, we evaluated Ki67 and Annexin V expression in DN4 and DP thymocytes. Although both DN4 and DP cells showed a lower expression of Ki67, the percentages of Ki67+ cells were higher in Arid1a-deficient cells compared with WT cells (Figure 5a,b). Interestingly, we found a significant increase of Annexin V+ cells in DP cells from Arid1a-deficient mice in comparison with that from WT mice (Figure 5c,d). Furthermore, both bone marrow chimera experiments (Figure 5e,f) and OP9-DL1 cell co-culture system (Figure 5g,h) confirmed a higher percentage of Annexin V+ cells in Arid1a-deficient DP thymocytes than WT cells. Taken together, these data demonstrate that the reduction of DP cells induced by Arid1a deficiency is due to increased apoptosis in the absence of proliferation defects.
Arid1a deficiency activated apoptosis-related pathways
To determine the mechanism underlying Arid1a-induced apoptosis in DP cells, we performed high-throughput RNA sequencing (RNA-Seq) to compare gene transcription between WT and Arid1a-deficient DP cells. The quality of sample and sequencing was validated based on mean of normalized counts, Pearson correlation across samples and RPKM distribution (Figure S11A–D). The analysis revealed 4162 upregulated genes and 1900 downregulated genes in Arid1a-deficient DP thymocytes compared with WT cells (Figure S11E).
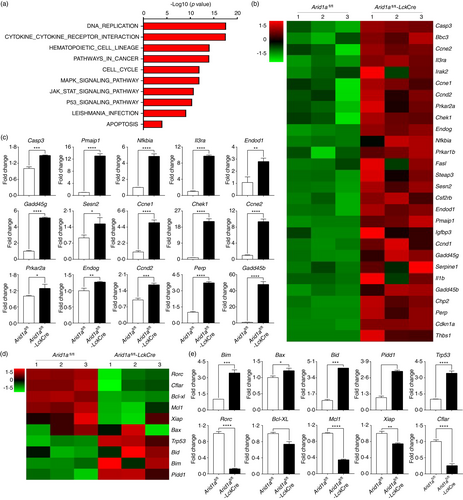
Kyoto encyclopaedia of genes and genomes (KEGG) pathways enriched two cell death-related pathways among the top 10 list, p53 signalling pathway and apoptosis pathway (Figure 6a). Heatmap revealed differential expression of genes belonging to the two pathways (Figure 6b,d). The gene expression changes were also validated by qPCR. Consistent with RNA-Seq data, Arid1a-deficient DP population displayed significantly increased expression of Casp3, Pmaip1 (alias Noxa), etc., compared with WT group (Figure 6c). Furthermore, we analysed canonical genes related to thymocyte survival. A number of pro-apoptotic genes showed increased expression including Bim, Bax, Bid, Pidd1 and Trp53. In addition, a list of genes supporting cell survival exhibited decreased transcription including Rorc, Bcl-XL, Mcl1, Xiap and Cflar (Figure 6d,e). Taken together, RNA-Seq analysis and qPCR results demonstrated that Arid1a suppresses the apoptosis pathway and activates cell survival mechanisms to protect DP cell from death.
DISCUSSION
The SWI/SNF complex is required for DP stage during the transition process in thymocyte development [20, 21. Astori et al. [26 reported that deletion of Arid1a in mice displayed a significant reduction of DP thymocytes, which is mainly due to the developmental block resided at the DN3-DN4 transition stage accompanied by reduced TCRβ expression. However, the mechanisms that Arid1a controls developmental block from DN to DP stages are still largely unclear. In this study, we further confirmed that deletion of Arid1a intrinsically impaired both DN3 to DN4, and DN4 to DP stage differentiation. Importantly, we demonstrated that the developmental block in DN3 to DN4 transitions is attributed to impaired pre-TCR signalling, while the defect in DP cells is caused by increased susceptibility to apoptosis.
Presumably, the SWI/SNF complex integrates 9–11 components to execute biological functions, and loss of each component may lead to a similar defect in thymocyte development. Indeed, deletion of either BRG1 [20-22, SRG3 [23-25 or Arid1a [26 caused a similar loss of DP thymocytes. Additionally, both Somasundaram's group and our study uncovered a new role of Arid1a in controlling the differentiation from DN3 to DN4 stages. It will be interesting to verify the functions of other components in the stage differentiation of early thymocyte development.
Thymocyte β-selection is an essential step allowing thymocyte transition from the DN3 to DN4 stage and maintaining adequate numbers of DP cells, which mainly relies on pre-TCR signal composed of a functional iTCRβ and pre-Tα. Pre-TCR signal regulates differentiation, proliferation and survival in the developmental process [29. Our results confirmed that Arid1a deficiency markedly impaired iTCRβ and pre-Tα expression in both DN3 and DN4 populations. But Arid1a-deficient DN3 and DN4 cells exhibited no defects in the rearrangement of TCRβ chain genes. Notch, ETS1 and GATA3 are known critical genes to participate in β-selection process [30-33; however, their expression did not alter in Arid1a-deficient DN3 and DN4 cells. Transgenic OT1-TCR expression was able to allow DN3 and DN4 cells to express relatively high level of iTCRβ, which completely rescued the developmental block during the DN3-DN4 transitions in Arid1a-deficient mice. It was also reported that p53 deletion could relieve the developmental block at the DN3-DN4 transition stage [34. However, p53 knockdown with shRNA retrovirus was unable to rescue the DN3 block in Arid1a-deficient thymocytes (Figure S12). Therefore, Arid1a controls the DN3-DN4 transition dependent on pre-TCR signals rather than p53 expression. Furthermore, forced OT1-TCR expression failed to rescue the DP population size supporting that Arid1a regulates DP thymocytes through other mechanisms.
The large population size of DP thymocytes is regulated by both cell proliferation and survival mechanisms. Arid1a-deficient DN and DP cells showed higher expression of Ki67, suggesting that there was no defect in cell proliferation. However, our data demonstrated significantly increased apoptosis in Arid1a-deficient DP cells. Of note, the reduction of thymic DP cells cannot be only explained by increased apoptosis, while it should partially result from the developmental block at DN stage. In contrast, Arid1a-deficient DN3 and DN4 cells exhibited similar apoptotic frequencies to WT cells. This is also supported by the finding that Brg1 also participates in the inhibition of immature thymocyte apoptosis [35, 36. Genes belonging to p53-regulated pathways and the Bcl-2 family, as well as other apoptosis-related genes, participate in the process [14, 37. Indeed, our RNA-Seq and qPCR analyses revealed the activation of p53 signalling and apoptosis pathway. In addition, the expression of several cell death-related genes (Bim, Bax, Bid and Trp53) was significantly increased, while the expression of cell survival-related genes (Rorc, Bcl-XL and Mcl1) was markedly decreased.
Taken together, our studies revealed a crucial role of Arid1a in the transition from DN3, DN4 to DP stages during thymocyte development. Arid1a controls DN3-to-DN4 transitions through pre-TCR signal and regulates DP population size by promoting cell survival. Future studies should investigate how Arid1a regulates iTCRβ and pre-Tα expression.
ACKNOWLEDGEMENT
We thank Prof. Enqi Liu from Laboratory Animal Center for mouse colony maintenance, and Prof. Chen Huang and Dr. Xiaofei Wang from Department of Cell Biology and Genetics for flow cytometric analysis and cell sorting.
CONFLICT OF INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
X.Y., L.L., X.W., Y.S., C.S., D.Z. and B.Z. analysed the data and wrote the original draft. X.Y., X.W., L.L., J.L. and W.L. performed the FACS analysis. Y.S., A.J., H.L., R.D. and C.Z. collected samples and provided bioinformatic analysis. C.S., L.S. and X.Z. discussed data analysis. C.S. and B.Z. generated the idea, designed the experiment and wrote the manuscript.




