The role of Toll-like receptor 10 in modulation of trained immunity
Summary
Toll-like receptor 10 (TLR10) is the only member of the human Toll-like receptor family with an inhibitory function on the induction of innate immune responses and inflammation. However, its role in the modulation of trained immunity (innate immune memory) is unknown. In the present study, we assessed whether TLR10 modulates the induction of trained immunity induced by β-glucan or bacillus Calmette–Guérin (BCG). Interleukin 10 receptor antagonist production was increased upon activation of TLR10 ex vivo after BCG vaccination, and TLR10 protein expression on monocytes was increased after BCG vaccination, whereas anti-TLR10 antibodies did not significantly modulate β-glucan or BCG-induced trained immunity in vitro. A known immunomodulatory TLR10 missense single-nucleotide polymorphism (rs11096957) influenced trained immunity responses by β-glucan or BCG in vitro. However, the in vivo induction of trained immunity by BCG vaccination was not influenced by TLR10 polymorphisms. In conclusion, TLR10 has a limited, non-essential impact on the induction of trained immunity in humans.
Abbreviations
-
- BCG
-
- bacillus Calmette–Guérin
-
- BSA
-
- bovine serum albumin
-
- IFN-γ
-
- interferon-γ
-
- IL
-
- interleukin
-
- IL-1Ra
-
- IL-1 receptor antagonist
-
- LPS,
-
- lipopolysaccharide
-
- mAb
-
- monoclonal antibody
-
- QTL
-
- quantitative trait loci
-
- SNP
-
- single-nucleotide polymorphism
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- tumor necrosis factor-α
Introduction
Toll-like receptors (TLRs) are pattern recognition receptors that are essential to provide a first line of defense upon invasion of pathogens. TLRs activate intracellular signaling pathways upon recognition of pathogen-associated molecular patterns, which results in activation of the innate immune response. Ten human TLRs have been described, and although the function of most TLRs has been studied extensively, there is relatively limited knowledge on the biological role of TLR10 during the human immune response.1, 2
Human TLR10 was discovered in 2001,3 and is located on chromosome 4 within the TLR2 gene cluster, together with TLR1 and TLR6. TLR10 is structurally similar to the other TLR family members and is phylogenetically most related to TLR1 and TLR6.3 TLR10 mRNA is highly expressed in lymphoid tissues,3 and is expressed in different immune cells, including B cells, dendritic cells,4-6 and monocytes.4, 7 Currently, there is no natural ligand identified for TLR10. TLR10 is able to homodimerize, as well as heterodimerize with TLR1 and TLR2.6, 7 Surprisingly, TLR10 does not induce the classical TLR downstream signaling pathway, even though it has been shown that it can associate with myeloid differentiation primary response 886 and protein kinase B.7
Although it was initially reported that TLR10 had a pro-inflammatory function,8, 9 the majority of reports have demonstrated anti-inflammatory properties of TLR10.4, 7, 10, 11 TLR10 single nucleotide polymorphisms (SNPs) strongly influence pro-inflammatory cytokine production in humans, and in hTLR10 transgenic mice, significantly lower interleukin-6 (IL-6) and KC (mouse analog of human IL-8) concentrations in plasma were found compared with wild-type mice.7 TLR10 has been shown to be involved in the induction of innate immune responses to influenza and is involved in Helicobacter pylori infection.9, 12, 13 Moreover, polymorphisms in TLR10 have been associated with various diseases, including complicated skin and skin-structure infections,14 tuberculosis,15 prostate cancer16 and Crohn's disease.17, 18
It is well documented that IL-1β is one of the crucial cytokines involved in innate immune memory and in the induction of trained immunity.19 Trained immunity is a process in which human innate immune cells such as monocytes can undergo extensive metabolic and epigenetic reprogramming upon certain infections or vaccinations, resulting not only in long-term enhanced immune responses and resistance to heterologous infections,20 but also in induction of maladaptive immune responses in inflammatory diseases when cells are stimulated by endogenous ligands.21 Due to the important modulatory role of TLR10 on innate immune responses, including the production of the anti-inflammatory cytokine IL-1 receptor antagonist (IL-1Ra),7 we hypothesized that TLR10 may also regulate the induction of trained immunity.
Materials and methods
Reagents
RPMI-1640 (Dutch modified; Gibco, Life Technologies, Waltham, MA) was used as culture medium supplemented with 5 μg/ml gentamicin (Centrafarm B.V., Etten-Leur, the Netherlands), 2 mm l-glutamine (Gibco), and 1 mm pyruvate (Gibco). Mouse anti-TLR10 monoclonal antibody (mAb) 3C10C5 (Sanbio, Tokyo, Japan) was used, and mouse IgG1 isotype control (R&D Systems, Minneapolis, MN) as control. Synthetic Pam3SK4 (Pam3Cys) was purchased from EMC Microcollections (Tübingen, Germany) and Borrelia burgdorferi, ATCC strain 35210, was cultured at 33° in Barbour–Stoenner–Kelley-H medium (Sigma, St Louis, MO) supplemented with 6% rabbit serum. Spirochetes were grown to late-logarithmic phase and motility was tested by dark-field microscopy. Spirochetes were counted using a Petroff–Hauser counting chamber. Upon centrifugation of the culture at 3000 g for 30 min, bacteria were harvested and subsequently washed twice using sterile phosphate-buffered saline (PBS), and diluted with medium to a concentration of 1 × 106 spirochetes per ml. Aliquots were kept at −20°. β-Glucan (β-1,3-(d)-glucan) was kindly provided by Professor David Williams (East Tennessee State University, Johnson City, TN), and bacillus Calmette–Guérin (BCG) vaccine was purchased from InterVax (Markham, ON, Canada). Interferon-γ (IFN-γ) was purchased from R&D Systems, and bovine serum albumin (BSA) was purchased from Sigma-Aldrich.
Blood samples
Peripgheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy blood donors (Sanquin, Nijmegen, the Netherlands). Venous blood of healthy volunteers was drawn and genotyped after informed consent (Radboudumc, Nijmegen, the Netherlands). TLR10 mRNA expression by RNA sequencing was measured in 30 healthy Dutch men who were randomly assigned to receive either BCG (SSI, Copenhagen, Denmark) or placebo (the diluent used to dissolve BCG) in a double-blind fashion.22 Blood was drawn before vaccination and 1 month later (see Supplementary material, Fig. S1a). Isolated monocytes at these time-points were lysed in TRIzol reagent and stored at −80°. The study was approved by the Arnhem-Nijmegen Medical Ethical Committee, NL50160.092.24.
TLR10 stimulation, cell surface expression in monocytes, and quantitative trait locus (QTL) mapping were studied in a cohort of healthy volunteers vaccinated with BCG (300BCG cohort). In this cohort, 321 healthy Dutch individuals (after exclusion of individuals who did not meet the inclusion criteria, see below) of Western European descent (139 men and 182 women, age range 18–71 years) were included and vaccinated with BCG (InterVax). Before vaccination, and 2 weeks and 3 months after vaccination, blood was drawn, and PBMCs were isolated (Fig. 1b). The effect of TLR10 SNP N241H on trained immunity in vitro was studied in the same cohort. This in vitro assay was performed with isolated PBMCs as described below. The 300BCG study was approved by the Arnhem-Nijmegen Medical Ethical Committee, NL58553.091.16. Volunteers were BCG naive and had not lived in an area where tuberculosis was endemic. Inclusion of volunteers and experiments was conducted according to the principles expressed in the Declaration of Helsinki. All volunteers gave written informed consent before any material was taken.
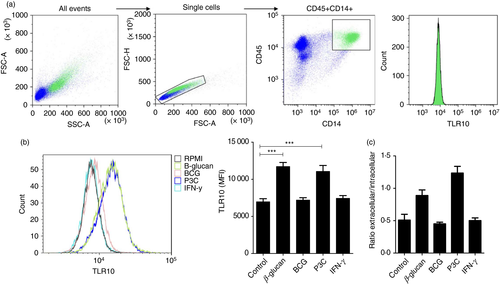
PBMC isolation and stimulation
Cells were isolated by density centrifugation on Ficoll-Paque (GE Healthcare, Chalfont St Giles, UK), washed three times in PBS and resuspended in culture medium. After isolation, 500 000 PBMCs were added to a round-bottom 96-well plate (Greiner, Kremsmünster, Austria). Anti-TLR10 (10 μg/ml) or IgG1 isotype control (10 μg/ml) was pre-incubated for 1 hr and subsequently Pam3Cys (10 μg/ml) or B. burgdorferi spirochetes (1 × 106/ml) were added for 24 hr at 37° and 5% CO2. For cross-linking of TLR10, anti-TLR10 or IgG1 isotype control (10 μg/ml) was coated to a flat-bottom 96-well plate (Corning, Corning, NY) for 2 hr, washed with PBS, and blocked for 1 hr with 1% BSA in PBS. After washing twice with PBS, 500 000 PBMCs were added in each well and subsequently incubated for 24 hr at 37° in 5% CO2.
Monocyte isolation and stimulation
Percoll monocytes were isolated by layering hyper-osmotic Percoll solution [48·5% Percoll (Sigma-Aldrich), 41·5% sterile H2O, 0·16 m filter-sterilized NaCl] on PBMCs. After 15 min centrifugation at 580 g, the interphase layer was isolated, cells were washed with PBS, and resuspended in culture medium. Percoll monocytes were added to a polypropylene tube (1 million per tube) and the stimuli or RPMI-1640 control were added. Cells were cultured for 24 hr at 37° in RPMI-1640 supplemented with 10% human pooled serum. To stimulate the cells, β-glucan (5 μg/ml), BCG (10 μg/ml), Pam3Cys (10 μg/ml) or IFN-γ (20 ng/ml) was used. After 24 hr, cells were used for flow cytometry experiments.
In vitro trained immunity assay
To increase the purity of Percoll-isolated monocytes, the monocytes were adhered to polystyrene flat-bottom plates (Corning) for 1 hr at 37° followed by washing with warm PBS. Next, cells were pre-incubated with culture medium supplemented with 10% human pooled serum as control, or together with IgG1 isotype control or anti-TLR10 antibody (10 μg/ml) for 1 hr. Subsequently, culture medium supplemented with 10% human pooled serum was added as a control, or together with either 2 μg/ml β-glucan, or 5 μg/ml BCG. After 24 hr, cells were washed with warm PBS and culture medium was added. Culture medium was refreshed after 3 days of incubation. On day 6, cells were re-stimulated with RPMI-1640, lipopolysaccharide (LPS) (10 ng/ml), or Pam3Cys (10 μg/ml). After 24 hr, supernatants were collected and stored at −20° until further use (see Supplementary material, Fig. S1c). In the in vitro system used, monocytes differentiated into macrophages. However, the monocytes previously trained with β-glucan or BCG undergo a different functional program characterized by increased responsiveness even long after the stimulus has been removed. This effect has been termed trained immunity.
Cytokine measurements
Cytokine production was measured in supernatants using commercial sandwich ELISA kits for human IL-6, tumor necrosis factor-α (TNF-α), IL-1β and IL-1Ra (R&D Systems), in accordance with the manufacturer's instructions.
Flow cytometry
Cells were washed with PBS supplemented with 1% BSA and thereafter labeled with fluorochrome-conjugated mAbs. After 30 min incubation at 4°, cells were washed, and subsequently incubated for 45 min at 4° with Fix'n'Perm (Thermo Fisher Scientific, Waltham, MA) and washed with Perm Buffer (10× diluted Perm Buffer; BD Biosciences, San Jose, CA). Next, cells for extracellular cell staining were measured using a flow cytometer (CytoFLEX; Beckman Coulter, Brea, CA). For intracellular staining, cells were then labeled with mAbs for intracellular staining for 15 min at room temperature. After washing with Perm Buffer (Thermo Fisher Scientific), these cells were measured. TLR10 expression was measured in a small subset of volunteers of the 300BCG cohort by adding antibodies to whole blood, followed by incubation for 10 min at room temperature and subsequent addition of lysis buffer (Thermo Fisher Scientific). Cells were incubated for 10 min, then measured on the CytoFLEX. The following mAbs were used: mouse anti-human CD45-allophycocyanin, CD45-knockout, CD14-phycoerythrin-Cy5 (Beckman Coulter) and TLR10-phycoerythrin (Thermo Fisher Scientific).
RNA sequencing
Monocytes were treated as described above, with either RPMI-1640 as a control, LPS 10 ng/ml or 5 μg/ml β-glucan. Monocytes (1·5 × 106) were seeded in six-well plates. At baseline, after 1 hr, 4 hr, 24 hr, 3 days, 6 days and 24 hr after LPS re-stimulation cells were lysed in TRIzol and stored at −80° until further analysis as described earlier.23 For BCG-vaccinated volunteers, monocytes were isolated before and 1 month after BCG vaccination from peripheral blood. From both experiments, total RNA was extracted from cells using the Qiagen RNeasy RNA extraction kit, using on-column DNaseI treatment. Ribosomal RNA was removed using the Ribo-Zero rRNA removal kit. RNA was then fragmented into 200-bp fragments by incubation for 4 min at 95° in fragmentation buffer (200 mm Tris–acetate, 500 mm potassium acetate, 150 mm magnesium acetate, pH 8·2). First-strand cDNA synthesis was performed using SuperScript III, followed by synthesis of the second cDNA strand. Library preparation was performed using the KAPA HyperPrep kit, using the USER enzyme to degrade the second cDNA strand. Quality of cDNA and the efficiency of ribosomal RNA removal were confirmed using quantitative RT-PCR using the IQ SYBR Supermix, with primers for GAPDH, 18S and 28S rRNA. TLR10 expression values (RPKM) were extracted from RNA-sequencing data. Raw data files have been deposited in the NCBI Gene Expression Omnibus under accession numbers GEO: GSE104149 respectively GSE85243.
Genetic analysis
To investigate whether genetic variation influences trained immunity responses, cytokine QTL mapping was conducted using the genotypes and ex vivo cytokine measurements upon Staphylococcus aureus stimulation before, and 2 weeks and 3 months after vaccination of healthy individuals of Western European ancestry from the 300BCG cohort (NL58553.091.16). DNA samples of 325 individuals were genotyped using the commercially available SNP chip, Infinium Global Screening Array MD v1.0 from Illumina (San Diego, CA). Genotype information on approximately 4 million SNPs was obtained upon imputation (MAF > 5% and R2 > 0·3 for imputation quality). Both genotype and cytokine data on innate memory responses were obtained for a total of 296 individuals. Genetic outliers, three samples due to medication use (of which one was identified as a genetic outlier), one sample due to onset of type 1 diabetes during the study, and 18 evening vaccinated individuals were excluded before QTL mapping. First, raw cytokine levels were log-transformed and ratios of cytokine production between the visits were taken as the change of cytokine levels. The cytokine changes were mapped to genotype data using a linear regression model with age and sex as covariates.
Statistics
Data were analyzed using a Wilcoxon signed-rank test or a Mann–Whitney U-test using GraphPad Prism software (version 5.03; GraphPad, San Diego, CA). Data are expressed as mean ± SEM of three or more independent experiments, and values of P < 0·05 were considered statistically significant.
Results
TLR10 expression on the cell surface of monocytes increases upon stimulation
Monocytes are important immune cells, and they have been shown to express TLR10.4, 7 We investigated TLR10 expression in monocytes stimulated for 24 hr with different stimuli. Total TLR10 expression gated on CD45+ CD14+ cells (Fig. 1a) was significantly increased when stimulated with β-glucan or Pam3Cys, but not BCG or IFN-γ (Fig. 1b). Total TLR10 expression in monocytes was higher than membrane-associated TLR10 expression (see Supplementary material, Fig. S2), indicating the presence of TLR10 in the cell. In addition, as seen in Fig. 1(c), upon TLR10 stimulation with β-glucan or Pam3Cys, the presence of TLR10 on the cell surface was mainly affected, and not intracellular TLR10 expression. Furthermore, the inhibitory function of TLR10 on the innate immune system was validated. PBMCs were stimulated with TLR2 ligands Pam3Cys or B. burgdorferi, which support heterodimer formation and subsequent activation of TLR10. An anti-TLR10 antibody was used to block the receptor: this resulted in increased pro-inflammatory cytokine production (see Supplementary material, Fig. S3a), supporting the concept that TLR10 is an anti-inflammatory receptor. On the other hand, TLR10 was stimulated by coating anti-TLR10 antibodies on the well (receptor cross-linking) and subsequently adding PBMCs, which results in production of anti-inflammatory cytokine IL-1Ra (see Supplementary material, Fig. S3b).
TLR10 expression during induction of trained immunity
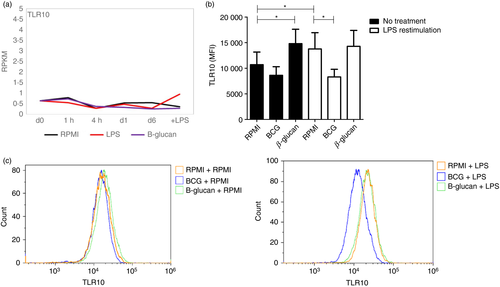
To identify if TLR10 is involved in the induction of trained immunity, we first examined TLR10 expression in monocytes using an in vitro trained immunity model.24 We induced trained immunity in vitro by incubating monocytes with β-glucan for 24 hr and re-stimulation at day 6 with LPS, as a non-specific stimulus. TLR10 mRNA expression was not affected during the induction of trained immunity (Fig. 2a). Thereafter, TLR10 protein expression was determined by flow cytometry upon β-glucan- and BCG-induced trained immunity in monocytes in vitro. On average, membrane-associated TLR10 expression on day 7 was fourfold higher compared with day 1 (see Supplementary material, Fig. S4), demonstrating up-regulation of TLR10 protein on the cell surface during differentiation of monocytes to monocyte-derived macrophages. As seen in Fig. 2(b), TLR10 expression is not affected upon LPS re-stimulation in β-glucan- and BCG-stimulated cells in vitro, whereas TLR10 expression in unstimulated cells was altered. Overall, these results show that TLR10 mRNA, as well as TLR10 protein expression, is not affected during β-glucan- and BCG-induced trained immunity in vitro.
TLR10 affects trained immunity response in monocytes
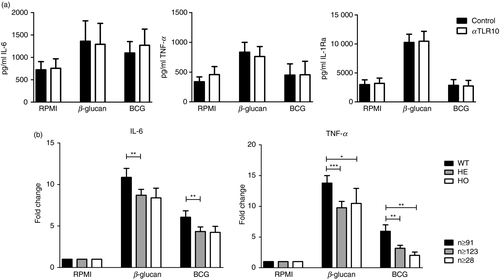
To assess whether TLR10 itself is able to modulate trained immunity, trained immunity was induced in monocytes in vitro by β-glucan or BCG in the presence or absence of an anti-TLR10 antibody. As seen in Fig. 3(a), addition of anti-TLR10 antibody did not significantly alter IL-6, TNF-α and IL-1Ra cytokine production upon Pam3Cys re-stimulation in β-glucan- and BCG-trained conditions. To further investigate whether TLR10 may influence trained immunity, we investigated the effect of TLR10 SNP N241H (rs11096957) on trained immunity. This missense mutation is associated with several diseases with an inflammatory pathophysiology,14, 16, 25 and individuals homozygous for this SNP show increased pro-inflammatory cytokine production.7 As seen in Fig. 3(b), individuals who are heterozygous or homozygous for this SNP show decreased IL-6 and/or TNF-α production upon secondary stimulation after β-glucan-, or BCG-induced trained immunity in vitro, suggesting that TLR10 positively affects trained immunity.
TLR10 expression in BCG-vaccinated healthy individuals
As BCG vaccination is known to induce trained immunity in vivo,19 TLR10 mRNA expression was assessed before and 1 month after BCG vaccination. As seen in Fig. 4(a), BCG vaccination did not affect TLR10 mRNA expression. However, 2 weeks after BCG vaccination, increased TLR10 cell surface expression was observed (Fig. 4b). Moreover, upon engagement of TLR10, more IL-1Ra was produced 2 weeks after BCG vaccination compared with before BCG vaccination (Fig. 4c), suggesting that TLR10 plays a role in BCG-induced trained immunity in vivo. To validate the findings of in vitro BCG-induced trained immunity, the effect of TLR10 genetic polymorphisms on cytokine production upon BCG-induced trained immunity was assessed in the 300BCG cohort from the Human Functional Genomics Study. In this study, cis cytokine- QTL mapping was performed in 296 healthy individuals of ex vivo cytokine production upon S. aureus stimulation before versus after BCG vaccination, representing a trained immunity response. No significant cis cytokine-QTL was observed for the TLR10 gene, indicating that TLR10 is not essential for the BCG-induced trained immunity response.

Discussion
Toll-like receptor 10 is the only anti-inflammatory member of the TLR family. This anti-inflammatory role is confirmed in the present study, in which blockade of the TLR10 receptor up-regulated pro-inflammatory cytokine production of PBMCs that were stimulated with TLR ligands. Furthermore, we expanded the understanding of the role of TLR10 in regulating innate immunity, by studying the role of TLR10 in trained immunity in vitro and in vivo. While a TLR10 missense SNP affected BCG- and β-glucan-induced trained immunity in vitro, no TLR10 genetic variant significantly affected the induction of trained immunity by BCG vaccination in healthy individuals.
Recently, it has been shown that TLR10 is mainly expressed in the endosomes of a human monocytic cell line. It has been suggested that intracellular TLR10 recognizes double-stranded RNA and thereby suppresses IFN type I production.26 Here we show that TLR10 is expressed both extracellularly and intracellularly in human CD45+ CD14+ monocytes. Additionally, upon stimulation, extracellular TLR10 expression increases whereas intracellular TLR10 expression is not affected. This suggests that TLR10 exerts its main biological function on the cell surface of monocytes, most likely as an anti-inflammatory receptor competing with ligands for other TLRs. Upon stimulation of PBMCs in the presence of a blocking anti-TLR10 antibody, increased production of pro-inflammatory cytokines IL-6, TNF-α and IL-1β was observed. In addition, we report an increased production of IL-1Ra, the inhibitor of IL-1 signaling, upon engagement and cross-linking of TLR10. These anti-inflammatory properties of TLR10 were also observed in earlier studies,4, 7, 10, 11 confirming that TLR10 functions as an anti-inflammatory receptor.
The importance of trained immunity in autoimmune and inflammatory diseases is becoming increasingly clear.27 It is therefore essential to identify the modulatory mechanisms that control the inappropriate induction of trained immunity, and we hypothesized that TLR10 may have such a regulatory role. TLR10 mRNA as well as TLR10 protein expression is not altered during the induction of β-glucan- and BCG-induced trained immunity in vitro. To investigate whether the induction of trained immunity is affected by TLR10, an anti-TLR10 antibody was added while inducing trained immunity in vitro. This tended to reduce pro-inflammatory cytokine responses in β-glucan- or BCG-induced trained immunity, but these results were not statistically significant. To provide a last argument towards a role, or the absence of it, for TLR10 in regulating trained immunity, we studied the effect of TLR10 SNP N241H on β-glucan- and BCG-induced cytokine production. This SNP is a missense mutation associated with a reduced risk of prostate cancer,16 complicated skin and skin structure infections,14 and osteoarthritis.25 We previously showed that healthy individuals carrying this SNP display increased pro-inflammatory cytokine production upon direct TLR2 stimulation, suggesting a reduction of the anti-inflammatory actions of TLR10.7 Surprisingly, in the current study, we demonstrate that healthy individuals who are heterozygous or homozygous carriers of this SNP show decreased cytokine production upon β-glucan- and BCG-induced trained immunity in vitro. This suggests that the effect of TLR10 on innate immune responses differs depending on the timing and setting of TLR stimulation: inhibiting pro-inflammatory responses upon direct stimulation, while promoting trained immunity.
Furthermore, to provide a definitive proof of the importance of TLR10 for the induction of trained immunity, we assessed its effects on trained immunity induced in vivo in a cohort of healthy individuals who received BCG vaccination. Confirming the in vitro experiments, the mRNA expression of TLR10 was not affected upon BCG-induced trained immunity in vivo. Yet, increased TLR10 expression on the cell surface of monocytes was observed, as well as an increased ability to produce IL-1Ra upon engagement of TLR10 ex vivo. However, TLR10 polymorphisms were not associated with the capacity of BCG vaccination to induce heterologous cytokine responses, a hallmark of trained immunity. This argues that while TLR10 expression is affected upon BCG vaccination, it does not significantly contribute to the process of trained immunity in vivo. Importantly, these analyses were performed on a relatively small cohort of 296 individuals, and larger cohorts may still identify small effects. However, a major role of TLR10 for the modulation of trained immunity is unlikely.
To conclude, we confirm anti-inflammatory properties of TLR10 and observed increased expression of TLR10 on the cell surface of monocytes upon direct stimulation. Furthermore, TLR10 moderately affects the induction of β-glucan- and BCG-induced trained immunity in vitro. However, genetic variation in the TLR10 gene does not regulate the induction of trained immunity by BCG vaccination in vivo, arguing against an essential role of TLR10 in this process.
Acknowledgements
This study was supported by a Veni grant (no. 016.176.006; MO) and an EFRO grant (to LABJ and MGN). MGN was partly supported by a Spinoza grant of the Netherlands Organization for Scientific Research. BN is supported by an NHMRC (Australia) CJ Martin Fellowship. We thank the UMCG Genomics Coordination Center, the UG Center for Information Technology and their sponsors BBMRI-NL and TarGet for storage and computer infrastructure.
Disclosure
The authors declare no conflict of interest.
Author contributions
The experiments were performed by VPM, RJWA, LCJB, VACMK, SJCFMM, JHP, LG, CDCCH and STK, and were analyzed by VPM, BN, MO and VM. The manuscript was written by VPM, LABJ, MGN and MO, and reviewed and edited by RJWA, BN, VM, LCJB, VACMK, SJCFMM, JHP, LG, CDCCH and STK.




