Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation
Summary
Human cathelicidin LL-37 protects against infections and endotoxin-induced inflammation. In a recent study we have shown that IG-19, an LL-37-derived peptide, protects in a murine model of arthritis. Cytokine interleukin-32 (IL-32) is elevated and directly associated with the disease severity of inflammatory arthritis. Therefore, in this study we examined the effects of LL-37 and IG-19 on IL-32-induced responses in human peripheral blood-derived mononuclear cells (PBMC) and macrophages. We showed that CD14+ monocytes are the primary cells that produce pro-inflammatory tumour necrosis factor-α (TNF-α) following stimulation of PBMC with IL-32. We demonstrated that LL-37 and IG-19 significantly suppress IL-32-induced production of pro-inflammatory cytokines, e.g. TNF-α and IL-1β, without altering chemokine production. In contrast, LL-37 and IG-19 enhance the production of the anti-inflammatory cytokine IL-1RA. Further mechanistic studies revealed that LL-37 and IG-19 suppress IL-32-mediated phosphorylation of Fyn (Y420) Src kinase. In contrast, IL-32-mediated phosphorylation of AKT-1 (T308) and MKP-1 (S359) is not suppressed by the peptides. LL-37 and IG-19 alone induce the phosphorylation of MKP-1 (S359), which is a known negative regulator of inflammation. Furthermore, the peptides induce the activity of p44/42 mitogen-activated protein kinase, which is known to phosphorylate MKP-1 (S359). This is the first study to demonstrate the regulation of IL-32-induced inflammation by LL-37 and its derivative peptide IG-19. The mechanistic results from this study suggest that regulation of immune-mediated inflammation by these peptides may be controlled by the dual phosphatase MKP-1. We speculate that LL-37 and its derivatives may contribute to the control of immune-mediated inflammatory diseases.
Abbreviations
-
- CHDPs
-
- cationic host defence peptides
-
- PBMC
-
- peripheral blood-derived mononuclear cells
-
- MDM
-
- monocyte-derived macrophages
-
- MAPK
-
- mitogen-activated protein kinase
Introduction
Cationic host defence peptides (CHDPs) are small peptides with < 50 amino acids, an overall charge of + 2 to + 7 and ≥ 30% hydrophobic residues. CHDPs are known to confer protection against bacterial, viral and parasitic infections, and modulate immune responses in the host.1-10 Research in the last two decades has demonstrated a wide range of immune functions mediated by CHDPs that influence both innate and adaptive immunity (reviewed in refs 11-13). The two best characterized families of CHDPs in mammals are cathelicidins and defensins. Mammalian cathelicidins are found in granules of neutrophils, myeloid precursors, epithelial cells, mast cells, lymphocytes and keratinocytes, and are expressed in a wide variety of tissues and body fluids. The only human cathelicidin is a 37-amino-acid peptide known as LL-37.14
LL-37-mediated processes of immune activation include facilitation of immune cell recruitment by chemotaxis,15-17 promotion of autophagy,18 phagocytosis,19 wound healing20 and influencing initiation of adaptive immune responses.21, 22 Most of these functions can be described as pro-inflammatory responses required for resolution of infections and tissue repair. However, LL-37 also plays a significant role in controlling pathogen-induced inflammation and maintaining immune homeostasis. Physiological concentrations of LL-37 suppress endotoxin-induced inflammatory responses by intervening in the Toll-like receptor-to-nuclear factor-κB (NF-κB) pathway.8, 23 Likewise, LL-37 was shown to confer protection in animal models of pathogenic sepsis.24, 25 Altered expression of LL-37 has also been reported in various chronic inflammatory diseases. For example, LL-37 is suppressed in Crohn's disease and atopic dermatitis, but elevated in psoriasis and systemic lupus erythematosus.26-29 However, the role of LL-37 in immune-mediated chronic inflammation remains largely unresolved. Recent studies have suggested that cathelicidins may be protective in certain immune-mediated inflammatory diseases.30-33 Consistent with this, in a recent study we demonstrated that an LL-37-derived peptide IG-19 can indeed alleviate the disease process in a murine model of inflammatory arthritis.34 The peptide IG-19 represents an internal sequence of LL-37 (amino acids 13–31) and was previously shown to be the minimum region required for the immunomodulatory functions mediated by LL-37.35 Therefore, in this study we aimed to investigate the underlying mechanism of the protective function of peptide IG-19 in inflammatory arthritis.34
A critical element in chronic inflammation is the loss of cytokine balance.36 Certain downstream effector cytokines such as interleukin-15 (IL-15), IL-18, IL-17 and IL-32 are found to be elevated in chronic inflammatory diseases.37-41 These ‘chronic inflammatory’ cytokines in turn induce the production of the other acute pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α) and IL-1β, resulting in the amplification and continuation of inflammation.36-41 In this study we examined the effect of peptides LL-37 and IG-19 on cellular responses induced in the presence of IL-32, a signature chronic inflammatory cytokine. Pro-inflammatory cytokine IL-32 is produced by immune cells such as mitogen-activated lymphocytes, activated natural killer cells, as well as by non-immune cells.42-45 IL-32 induces robust production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and chemokines [growth regulated oncogene-α (GRO-α), IL-8 and macrophage inflammatory protein 2], induces histamine release, and activates NF-κB and p38 mitogen-activated protein kinase (MAPK) signalling pathways.41, 46-48 There are six splice variants of IL-32 and the isoform IL-32γ is the most biologically active isoform.49 In a previous study we had shown that IL-32γ induces a significant production of inflammatory cytokines in immune cells such as macrophages.48 In this study we showed that following stimulation of peripheral blood-derived mononuclear cells (PBMC) with IL-32γ, CD14+ monocytic cells are the primary cell types that produce TNF-α. Therefore, in this study we examined the effect of the peptides LL-37 and IG-19 on IL-32γ-induced downstream protein production and signalling intermediates in human PBMC and macrophages. This is the first study to demonstrate that LL-37 and its derivative peptide IG-19 regulate cytokine IL-32γ-mediated inflammation, suggesting a potential role for these peptides in the control of immune-mediated inflammation in chronic inflammatory diseases. This study also demonstrates that the regulatory mechanism of LL-37 and its derivative peptide IG-19 in controlling IL-32-induced signalling events and downstream inflammation may be mediated by the dual phosphatase MKP-1.
Materials and methods
Cell isolation and culture
Venous blood was collected from healthy volunteers in accordance with a protocol approved by the University of Manitoba Ethics Review Board. Blood was diluted 1 : 1 with complete RPMI-1640 medium [containing 1 mm sodium pyruvate and 10% (volume/volume) fetal bovine serum]. Diluted blood was separated over Ficoll-Paque Plus (GE Healthcare Life Sciences, Baie d’Urfe Quebec, Canada). The buffy coat was isolated, washed twice in complete RPMI-1640 medium (300 g for 10 min). Human PBMC obtained from the buffy coat were seeded (1 × 106 cells per well) into 24-well plates and rested for 2 hr at 37° in a humidified 5% CO2 incubator before stimulations. Human monocyte-derived macrophages (MDM) were generated as previously described.50 Briefly, human PBMC (3 × 106 cells per well) were seeded into 24-well plates for 16 hr at 37° in a humidified 5% CO2 incubator. The non-adherent cells were removed and the adherent cells were differentiated in complete RPMI-1640 medium supplemented with 50 ng/ml of recombinant human macrophage colony-stimulating factor for an additional 6 days before stimulation. Human monocytic THP-1 cells (ATCC TIB-202) were cultured in complete RPMI-1640 medium and maintained at 37° in a 5% CO2 humidified incubator. THP-1 cells were differentiated into plastic-adherent macrophage-like cells with PMA (Sigma-Aldrich, Oakville, ON, Canada) as previously described.8 The plastic-adherent THP-1 macrophages were rested in complete RPMI-1640 medium for 24 hr before the addition of the various stimulants. Tissue culture (TC) supernatants were centrifuged at 250 g for 5 min to obtain cell-free samples, aliquoted and stored at −20° until needed. Cellular cytotoxicity was determined by monitoring the release of the enzyme lactate dehydrogenase in the TC supernatants after 24 hr of stimulation, using a colorimetric detection assay from Roche Diagnostic (Laval, QC, Canada) as previously described.8, 51 Haemolytic activity of the peptides was determined in human erythrocytes as previously described.52
Peptides and stimulants
Peptides LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES), a scrambled LL-37, sLL-37 (RSLEGTDRFPFVRLKNSRKLEFKDIKGIKREQFVKIL) and IG-19 (IGKEFKRIVQRIKDFLRNL-NH2) were obtained from CPC Scientific (Sunnyvale, CA). The peptides were re-suspended in endotoxin-free water and stored at −20° until needed. Recombinant human IL-32γ and macrophage colony-stimulating factor was obtained from R&D Systems (Minneapolis, MN).
Enzyme-linked immunosorbent assay
Production of cytokines TNF-α, IL-1β and IL-6 was monitored using specific antibody pairs from eBioscience (San Diego, CA), and interleukin-1-receptor antagonist (IL-1RA), GRO-α and IL-10 were monitored using antibody pairs from R&D Systems, as per the manufacturers’ instructions. Serial dilutions of the recombinant human cytokines were used to establish a standard curve for the evaluation of the cytokine concentrations in the TC supernatants.
Flow cytometry
Freshly isolated human PBMC (1 × 106 cells per ml) were stimulated with IL-32γ (20 ng/ml) in the presence and absence of 5 μm peptides (LL-37, IG19 or sLL-37) for 18 hr at 37° in 5% CO2 in the presence of BD GolgiPlug (BD Bioscience, Mississauga, ON, Canada). Unstimulated cells were used as a negative control and cells stimulated with lipopolysaccharide (10 ng/ml) were used as a positive control. Adherent cells were detached using Versene (Gibco®, Life technologies, Burlington, ON, Canada). Adherent and non-adherent cells were washed twice with cold PBS, followed by two washes with staining buffer (PBS + 3% fetal bovine serum). Cells were incubated in human FcR-binding inhibitor (eBioscience) on ice for 20 min, followed by staining with extracellular antibodies (anti-human CD3-phycoerythrin/Cy7, anti-human CD14-eFluor450 and anti-human CD19-allophycocyanin) obtained from eBioscience, on ice for 30 min in the dark. Cells were fixed and permeabilized with BD Cytofix/Cytoperm solution according to the manufacturer's protocol, followed by incubation with anti-human TNF-α-FITC antibody (eBioscience) on ice for 30 min, in the dark. The stained cells were washed with BD Perm/Wash Buffer (BD Bioscience) twice, resuspended in staining buffer and analysed by flow cytometry. UltraComp eBeads from eBioscience were used for compensation. Acquisition of cell samples was done on a FACSCantoII cytometer (Becton Dickinson, Mountain View, CA) using FACSDiva software (Becton Dickinson). Data were analysed with FlowJo (Treestar, CostaMesa, CA). The production of human chemokines [IL-8, Regulated on Activation Normal T Cell Expressed and Secreted (RANTES), monokine induced by interferon-γ (MIG), monocyte chemoattractant protein 1 (MCP-1) and interferon-γ-inducible protein 10 (IP-10)] in the TC supernatants were determined by using a preconfigured multiplex BD™ Cytometric Bead Array kit employing a FACS Calibur™ flow cytometer (BD Biosciences) as per the manufacturer's instructions and as previously described by us.51
Western blots
Plastic adherent macrophage-like THP-1 cells or human MDM in RPMI-1640 medium containing 1% (volume/volume) fetal bovine serum were stimulated with IL-32γ (20 ng/ml) with or without 5 μm of the peptides for 15 min. Total cell lysates were prepared in lysis buffer containing 20 mm Tris–HCl pH 7·5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm sodium fluoride, 1 mm sodium orthovanadate, 2·5 mm sodium pyrophosphate, protease inhibitor cocktail (Sigma-Aldrich) and 1% (volume/volume) Triton X-100. Total protein concentration was determined using a micro-bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL) with a BSA (Sigma-Aldrich) standard curve. Equal amounts of protein (50 μg) were resolved on 4–12% NuPAGE Bis-Tris gels (Invitrogen Corporation, Burlington, ON, Canda), followed by transfer to nitrocellulose membranes (Millipore, Billerica, MA). Membranes were blocked with TBST (20 mm Tris–HCl pH 7·5, 150 mm NaCl, 0·1% Tween-20) containing 3% BSA. Phosphorylation of Akt-1 (T308), MKP-1 (S359) and Fyn (Y420) was determined using anti-human phospho-site-specific antibodies (Cell Signaling Technology, Danvers, MA). Antibodies to paired total Akt-1, Fyn and MKP-1 (Cell Signaling Technology) were used to examine protein loading amounts. The membranes were probed with the various antibodies as indicated in TBST containing 1% BSA. Affinity purified horseradish peroxidase-linked secondary antibodies were used for detection, and the membranes were developed with an Amersham ECL detection system (GE Healthcare, Baie d'Urfe, QC, Canada) according to the manufacturer's instructions.
p44/42 MAPK activity assay
Differentiated THP-1 cells were stimulated with IL-32γ (20 ng/ml) with or without 5 μm of either LL-37 or IG-19 peptide for 5–30 min as indicated. Cell lysates were obtained by sonicating cells on ice in the cell lysis buffer from the p44/42 MAPK activity assay kit (Cell Signaling Technology) containing 1 mm PMSF. A micro-BCA assay (Thermo Scientific) was used to determine the protein concentration for each sample. Kinase activity specific to p44/42 MAPK was monitored using the p44/42 MAPK activity assay kit as per the manufacturer's instructions. Briefly, 150 μg of protein from each sample was used for immunoprecipitation, employing an immobilized phospho-p44/42 MAPK antibody at 4° overnight, with gentle rocking. The eluate was incubated with p44/42 MAPK substrate Elk-1 in the presence of ATP at 30° for 30 min. Subsequent phosphorylation of the Elk-1 substrate was determined by probing immunoblots with anti-phospho Elk-1 (Ser383) antibody.
Results
IL-32γ-induced pro-inflammatory cytokines are significantly suppressed by LL-37 and IG-19 in human PBMC
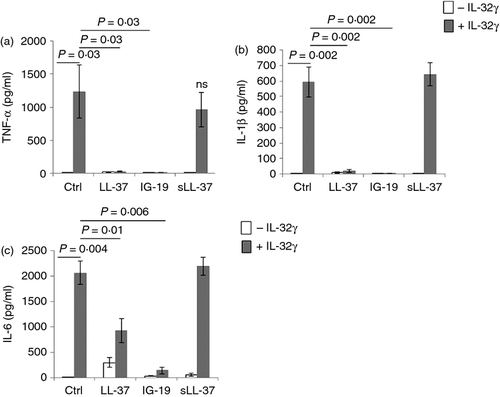
Cytokine IL-32 induces the production of pro-inflammatory cytokines, e.g. TNF-α and IL-1β in blood-derived mononuclear cells.41, 48, 53 Therefore, in this study we monitored the effect of the peptides LL-37 and IG-19 on IL-32γ-induced production of pro-inflammatory cytokines in human PBMC. As the isoform IL-32γ is the most biologically active isoform of the cytokine,49 we used recombinant human IL-32γ to stimulate the cells. It has been previously demonstrated that as much as 20 ng/ml of IL-32γ is found in the synovial fluid of patients with active rheumatoid arthritis.54 Furthermore, we have previously shown that 20 ng/ml of IL-32γ induces significant pro-inflammatory responses in macrophages.48 Therefore, we stimulated human PBMC with 20 ng/ml of IL-32γ in the presence and absence of each of the peptides LL-37, IG-19 or sLL-37 (5 μm) for 24 hr. The concentration of the peptides was based on previous studies demonstrating that 5 μm is a physiologically relevant concentration for studying the immunomodulatory functions of LL-37 and its derivative peptides, including the peptide IG-19.8, 35, 55 Human PBMC did not exhibit increased cytotoxicity, as measured by lactate dehydrogenase release following the various stimulations (data not shown). Also, the peptides did not induce haemolytic activity (data not shown).
We showed that LL-37 and IG-19 abrogated IL-32γ-induced TNF-α production (Fig. 1a) and IL-1β production (Fig. 1b) by > 97% in PBMC. Interleukin-32γ-induced IL-6 production was also significantly suppressed by ~ 50% in the presence of LL-37, and by > 93% in the presence of IG-19 (Fig. 1c). In contrast, the scrambled peptide sLL-37 did not mediate these anti-inflammatory effects in human PBMC (Fig. 1). Furthermore, IL-32γ-induced chemokine production, such as that of GRO-α, IL-8, RANTES and MCP-1, was not significantly altered in the presence of either LL-37 or IG-19 in human PBMC (data not shown).
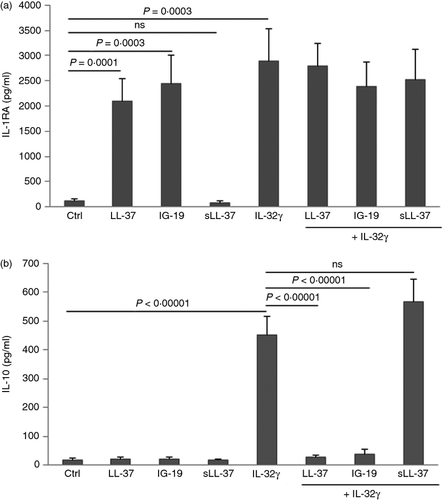
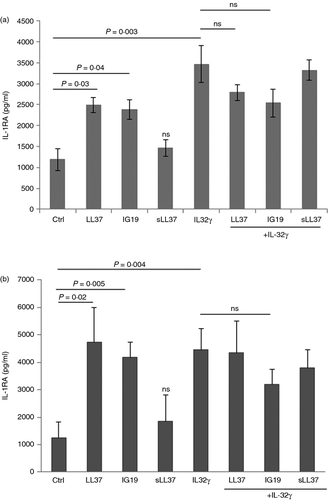
LL-37 and IG-19 induce the production of IL-1RA in PBMC
We have previously shown that the anti-inflammatory cytokine IL-1RA is induced by cathelicidin-derived small peptides that modulate immune-mediated inflammation.51 Moreover, previous studies have shown that the anti-inflammatory cytokine IL-10 is induced by LL-37.56 However, there are also studies that demonstrate an inverse correlation between IL-10 and LL-37.57, 58 Therefore, in this study we examined the production of anti-inflammatory cytokines IL-1RA and IL-10 following stimulation of PBMC with IL-32γ (20 ng/ml) in the presence and absence of the peptides LL-37 or IG-19 (5 μm). The TC supernatants were monitored for the production of IL-1RA and IL-10 by ELISA, after 48 hr. Production of IL-1RA was significantly enhanced between eightfold and 15-fold compared with that in unstimulated cells by LL-37 and IG-19 alone (Fig. 2a). Moreover, IL-32-induced IL-1RA was not suppressed in the presence of the peptides (Fig. 2a). In contrast, the peptides did not enhance the production of IL-10, and IL-32γ-induced IL-10 production was significantly suppressed in the presence of either LL-37 or IG-19, in PBMC (Fig. 2b).
Flow cytometry analysis of IL-32γ-induced TNF-α production by PBMC
To further determine the target cells that respond to IL-32γ within the PBMC population, we stimulated human PBMC with IL-32γ (20 ng/ml), in the presence and absence of 5 μm peptides (either LL-37, IG-19 or sLL-37), for 18 hr at 37°. The PBMC were stimulated with bacterial lipopolysaccharide as a positive control. Cells were stained for the presence of surface markers with anti-human CD3-phycoerythrin/Cy7 (for T cells), anti-human CD14-eFluor450 (for monocytes) and anti-human CD19-allophycocyanin (for B cells). Following permeabilization, the cells were stained with anti-human TNFα-FITC. Interleukin-32γ-induced TNF-α was abrogated in the presence of either LL-37 or IG-19, but not in the presence of sLL-37, as detected by gating on cellular subsets positive for TNF-α (Fig. 3a). This is consistent with previous studies demonstrating that monocytes/macrophages are required for the immunomodulatory activity of cathelicidin-derived peptides.59, 60
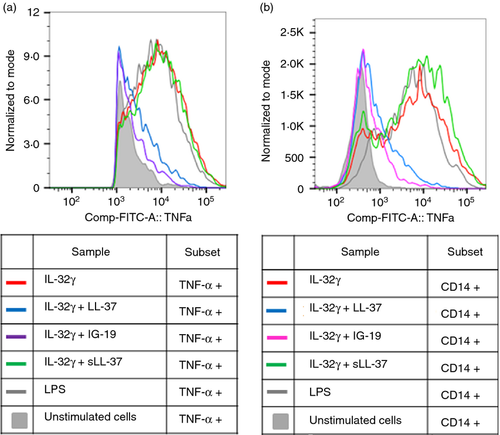
Further gating on the different cellular subsets showed that IL-32γ-induced TNF-α was not detected in either CD3+ T cells or CD19+ B cells (Fig. S1 and S2). We demonstrated that CD14+ monocytes were the target cell type that produced TNF-α after stimulation of PBMC by IL-32γ (Fig. 3b). This is consistent with our previous study demonstrating that IL-32γ induces robust pro-inflammatory cytokine production and NF-κB activation in human macrophages.48 We also showed that IL-32γ-induced TNF-α production in CD14+ monocytes was significantly suppressed to background levels in the presence of either LL-37 or IG-19, but not sLL-37 (Fig. 3b). Based on these results, we further focused on investigating the activity of the peptides on IL-32γ-induced responses in macrophages as follows.
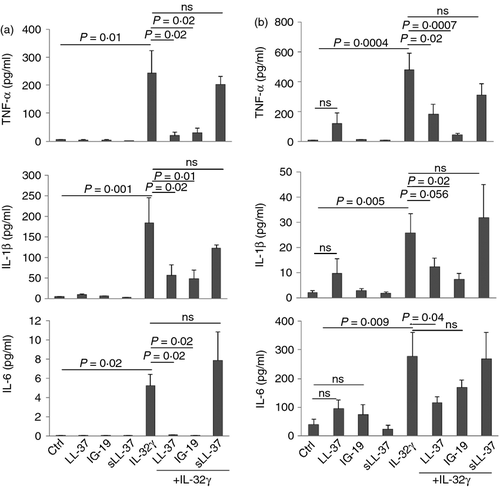
IL-32γ-induced pro-inflammatory cytokine production is significantly suppressed in the presence of LL-37 and IG-19 in macrophages
We evaluated IL-32γ-induced cytokine production in plastic-adherent, differentiated THP-1 cells,8 in the presence and absence of the peptides LL-37, IG-19 or sLL-37. Plastic adherent differentiated THP-1 cells display distinct markers and inflammatory cytokine-induced responses similar to those of PBMC-derived macrophages.8, 61, 62 Furthermore, to determine the effect of these peptides in primary cells, we also evaluated IL-32γ-induced pro-inflammatory cytokine production in human MDM in the presence and absence of the peptides. These cells were stimulated with IL-32γ (20 ng/ml) in the presence or absence of either LL-37, IG-19 or sLL-37 (5 μm). The TC supernatants were monitored for the production of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 after 24 hr. Similar to the responses in human PBMC (Fig. 1), LL-37 and IG-19 significantly suppressed the production of IL-32γ-induced TNF-α, IL-1β and IL-6 in THP-1 macrophages (Fig. 4a). Interleukin-32γ-induced TNF-α and IL-1β production was also significantly suppressed by LL-37 and IG-19 in human MDM (Fig. 4b). However, the suppression of IL-32γ-induced IL-6 production in human MDM was not as robust as that seen in THP-1 macrophages (Fig. 4), in the presence of the peptides. In contrast, the scrambled peptide sLL-37 did not significantly suppress IL-32γ-induced pro-inflammatory cytokine production in either THP-1 macrophages or MDM (Fig. 4a,b respectively). Similar to that observed in human PBMC, IL-32γ-induced chemokine responses such as GRO-α and MCP-1 were not altered in the presence of the peptides in macrophages (data not shown).
We further monitored the production of anti-inflammatory cytokines IL-1RA and IL-10 in THP-1 macrophages and human MDM. LL-37 and IG-19 alone significantly enhanced the production of IL-1RA twofold in THP-1 macrophages (Fig. 5a) and approximately fourfold in human MDM (Fig. 5b), compared with unstimulated control cells, whereas sLL-37 did not significantly induce IL-1RA in either THP-1 or MDM (Fig. 5). Furthermore, IL-32γ-induced IL-1RA was not significantly suppressed in either THP-1 macrophages or MDM by the peptides (Fig. 5). These results were consistent with the results in human PBMC (Fig. 2). Interleukin-10 was not detected in the TC supernatants from THP-1 macrophages. Similar to that in PBMC, the peptides did not significantly induce IL-10 production in MDM, and IL-32γ-induced IL-10 was suppressed by the peptides in human MDM (data not shown).
Overall, our results demonstrated that LL-37 and IG-19 significantly suppress IL-32γ-induced pro-inflammatory cytokines, and induce anti-inflammatory cytokine IL-1RA, in human PBMC and macrophages. The results taken together also indicated that regulation of IL-32-mediated inflammation by these peptides may be independent of suppression of chemokine production and consequent leucocyte recruitment, and also independent of IL-10. To delineate the mechanisms of the regulatory role of LL-37 and IG-19, we investigated their effects on IL-32γ-induced signalling events as follows.
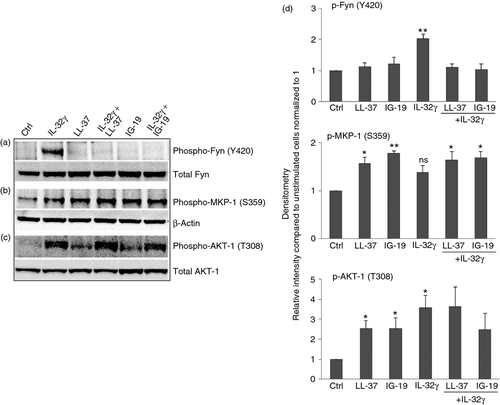
LL-37 and IG-19 alter IL-32γ-mediated specific protein phosphorylation
Post-translational modifications such as phosphorylation of proteins are a critical mechanism in the regulation of cellular processes.63 Therefore, to further delineate the mechanism of action of LL-37 and IG-19 in suppressing IL-32γ-induced pro-inflammatory cytokines, we examined the effects of these peptides on IL-32γ-induced protein phosphorylation events. We have previously used a kinome array to profile kinase activities mediated by IL-32γ in macrophages.48 From our previous kinome analyses (deposited in NCBI's Gene Expression Omnibus database under series accession number GSE28649) we selected three proteins, Akt-1 (T308), MKP-1 (S359) and Fyn (Y420), which were all significantly phosphorylated in response to IL-32γ,48 for further investigation (Table 1). We examined these phosphorylation events in the presence and absence of peptides LL-37 or IG-19 in differentiated THP-1 macrophages. These cells were chosen because we have demonstrated that IL-32γ-induced responses in differentiated THP-1 macrophages are similar to those of human PBMC and MDM, in the presence and absence of the peptides (Figs 1-5).
| Phosphoprotein | Kinase target | IL-32γ-induced relative fold change | P-value |
|---|---|---|---|
| Fyn | Y420 | 3·43 | P < 0·01 |
| Akt-1 | T308 | 3·26 | P < 0·01 |
| MKP-1 | S359 | 2·52 | P < 0·03 |
- Human macrophage-like THP-1 cells were stimulated with IL-32γ (20 ng/ml) for 15 min. A kinome screen using peptide arrays representing 300 phosphorylation targets of kinase activity was employed for quantifying kinase activity.48 Table shows proteins that were phosphorylated ≥ 2·5 fold (P < 0·05) in the presence of the cytokine IL-32γ compared with unstimulated cells
In this study, we demonstrated using Western blots that Akt-1 (T308) and Fyn (Y420) were significantly phosphorylated, whereas the phosphorylation of MKP-1 (S359) was modest (not statistically significant), in response to IL-32γ after 15 min (Fig. 6). These results were largely consistent with our kinome analyses (Table 1). We further showed that IL-32γ-mediated phosphorylation of Fyn (Y420) was abrogated in the presence of the peptides either LL-37 or IG-19 (Fig. 6a,d). Moreover, the peptides LL-37 or IG-19 alone each significantly enhanced the phosphorylation of MKP-1 (S359) by > 1·8-fold, and that of AKT-1 (T308) by 2·5-fold, compared with unstimulated cells (Fig. 6). Interleukin-32γ-mediated phosphorylation of AKT-1 (T308) was not significantly suppressed by the peptides (Fig. 6a,d). Moreover, the modest enhancement of IL-32-mediated phopsho-MKP-1 (S359) was enhanced by the presence of the peptides to levels that were statistically significant compared with unstimulated cells (Fig. 6b,d).
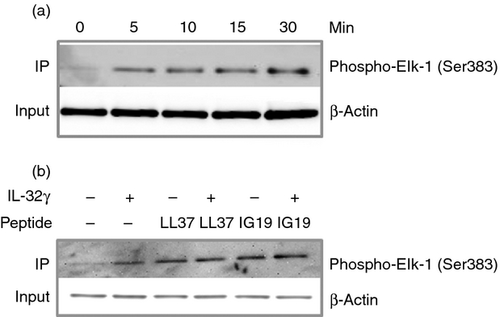
Peptides LL-37 and IG-19 activate p44/42 MAPK
Phosphorylation of MKP-1 (S359) is known to be mediated by the activity of p44/42 MAPK, which results in the stabilization of MKP-1.64 As we have already shown that the peptides significantly induce the phosphorylation of MKP-1 (Fig. 6b,d), we further employed a p44/42 MAPK activity assay (Cell Signaling Technology) to interrogate the molecular mechanisms upstream of the phosphorylation of MKP-1 (S359). We performed a kinetic study to determine the optimal time point for IL-32γ-mediated p44/42 MAPK activation. Differentiated THP-1 macrophages were stimulated with IL-32γ (20 ng/ml) for 5 to 30 min, followed by immunoprecipitation of the cell lysates with phospho-p44/42 MAPK antibody. The activity of p44/42 MAPK was further determined by measuring the phosphorylation of the substrate Elk-1 in the presence of ATP, by probing immunoblots with anti-phospho Elk-1 (Ser383) antibody. We showed that the cytokine IL-32γ induced the activity of p44/42 MAPK after 5 min (Fig. 7a). We selected 10 min as the optimal time-point for further investigation. We demonstrated that LL-37 and IG-19 alone induced p44/42 MAPK activation, and that IL-32γ-induced activation of p44/42 MAPK was not suppressed in the presence of the peptides (Fig. 7b).
Discussion
The role of LL-37 to regulate pathogen-induced inflammation is now well established.8, 23 In addition, recent studies have also demonstrated that partial fragments of LL-37, including the peptide IG-19, can suppress bacterial endotoxin-induced inflammatory cytokines.35, 65 However, the role of LL-37 and its derivatives in regulating sterile inflammation has not been well defined. Recent studies have suggested that cathelicidins may contribute to the regulation or control of immune-mediated inflammation. For example, LL-37 has been shown to intervene in the activation of inflammasome,66 and vitamin D-mediated induction of LL-37 has been hypothesized to be protective in chronic inflammatory diseases.30 Consistent with this, it has been demonstrated that the rat cathelicidin, rCRAMP, can heal gastric ulcers.33 Moreover, in a recent study we demonstrated that the LL-37-derivative peptide IG-19 alleviates the disease process in a murine model of inflammatory arthritis.34 Therefore, in this study we examined the activity of LL-37 and IG-19 to regulate immune-mediated inflammation, in particular the inflammatory responses induced by the cytokine IL-32. Pro-inflammatory cytokine IL-32 level is directly correlated with the severity and progression of chronic inflammatory arthritis and is therefore an emerging therapeutic target.37-42, 67, 68 This is the first study to demonstrate that the human cathelicidin LL-37 and its derivative peptide IG-19 can suppress IL-32γ-induced inflammatory cytokine production in immune cells such as PBMC and macrophages. The results from this study suggest that LL-37 and its derivatives regulate immune-mediated inflammation, and that their anti-inflammatory function may be independent of immune cell recruitment and IL-10. The mechanistic results from this study show that specific IL-32γ-induced phosphorylation events are altered by LL-37 and IG-19, and that the dual phosphatase MKP-1 may be a critical mediator of the regulatory functions of these peptides.
In this study, we showed that the IL-32γ-induced pro-inflammatory acute cytokines TNF-α, IL-1β and IL-6 were all significantly suppressed by LL-37 and IG-19 (Figs 1 and 4). Cytokines TNF-α, IL-1β and IL-6 are all critical mediators of inflammation known to contribute to cartilage and bone destruction within the synovial microenvironment in inflammatory arthritis.69-71 We also demonstrated that the target cell within the PBMC population that responds to IL-32γ is the CD14+ monocytic cells (Fig. 3). It is known that monocyte/macrophage-derived pro-inflammatory mediators activate stromal cells, for example the synoviocytes in arthritic joints,72, 73 hence these cells are key players in chronic inflammatory disorders. Therefore, suppression of IL-32γ-induced pro-inflammatory cytokines by LL-37 and IG-19, in PBMC and macrophages, strongly supports our previous study demonstrating the protective role of IG-19 in a murine model of inflammatory arthritis.34 The involvement of macrophages in the regulation of IL-32-mediated sterile inflammation by LL-37 and IG-19, as demonstrated in this study, is also consistent with previous studies showing that macrophages are required for the immune-modulatory function of cathelicidin-derived small peptides to protect against infections.59, 60 In addition, previous studies have shown that small internal fragments of LL-37 can control endotoxin-induced pro-inflammatory cytokines in blood-derived mononuclear cells.35, 65 Hence there is a possibility that if IG-19 is generated by alternative processing of LL-37 in vivo, then this naturally occurring fragment may be contributing to the cathelicidin-mediated anti-inflammatory activities. Although alternative processing of LL-37 by proteases to smaller peptides in body fluids has been reported in previous studies,74, 75 the peptide IG-19 has not yet been specifically identified as a natural degradation product of LL-37.
In this study, we further showed that the anti-inflammatory mediator IL-1RA is induced by LL-37 and IG-19 alone (Fig. 2). Interleukin-1RA is an endogenous inhibitor of IL-1β, and the recombinant IL-1RA (Anakinra) is used as a biological therapy for rheumatoid arthritis.76 Therefore, induction of IL-1RA by LL-37 and IG-19 supports the findings that IG-19 plays a protective role in inflammatory arthritis.34 However, in this study, IL-10 levels were not enhanced in the TC supernatants of either PBMC or MDM stimulated with LL-37 or IG-19. This is consistent with previous studies demonstrating an inverse correlation of LL-37 with IL-10.57, 58 However, we have previously shown that LL-37 (at a concentration twice as high as that used in this study) can induce IL-10 production in human PBMC.56 This suggests that the effect of LL-37 on IL-10 production may vary depending on concentration, cell type, the inflammatory stimuli and the kinetics of response.56-58 Furthermore, in this study we showed that IL-32-induced IL-10 was suppressed by both LL-37 and IG-19 in PBMC and macrophages. This is consistent with our recent study where we have demonstrated that even though the peptide IG-19 is protective in a murine model of inflammatory arthritis, serum levels of IL-10 are reduced upon administration of IG-19 compared with the arthritic mice.34 It should also be noted that even though IL-10 is a cytokine that limits inflammation, the therapeutic potential of IL-10 for chronic inflammatory autoimmune diseases has yet to be fully established.77 Taken together, our results indicate that the ability of LL-37 and IG-19 to control IL-32γ-induced inflammation may be independent of IL-10 production.
In this study, we showed that while LL-37 and IG-19 suppress IL-32-induced production of pro-inflammatory acute cytokines, e.g. TNF-α, chemokine production is not suppressed by the peptides. This suggests that the anti-inflammatory function of LL-37 and IG-19 in regulating IL-32-mediated inflammation may be independent of chemokine-mediated leucocyte recruitment. This is consistent with previous studies demonstrating that LL-37 and synthetic cathelicidin-derived peptides maintain chemokine responses in the presence of pathogenic or endotoxin stimuli.8, 59, 60 Chemokines play an important role in immune surveillance and the recruitment of immune cells to sites of infection for protection against microbial assault.78 Therefore, our results suggest that LL-37 and its derivative IG-19 have the potential to aid in the recruitment of immune cells to the site of infection even under immune-mediated inflammatory conditions, while selectively limiting the production of acute pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6. These results strongly support the idea that cathelicidin-derivative peptides may be beneficial in controlling inflammatory disorders without compromising host resistance to infections. The dual potential of these peptides to control both inflammation and infections makes them attractive for development as anti-inflammatory therapeutics.
The network of signalling pathways induced by the cytokine IL-32 has not been completely delineated. We have previously used a kinome array to profile kinase activities induced in the presence of cytokine IL-32γ.48 From our previous study we selected three proteins that showed enhanced phosphorylation in response to IL-32γ (Table 1). In this study, we confirmed our kinome analysis and conclusively showed that the Fyn protein tyrosine kinase (PTK) and Akt-1 were significantly phosphorylated, and that the MKP-1 dual phosphatase was modestly phosphorylated, in response to IL-32γ (Fig. 6), demonstrating the involvement of these signalling pathways in IL-32-mediated downstream responses. We further showed that LL-37 and IG-19 abrogate IL-32γ-mediated phosphorylation of Fyn (Y420) (Fig. 6a,d). Fyn is a member of the Src PTK family, known to mediate intracellular signalling in inflammation.79 Therefore, pharmacological inhibition of Src PTK has been shown to be beneficial in sepsis, myocardial infarctions and other diseases.79 This suggests that the human cathelicidin LL-37, and in particular its small synthetic derivative IG-19, may be beneficial as pharmacological inhibitors for immune-mediated inflammatory disorders by intervening with Fyn PTK-mediated inflammation. Our results clearly indicate that LL-37 and IG-19 regulate IL-32-mediated inflammatory responses by intervening with the activation of specific signalling pathways such as the Fyn Src PTK.
In contrast to Fyn PTK activity, LL-37 and IG-19 did not suppress IL-32γ-induced phosphorylation of Akt-1(T308); moreover, the peptides significantly enhanced phospho-AKT-1 (T308) levels compared with unstimulated cells (Fig. 6c,d). Previous studies have shown that the Akt-1/phosphoinositide 3-kinase pathway contributes to the control of endotoxin-induced inflammation in monocytic cells, and that Akt-1 knockout results in hyper-responsiveness to endotoxin and elevated production of pro-inflammatory mediators.80-82 However, limited data are available on the biological significance of phospho-AKT-1 (T308), it is difficult to speculate how this phospho-specific site contributes to the anti-inflammatory role of the peptides, which can be investigated in future studies.
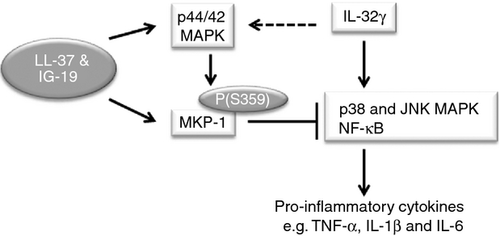
Finally, in this study we showed that LL-37 and IG-19 induce the phosphorylation of MKP-1 (S359), and do not suppress IL-32-induced phosphorylation of MKP-1 (Fig. 6b,d). We further demonstrated that the upstream MAPK p44/42 activation, which is known to phosphorylate and stabilize MKP-1,64 was also enhanced by LL-37 and IG-19 (Fig. 7). MKP-1 (also known as DUSP-1) is a MAPK phosphatase, demonstrated to limit Toll-like receptor-mediated inflammation, inactivate MAPK p38 and Jun N-terminal Kinase (JNK) signalling and suppress NF-κB signalling. Hence, MKP-1 is a critical negative regulator of inflammation.83-85 Consistent with this, knockout of MKP-1 enhances activation of p38 MAPK and JNK, enhances production of pro-inflammatory cytokines and shows an accelerated disease progression of auto-inflammatory rheumatoid arthritis.83, 86-88 MKP-1 activity inhibits mRNA transcription of inflammatory cytokines such as IL-6 and TNF-α.84 This is consistent with our results demonstrating that LL-37 and IG-19 induces MKP-1 (S359) phosphorylation (Fig. 6). Moreover, IL-32γ is known to activate p38 and JNK, MAPK, and NF-κB pathways to enhance the production of downstream pro-inflammatory cytokines such as TNF-α.48, 89, 90 Taken together, we hypothesize that the dual phosphatase MKP-1 plays a critical role in the regulatory mechanism of LL-37 and IG-19 by inhibiting the activation of IL-32γ-induced p38, JNK MAPK and NF-κB mediated signaling, resulting in the control of IL-32γ-induced production of pro-inflammatory downstream cytokines (Fig. 8).
This is the first study to demonstrate that the human cathelicidin LL-37 and its derivative peptide IG-19 can control chronic inflammatory cytokine IL-32γ-mediated inflammation in immune cells. This study provides mechanistic data to suggest that the regulatory activity of LL-37 and its derivative IG-19 in an immune-mediated inflammatory environment may involve blocking Fyn PTK activation, while promoting the activity of the dual phosphatase MKP-1 to control inflammation. The results from this study strongly support further research into the development of cathelicidin-derived small peptides for the control of immune-mediated chronic inflammatory diseases.
Acknowledgements
KGC performed the experiments, SN designed and performed the kinome analyses, NM designed the study, and KGC and NM wrote the paper. The authors also gratefully acknowledge the technical and intellectual input of Dr Christine Zhang, Flow Cytometry Core Facility at the University of Manitoba. This research was supported with funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Manitoba Health Research Council (MHRC). KGC is supported by a studentship from MHRC.
Disclosures
The authors declare no conflict of interest.




