Autosomal dominant hypercalciuric hypocalcaemia: the calcium-sensing receptor in renal calcium homeostasis and the impact of renal transplantation
Funding: None.
Conflict of interest: None.
Abstract
Calcium-sensing receptors (CaSRs) are G protein-coupled receptors that help maintain Ca2+ concentrations, modulating calciotropic hormone release (parathyroid hormone (PTH), calcitonin and 1,25-dihydroxyvitamin D) by direct actions in the kidneys, gastrointestinal tract and bone. Variability in population calcium levels has been attributed to single nucleotide polymorphisms in CaSR genes, and several conditions affecting calcium and phosphate homeostasis have been attributed to gain- or loss-of-function mutations. An example is autosomal dominant hypercalciuric hypocalcaemia, because of a missense mutation at codon 128 of chromosome 3, as reported in our specific case and her family. As a consequence of treating symptomatic hypocalcaemia as a child, this female subject slowly developed progressive end-stage kidney failure because of nephrocalcinosis and nephrolithiasis. After kidney transplantation, she remains asymptomatic, with decreased vitamin D and elemental calcium requirements, stable fluid and electrolyte homeostasis during intercurrent illnesses and has normalised urinary calcium and phosphate excretion, reducing the likelihood of hypercalciuria-induced graft impairment. We review the actions of the CaSR, its role in regulating renal Ca2+ homeostasis along with the impact of a proven gain-of-function mutation in the CaSR gene resulting in autosomal dominant hypercalciuric hypocalcaemia before and after kidney transplantation.
Introduction
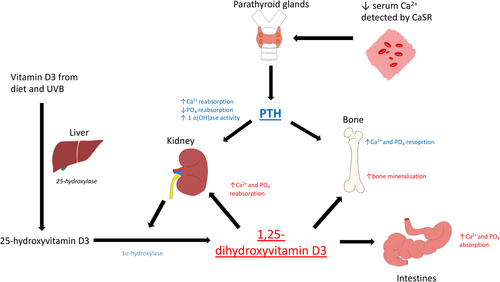
Ionised calcium (Ca2+) plays a vital role in a plethora of physiological processes, including muscle contraction, nerve conduction, intracellular transport, hormone secretion and bone turnover.1 Homeostasis is achieved by balancing dietary intake, gastrointestinal (GI) absorption, bone metabolism and renal reabsorption, which in turn are mediated primarily by parathyroid hormone (PTH), 1,25-dihydroxyvitamin D (calcitriol) and calcitonin (CT)1 (Fig. 1). Calcium-sensing receptors (CaSRs) are found in a multitude of tissues, including the parathyroid gland, kidneys, intestines and bone (among others) and are vital in tightly regulating Ca2+ in response to small deviations in concentration generated by processes such as the release of calciotropic hormones.2-4
Index case
Ms X first presented as a child with seizures resulting from hypocalcaemia with low/normal PTH levels. She had a strong family history of hypocalcaemia with an autosomal dominant inheritance pattern. Her condition was initially labelled as isolated hypoparathyroidism, and she was established on long-term vitamin D and calcium supplementation. Throughout her youth, she experienced infrequent episodes of symptomatic hypocalcaemia with symptom resolution when serum calcium concentrations trended to the low normal range.
By age 26, she had developed chronic kidney disease (CKD) with a serum creatinine of 170 μmol/L (estimated glomerular filtration rate (eGFR) 35 mL/min/1.73 m2). She suffered paroxysmal hypocalcaemic carpopedal spasms, nephrocalcinosis, polydipsia and polyuria, whereas urinalysis demonstrated persistently elevated urinary calcium/creatinine ratio (mean 0.78, range 0.32–1.07). Management comprised vitamin D supplementation (Alfacalcidol 0.5 micrograms twice daily) and calcium carbonate (600–1200 mg elemental calcium a day). The hypercalciuria and associated diuresis precipitated problems with fluid homeostasis. Intercurrent illness often resulted in hospital admissions with nausea, tetany, paraesthesia and hypovolaemia requiring intravenous fluid and calcium replacement.
In 1995, her family were one of six enrolled into a kindred study with a constellation of autosomal dominant hypocalcaemia, hypercalciuria, low-normal PTH concentrations and a previous diagnosis of isolated hypoparathyroidism.5 DNA sequence analysis of the CaSR gene located on chromosome 3 revealed a heterozygous missense mutation at codon 128, resulting in a leucine (Leu) residue being replaced by a phenylalanine (Phe) (TTC to CTC). Cloned mutant CaSR cDNA was then transfected into human embryonic kidney (HEK-293) cells. The impact of these mutations was assessed in terms of intracellular inositol phosphate levels in response to different extracellular calcium concentrations. Ms X's hereditary Phe128Leu mutation demonstrated an important gain-of-function conformational change to the extracellular domain of the CaSR with a leftward shift of her calcium-response curve.
Over 30 years, the CKD slowly progressed to end-stage kidney disease (ESKD) at age 58. The serum calcium was maintained at 1.8–2.0 mmoL/L on calcitriol 0.25 μg and 500–1000 mg elemental calcium daily. She was commenced on continuous ambulatory peritoneal dialysis (CAPD); the dialysate bags were supplemented with calcium and oral vitamin D replacement was stopped. Calcium concentrations on dialysis were consistently 2.0–2.1 mmoL/L, phosphate averaged 2.3 mmol/L and PTH: 2.8 pmoL/L.
After 14 months on CAPD, Ms X received a kidney from a non-directed altruistic donor. There was immediate graft function with 2.5 L urine within the first 12 h. The serum creatinine fell rapidly to 122 μmoL/L. Day 1 after transplant the serum calcium dropped to 1.7 mmoL/L requiring intravenous replacement. Calcitriol 0.25 μg/daily and elemental calcium 1000 mg twice daily were re-commenced aiming for serum concentrations between 1.9 and 2.1 mmol/L. By day 11 after the operation, the urinary calcium:creatinine ratio was 0.29, falling to 0.14 at 2 months and 0.06 at 2 years. In the weeks following transplantation, the calcium levels rose to 2.1, necessitating reduction of calcitriol to 0.25 μg three times weekly and calcium carbonate 500 mg to once daily. This regimen has subsequently maintained her serum Ca2+ at 1.9–2.0 mmol/L. The average post-transplant PTH concentration was 2.98 pmoL/L compared with 2.07 pmoL/L before transplant and the serum phosphate levels remained within 1.5–2.2 mmol/L (Table 3). After 3 years since the transplant, her urine phosphate:creatinine ratio remains within the normal range at 1.1 (median 1.34, range 0.38–2.48)6 compared with 2.56 prior to her transplant. The serum creatinine remains at 76 μmoL/L with an eGFR of 73 mL/min/1.73 m2. The current immunosuppression regimen is cyclosporin 50 mg twice daily, mycophenolate mofetil 500 mg mane and 1 g in the evening, and prednisone 5 mg daily.
The physiology of calcium regulation
Calcium-sensing receptor
The CaSR, first cloned from bovine parathyroid gland cells in 1993, is a G protein-coupled receptor with three domains.7 In addition to Ca2+, CaSRs bind divalent and trivalent cations (e.g. Mg2+ and Gd 3+) and organic molecules such as amino acids and aminoglycoside antibiotics.8 After agonist binding, the 250-amino-acid 7 transmembrane domains (TMD) and the approximately 200-amino-acid intracellular domain (ICD) undergo conformational changes activating G proteins Gq/11, G12/13 and Gi.9, 10 These mediate cellular signalling pathways, including activation of phospholipases A2, C and D, mitogen-activated protein kinases (MAPK) and inhibition of adenylate cyclase with calciotropic and non-calciotropic outcomes.9
Parathyroid hormone
PTH is an 84-amino-acid polypeptide secreted in response to reduced Ca2+ concentrations. Through actions mediated by the CaSR, PTH levels may rise rapidly (within seconds to minutes) by exocytosis and reduced breakdown or slowly (over days to weeks) by increasing PTH gene expression through mRNA stabilisation and parathyroid cellular hyperplasia.11 Under normal circumstances, small decreases in ionised calcium concentrations detected by parathyroid chief cell CaSRs cause significant elevations in PTH. Acting through receptors throughout the body, PTH then increases Ca2+ levels by (i) mobilising bone stores and stimulating osteoblast-mediated bone remodelling, (ii) upregulating 1-alpha hydroxylase production in proximal tubule epithelial cells, increasing calcitriol and active intestinal Ca2+ absorption and (iii) enhancing renal tubular reabsorption.12 In the thick ascending limb (TAL) of the loop of Henle, PTH inhibits claudin-14 expression, itself an inhibitor of claudin-16 and claudin-19, which constitute a paracellular cation channel.13 The hormone also exerts an effect on the distal nephron transcellular Ca2+ transport by upregulating proteins TRPV5 and calbindin-D28K (also stimulated by calcitriol), plasma membrane calcium transporting adenosine triphosphatase 1b (PMCA1b) and plasma membrane Na+/Ca2+ exchanger (NCX1).1 For more rapid fine-tuning of calcium absorption, PTH stimulates secondary messenger protein kinases A and C, activating the epithelial Ca2+ channels (transient receptor potential channel family ‘vanilloid’ – TRPV) TRPV5 and TRPV6.14
CaSRs can be found in thyroid C cells, which release calcitonin in response to raised Ca2+.15 In contrast to PTH, calcitonin reduces renal tubular reabsorption and bone remodelling but has less significant hypocalcaemic effect in humans than in Muridae.16 In the GI tract, CaSRs stimulate the secretion of gastrin from the stomach and cholecystokinin from the small intestine and increase luminal TRPV6 expression for apical calcium uptake. Their presence on osteoblasts and osteoclast precursors allows localised control of bone resorption, whereas in breast tissues, CaSRs regulate the calcium content of milk. Discovery in tissues not involved in calcium homeostasis, including haemopoietic stem-cell precursors, keratinocytes and the pancreas, hint at a range of actions yet to be described.17
CaSRs in the kidney
The role of CaSRs in the kidney can broadly be thought of as modulating the effects of calciotropic hormones directly and indirectly. Hypercalcaemia inhibits PTH secretion and downregulates 1-alpha-hydroxylase activity in the distal tubules.10 CaSRs, located on both apical and basolateral membranes at different points throughout the nephron, exert localised, direct influence over calcium and phosphate homeostasis, diuresis, renin production and urinary acidification in response to extracellular Ca2+.10, 18
Calcium handling in the kidney
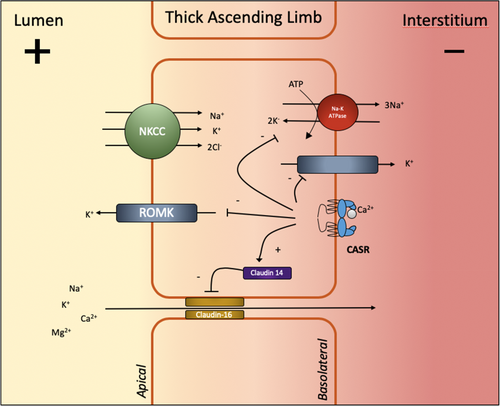
Ionised calcium (45% of plasma Ca2+) is freely filtered. Approximately 65% is reabsorbed passively in the proximal tubule.19 A further 20% is passively absorbed in the TAL of the loop of Henle predominantly through paracellular claudin-16 cation channels. This process is driven by a positive electrochemical gradient established by apical NKCC co-transporters and basolateral Na-K-ATPase.20 Activation of CaSRs in the basolateral membrane during hypercalcaemia diminishes this electrical gradient by inhibiting the renal outer medullary K+ channel (ROMK) and Na-K-ATPase pump channels through the production of a cytochrome P450-related arachidonate metabolites (Fig. 2).21 CaSR signalling also leads to lysosomal translocation of claudin-16 and upregulation of claudin-14 further impeding paracellular cation transport.22, 23 By reducing intracellular cAMP concentrations, activated CaSRs reduce transcellular PTH-mediated transport through ATP-dependent PMCA-1b channels.24
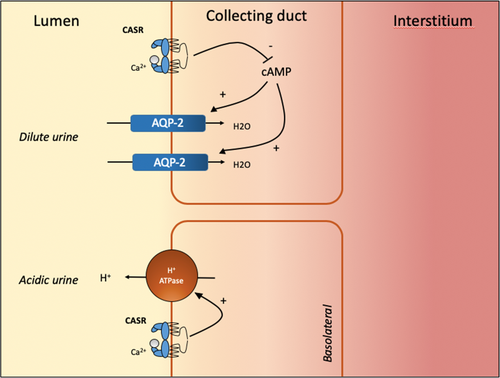
In the distal tubules, the remaining 15% of filtered calcium ions are reabsorbed through transcellular pathways modulated by PTH, calcitriol and calcitonin.25 Renal Ca2+ clearance in the distal convoluted and connecting tubules is therefore finely adjusted depending upon circulating concentrations. Mouse models show that in isolated medullary collecting duct samples exposed to CaSR ligands (including Ca2+), H+ ion secretion through H+-ATPase increases while AQP-2 channels decrease (Fig. 3).26 The CaSR therefore plays a vital role in the polyuria and urinary acidification consequent to hypercalcaemia, reducing the risk of nephrocalcinosis and stone formation.27 Chronic hypercalcaemia may cause nephrogenic diabetes insipidus by reducing AQP-2 expression by a post-transcriptional mechanism. By reducing TAL NaCl transport, the CaSR increases natriuresis while reducing the medullary osmotic gradient necessary for urinary concentration and further contributing to polyuria.27
Disorders of the CaSR
Variability in baseline calcium concentrations in the normal population can be partly attributed to single nucleotide polymorphisms in exons encoding the CaSR.28 Alterations in protein structure, proliferation and downstream signalling components may alter CaSR function and can be divided into those causing ‘gain’ or ‘loss’ of function4 and then further categorised into inherited and acquired (Tables 1 and 2).
| Loss-of-function CaSR disorders |
|---|
| Inherited |
| Familial hypocalciuric hypercalcaemia |
| Neonatal severe primary hyperparathyroidism |
| Neonatal primary hyperparathyroidism |
| Acquired |
| Acquired hypocalciuric hypercalcaemia |
| Primary hyperparathyroidism |
| Secondary (uraemic) hyperparathyroidism |
| Gain-of-function CaSR disorders |
|---|
| Inherited |
| Autosomal dominant hypercalciuric hypocalcaemia |
| Autosomal dominant hypercalciuric hypocalcaemia with Bartter syndrome type V |
| Acquired |
| Autoimmune hypoparathyroidism |
| Time | Serum Ca2+ mmol/L | Serum Phos mmol/L | Treatment | Serum PTH pmol/L | Serum Cr μmol/L | Urine Ca2+:Cr ratio |
|---|---|---|---|---|---|---|
| Before transplant | 2.1 | 1.2 | CAPD Nil other replacement | 3.2 | 574 | 0.55† |
| 2 days after | 1.7 | 1.5 | Calcitriol 0.25 mcg daily 1 g elemental Ca2+ BD | ‡ | 122 | 0.29 |
| 2 months after | 2.1 | 1.9 | Calcitriol 0.25 mcg three times weekly 500 mg elemental Ca2+ daily | 5.9 | 86 | 0.14 |
| 2 years after | 2 | 2.2 | Calcitriol 0.25mcg three times weekly 500 mg elemental Ca2+ daily | 2.2 | 87 | 0.06 |
| 3 years after | 1.9 | 1.5 | Calcitriol 0.25 mcg three times weekly 500 mg elemental Ca2+ daily | ‡ | 76 | 0.10 |
- † Most recent value – not measured during period immediately prior to transplant.
- ‡ No value available for serum PTH at this time.
- The data show a significant improvement in renal function and calcium handling.
- PTH, parathyroid hormone.
Acquired loss- and gain-of-function mutations
Inactivating auto-antibodies against CaSRs reduces the stimulation of downstream messengers – phospholipase C and MAPK in response to hypercalcaemia. PTH levels are inappropriately elevated because of reduced inhibition. Termed ‘acquired hypocalciuric hypercalcaemia’, this condition may be associated with other autoimmune diseases, including Hashimoto thyroiditis.29 There have been case reports of individuals with activating auto-antibodies against the ECD domain of the CaSR, which occurred either spontaneously or in the context of a polyglandular autoimmune syndrome type 1.30 In primary or severe secondary hyperparathyroidism, levels of CaSR mRNA and protein are reduced by approximately 50% by decreased expression of one of the two CaSR promoters.31, 32
Inherited loss of function
Loss-of-function mutations of the CaSR cause hypercalcaemia by right-shifting the homeostatic response. Higher concentrations of Ca2+, compared with the wild type, are then necessary to suppress PTH release and reduce renal tubular Ca2+ reabsorption.17 The severity of the patient's phenotype demonstrates a gene dose effect where heterozygotes manifest familial hypocalciuric hypercalcaemia, while homozygotes develop severe neonatal primary hyperparathyroidism.33
Familial hypocalciuric hypercalcaemia (FHH) is characterised by autosomal dominant inheritance, mild hypercalcaemia (and hypermagnesaemia) and inappropriately high PTH levels (with relative hypocalciuria).34 Over 100 different genetic defects have been identified with a majority of missense or nonsense mutations on the long arm of chromosome 3 or the long or short arms of chromosome 19, which alter the CaSR or its coupled G protein.34, 35 Often an incidental finding, carriers are usually asymptomatic displaying some resistance to the effects of hypercalcaemia.34 Divalent serum calcium levels usually lie between 2.6 and 2.9 mmol/L, although levels as high as 3.5 mmol/L have been demonstrated and may arise from heterodimerisation of mutant CaSRs with wild-type receptors.36 An inactivated CaSR allele results in (i) higher calcium levels (by 10–20%), inhibiting PTH release from parathyroid chief cells, (ii) reduced renal tubular calcium reabsorption and (iii) inhibition of urinary dilution of urine by hypercalcaemia.37 For diagnosis, vitamin D/dietary calcium deficiency and the effects of medications such as thiazide diuretics must be excluded.4
Neonatal severe primary hyperparathyroidism (NSHPT) arises in individuals homozygous for mutant alleles (usually associated with consanguinity), those compound-heterozygous for different inactivating alleles or where the mutant allele exploits negative dominance in heterodimers.4 Calcium levels are routinely far greater than in FHH, and the condition is associated with significant hyperparathyroidism, bone demineralisation and fractures.4, 35 Unless properly managed, usually by immediate surgical parathyroidectomy, NSHPT can be fatal.
Activating mutations of CaSR
Autosomal dominant hypercalciuric hypocalcaemia arises from gain-of-function mutations either to the CaSR or its coupled G alpha 11 protein.4, 35 Despite the name, inheritance may be autosomal recessive, and, rarely, mutations may occur de novo.38 Most cases are because of point mutations affecting the extracellular domain; however, cases involving the transmembrane domain and large section deletions from the carboxy tail have been demonstrated.38 Individuals are typically heterozygotes, although homozygotes are no more severely affected. Calcium levels range from mildly reduced to profoundly low (<1.25 mmol/L). Similar to FHH, affected individuals display partial resistance to their Ca2+ derangement but in severe cases present with seizures, altered mental state and muscle spasms.4 Biochemically, the condition is characterised by hypocalcaemia, hypomagnesaemia, hyperphosphataemia, inappropriately low PTH levels and normal to high urinary calcium excretion.17 CaSR-mediated inhibition of Na-K-2Cl channels in the cortical TAL can cause NaCl wasting precipitating hypovolaemia, hyperreninaemia, hyperaldosteronaemia and hypokalaemia in a phenotype closely resembling Bartter syndrome.39
Patients with autosomal dominant hypocalcaemic hypercalciuria should only be treated if symptomatic and only up until the point at which symptoms resolve, regardless of calcium level achieved. Some experience symptoms associated with hypercalcaemia even as serum levels approach low-normal. Vitamin D and calcium replacement exacerbate hypercalciuria, resulting in nephrocalcinosis, nephrolithiasis and chronic kidney impairment.8 Diagnosing ADHH based on biochemical parameters has proven unreliable and with many carriers asymptomatic and variable penetrance, establishing a clear family history can be challenging.8, 40 Therefore, genetic analysis is often necessary to differentiate from other forms of hypoparathyroidism.
Discussion
Although seven members of Ms X's family were included in the kindred study, only one sister has manifested symptoms of hypocalcaemia requiring treatment at an early age. That sister has undergone left nephrectomy for nephrolithiasis and now has an eGFR of 16 mL/min/1.73 m2 at age 72 with only slowly progressive renal impairment (eGFR was 20 mL/min/1.73 m2 20 years previously) and with calcium and phosphate concentrations tightly controlled in the range 1.9–2.0 on calcitriol 0.25 μg twice a week. This sister has not suffered the same problems with intercurrent illness and remains asymptomatic from both hypocalcaemia and renal impairment.
This is the first reported case of an individual with ESKD and autosomal dominant hypercalciuric hypocalcaemia successfully treated with a renal transplant. The ‘wild type’ kidney has normally functioning CaSRs. There is no hypercalciuria, and her serum calcium concentrations are far easier to control on lower doses of elemental calcium and calcitriol. Since transplantation there have been no further hospital admissions with hypocalcaemia despite intercurrent illnesses that previously required inpatient care. With urinary calcium excretion now within the normal range, it appears that the risk of reduced kidney function from nephrocalcinosis or nephrolithiasis is extremely low.
Nevertheless, despite wild-type CaSRs in the graft kidney, gain-of-function mutations persist in other tissues, most significantly the parathyroid gland. Consequently, Ms X continues to display relative hypoparathyroidism with a post-transplant mean PTH concentration of 2.98 pmol/L. Serum phosphate concentrations are closely regulated by the actions of PTH and FGF-23 on renal reabsorption in the proximal tubule through the sodium phosphate co-transporter (NPT2A).41 PTH and fibroblast growth factor-23 (FGF-23) suppress NPT2A expression, reducing phosphate reabsorption. Therefore, gain-of-function CaSR-mediated suppression of serum PTH levels may explain in part Ms X's ongoing hyperphosphataemia. A subsequent one-off assessment of Ms X's FGF23 serum concentration 3 years after transplant demonstrated an elevated FGF23 concentration of 432.8 pg/mL. Normally, FGF23 concentrations fall rapidly after transplantation to a normal range, especially with live donor kidneys.42 As FGF23 does not require PTH specifically for its actions, this elevated FGF23 concentration could explain the relatively normal phosphate excretion that was observed.43 The elevated FGF23 may also be an appropriate response to increased dietary phosphate intake and elevated serum phosphate in the setting of low serum PTH concentrations.
Studies in rats that underwent parathyroidectomy demonstrated reduced expression of transcellular calcium transport proteins TRPV5, calbindin-D28k and NCX1.14 Relative reductions in PTH reduce adenylate cyclase-mediated activation of TRPV5, increase claudin-14 expression (inhibiting paracellular cation transit) and reduce the production of calcitriol by inhibiting 1-alpha-hydroxylase.44, 45 With only 15% of filtered calcium reabsorbed actively in the distal tubule, the remaining 85% is reabsorbed either passively in the proximal tubule (the mechanism of which remains unclear) or in the TAL through paracellular channels largely regulated by renal CaSRs and circulating calcium levels. Consequently, we postulate that the wild-type CaSRs in Ms X's graft respond to aberrations in calcium homeostasis brought about by the leftward shifted receptors in her parathyroid gland, reducing the requirement for elemental calcium and calcitriol replacement since the transplant.
The long-term consequences of suppression of the parathyroid gland with a normally functioning ‘wild-type’ kidney remain to be seen. It is not unreasonable to expect phenotypic similarities to primary hypoparathyroidism. With tight control of calcium concentrations between Ms X's asymptomatic limits (1.8–2.1), acute manifestations of low serum PTH and hypocalcaemia, that is, tetany, fatigue and seizures are unlikely. However, chronic hypocalcaemia in the context of primary hypoparathyroidism is associated with cataracts, increased bone mineral density and basal ganglia calcification, causing extrapyramidal symptoms.46-48 On the other hand, calcium and vitamin D replacement, the remaining gain-of-function CaSRs in other tissues including brain and bone and the reduced symptoms of hypocalcaemia may reduce the likelihood of these complications.
Conclusion
This review outlines the current understanding of autosomal dominant hypercalciuric hypocalcaemia associated with gain-of-function mutations in the CaSR or the coupled G protein. Although understanding has improved, this patient cohort continues to provide a diagnostic and therapeutic challenge. Routine lab tests are insufficient to reliably discriminate between causes of hypoparathyroidism. ADHH displays variable penetrance with a large proportion of individuals remaining asymptomatic, as shown in this patient's family. Thus, it has been postulated that a third of people with unexplained hypoparathyroidism may exhibit undiagnosed gain-of-function mutations of the CaSR gene.4 Unfortunately, misdiagnosis begets mismanagement with overly aggressive calcium and vitamin D replacement causing nephrocalcinosis, nephrolithiasis, nephrogenic diabetes insipidus and progressive CKD.4, 34 Further investigations are planned to examine the role of FGF-23 in the index case and in the extended family to enhance understanding of the interactions between PTH, the CaSR and FGF-23 in calcium and phosphate handling in ADHH.
This case is the first to demonstrate the effects of providing normal CaSR in a kidney transplant to a patient with autosomal dominant hypercalciuric hypocalcaemia. After 3 years since the transplant, Ms X remains asymptomatic, with reduced requirements for calcium and vitamin D. Serum Ca2+ is maintained at the lower end of normal in order to maintain reduced renal Ca2+ excretion, minimising the risk of graft injury from hypercalciuria. This suggests that renal transplantation is a safe and appropriate option for ESKD with autosomal dominant hypercalciuric hypocalcaemia.
Acknowledgements
Open access publishing facilitated by University of Otago, as part of the Wiley - University of Otago agreement via the Council of Australian University Librarians.




