ANZTCT consensus position statement on ruxolitinib in steroid-refractory acute and chronic graft-versus-host disease
Funding: Medical writer assistance was funded by Novartis through Wiley and neither ANZTCT nor the authors received any funding for this work.
Conflict of interest: The authors declare any conflicts of interest as follows: N. Hamad: Advisory boards and honoraria: Novartis Australia, Roche Australia, Takeda Australia, AbbVie, Janssen Australia, Astellas Pharma Inc. and Jazz Pharmaceuticals. I. Bilmon: Advisory board attendance: Pfizer Australia and AbbVie Australia. L. Chee: Honoraria and advisory board attendance for Novartis Australia, advisory board attendance for Otsuka Australia Pharmaceutical. A. Henden: Advisory/educational activities for Novartis Australia, Astellas Pharma Inc., Gilead and AstraZeneca Australia. Anna M. Johnston: Consulting/advisory role with Roche Australia, BeiGene, Novartis Australia and Sanofi. D. Purtill: Honoraria and advisory boards: Novartis Australia, Gilead, BMS Celgene, Jazz Pharmaceuticals and Bastion Medical Education (all honoraria paid to institution). A. Bajel: Advisory roles with AbbVie, Amgen Australia, Astellas Pharma Inc., Pfizer Australia, Takeda Australia, Novartis Australia and Gilead; speaker fees from Amgen Australia. Siok-Keen Tey: Advisory board attendance: Link Healthcare and MSD Australia. D. Yeung: Honoraria and advisory boards: Novartis Australia, Pfizer Australia, Amgen Australia and Takeda Australia; research funding: Novartis and Bristol-Myers Squibb. T. Cole: None. C. Lewis: Educational activities for Novartis Australia. J. Butler: Honoraria: Janssen Australia, Novartis Australia; Consulting or Advisory Role: Gilead Sciences, Janssen Australia, Novartis Australia; Speakers' Bureau: Gilead Sciences, Novartis Australia, Janssen Australia, Roche Australia and Takeda Australia.
Abstract
This position paper provides an overview of the assessment and management of both acute and chronic graft-versus-host disease (GvHD). There is a focus on the use of ruxolitinib, a selective inhibitor of Janus kinase (JAK)1 and JAK2, for the treatment of corticosteroid-refractory and corticosteroid-dependent GvHD.
Introduction
While haemopoietic stem cell transplantation (HSCT) is the only curative therapy for many haematological malignancies and disorders, graft-versus-host disease (GvHD) poses significant morbidity and mortality.1, 2 Acute GvHD (aGvHD) develops in approximately 50% of patients undergoing allogeneic HSCT,2 and chronic GvHD (cGvHD) develops in 30–70% of patients.1 While data from the Australia and New Zealand Transplant and Cellular Therapies (ANZTCT) registry show sustained and significant reductions in the incidence of GvHD between 2001 and 2015, for transplants during 2011–2015, cumulative incidences of grades II–IV aGvHD, grades III–IV aGvHD and cGvHD at 3 years were 31%, 14% and 52% respectively.3
The standard first-line treatment for both acute and chronic GvHD is high-dose systemic corticosteroids.1, 2 However, response rates are variable2 and there is a higher risk of poor outcomes in patients with corticosteroid-refractory or corticosteroid-dependent disease.1, 2
ANZTCT developed this consensus position paper to support best practices in GvHD management in Australia and New Zealand. This manuscript was developed using a modified Delphi method and in accordance with the ANZTCT policy for consensus practice/position statement development. The relevant literature has been reviewed and selected by the expert authors. The authorship group includes ANZTCT board members, who have sought representatives for geographic representation and gender equity and diversity principles.
We provide an overview of the assessment and management of both acute and chronic GvHD with a focus on the use of ruxolitinib, a selective inhibitor of Janus kinase (JAK)1 and JAK2, for the treatment of corticosteroid-refractory and -dependent GvHD.
Ruxolitinib is approved in Australia for GvHD, on the basis of the REACH22 and REACH31 studies. These trials enrolled patients with acute and chronic GvHD respectively, and in both trials, patients were aged 12 years and older and were steroid-refractory or -dependent. In REACH2, patients with aGvHD were more likely to respond to ruxolitinib compared with ‘best available treatment’ (BAT) (odds ratio (OR), 2.64; 95% confidence interval (CI), 1.65–4.22; P < 0.001). Similarly, in REACH3, ruxolitinib-treated patients with cGvHD were more likely to respond compared with those treated with BAT (OR, 2.99 (95% CI, 1.86–4.80); P < 0.001). In REACH3, there was a higher overall response with ruxolitinib compared with BAT at week 24 (49.7% vs 25.6%), regardless of the organs involved.1 In addition, ruxolitinib led to a higher best overall response (76.4% vs 60.4%), longer duration of response and longer failure-free survival compared with BAT.1
In Australia, ruxolitinib is indicated for the treatment of patients aged 12 years and older with acute or chronic GvHD who have inadequate responses to corticosteroids.4 In New Zealand, ruxolitinib is yet to be approved by the New Zealand Medicines and Medical Devices Safety Authority for use in GvHD but is approved for other indications.5
Paediatric considerations
Children undergo HSCT for a wider range of indications, including primary immunodeficiency, haemoglobinopathies and metabolic disorders,6, 7 where GvHD is highly undesirable as the graft-versus-malignancy effect is of no relevance. Despite techniques pioneered to reduce the risk of GvHD, such as T cell receptor alpha/beta depletion or use of post-transplant cyclophosphamide, GvHD remains a problem causing significant morbidity and mortality, with no clear second-line agent after corticosteroids.7, 8 Similar to the adult setting, a range of agents has been trialled with variable success. Children are often excluded from clinical trials, and those under 12 years of age were not included in the REACH studies. Despite this, there are reports of successful use of ruxolitinib in younger children, with doses adjusted for weight.6, 8 As in older children and adults, appropriate anti-infective prophylaxis is required if ruxolitinib is used.6
Acute graft-versus-host disease
Acute GvHD classically occurs within the first 100 days following allogeneic transplant, although late-onset aGvHD and overlap syndrome are frequently seen.9 The diagnosis and staging of aGvHD is achieved with clinical, laboratory and biopsy assessment of target organs – the skin, liver and gastrointestinal tract.10 Clinical assessment for maculopapular rash, jaundice and symptoms of gastrointestinal involvement and testing for liver enzyme elevations are used to stage aGvHD (Table 1). Biopsy is recommended for confirmation of the diagnosis and exclusion of important differential diagnoses, such as drug toxicity and infection-related complications, although this should not generally delay treatment.11
| Stage | Skin (active erythema only) | Liver (bilirubin) | Upper GI | Lower GI (stool output/day) |
|---|---|---|---|---|
| 0 | No active (erythematous) GvHD rash | <34.2 μmol/L (<2 mg/dL) | No or intermittent nausea, vomiting or anorexia | Adult: <500 mL/day or < 3 episodes/day |
| Child: <10 mL/kg/day or < 4 episodes/day | ||||
| 1 | Maculopapular rash <25% BSA | 34.2–51.3 μmol/L (2–3 mg/dL) | Persistent nausea, vomiting or anorexia | Adult: 500–999 mL/day or 3–4 episodes/day |
| Child: 10–19.9 mL/kg/day or 4–6 episodes/day | ||||
| 2 | Maculopapular rash 25–50% BSA | 53.0–102.6 μmol/L (3.1–6 mg/dL) | Adult: 1000–1500 mL/day or 5–7 episodes/day | |
| Child: 20–30 mL/kg/day or 7–10 episodes/day | ||||
| 3 | Maculopapular rash >50% BSA | 104.3–256.5 μmol/L (6.1–15 mg/dL) | Adult: >1500 mL/day or >7 episodes/day | |
| Child: >30 mL/kg/day or >10 episodes/day | ||||
| 4 | Generalised erythroderma (>50% BSA) plus bullous formation and desquamation >5% BSA | >256.5 μmol/L (>15 mg/dL) | Severe abdominal pain with or without ileus or grossly bloody stool (regardless of stool volume). |
- BSA, body surface area; GI, gastrointestinal.
- Overall clinical grade (based on most severe target organ involvement): Grade 0: No stages 1–4 of any organ. Grade I: stages 1–2 skin without liver, upper GI, or lower GI involvement. Grade II: Stage 3 rash and/or stage 1 liver and/or stage 1 upper GI and/or stage 1 lower GI. Grade III: stages 2–3 liver and/or stages 2–3 lower GI, with stages 0–3 skin and/or stages 0–1 upper GI. Grade IV: Stage 4 skin, liver, or lower GI involvement, with stages 0–1 upper GI.
- Adapted from Harris et al.,10 with permission from RightsLink/Elsevier.
Staging is recommended according to the Mount Sinai Acute GvHD International Consortium (MAGIC) criteria10, which is a modification on the previously used Glucksberg criteria.12 Bedside electronic resources may help facilitate scoring and grading – one example is the eGvHD app,13 which was developed in collaboration with the European Society for Blood and Marrow Transplantation and the National Institutes of Health (NIH).
Many patients with aGvHD will remain on prophylactic agents such as calcineurin inhibitors and mycophenolate mofetil, and appropriate dosing should be ensured.11
Treatment of grade I aGvHD (stage I or II skin involvement) consists of mid- or high-potency topical corticosteroids11 twice daily, skin moisturisers and hypoallergenic sun protection. Antihistamines may be beneficial in treating pruritus. The standard initial treatment of choice for patients with grade II disease or higher is systemic, high-dose corticosteroids.2 Methylprednisolone 2 mg/kg/day or equivalent is recommended for first-line therapy.11 In grade II disease isolated to the skin or upper gastrointestinal tract, 1 mg/kg/day methylprednisolone or prednisone may be considered.11
For patients with gastrointestinal involvement, adjunctive treatment with a non-absorbable oral corticosteroid (e.g. budesonide 3 mg three times daily) is recommended. However, gastrointestinal infection should be excluded with cultures and viral testing of stool and peripheral blood. Biopsies should be performed on a case-by-case basis, with immunohistochemistry staining for infection (e.g. cytomegalovirus (CMV)). Recommended supportive care for those with gastrointestinal involvement includes dietitian review to ensure nutritional requirements are met and early supplementary nutritional support.14 Octreotide has been studied in a single-arm trial for the treatment of diarrhoea in patients with GvHD, and it was noted that this agent should be ceased within 24 h of symptom resolution to avoid ileus.15
In patients who respond to corticosteroids, treatment is continued for 1 to 2 weeks before slowly and cautiously tapering the corticosteroid dose over a period of weeks,11 with ongoing monitoring of response for early detection of a flare of GvHD. Patients whose disease progresses after at least 3 days of high-dose steroid use, who lack response (absence of partial response (PR) or better) after 7 days or who relapse when corticosteroids are tapered are considered corticosteroid-refractory2, necessitating second-line treatment (Fig. 1).
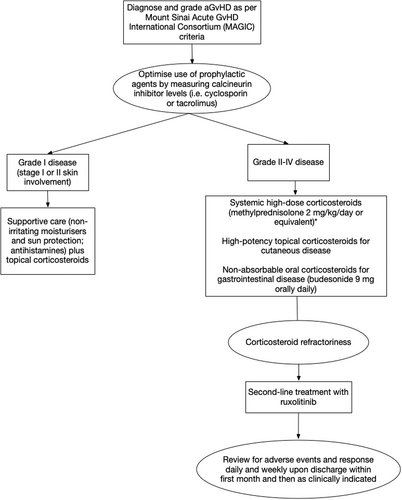
Treatment of steroid-refractory aGvHD
Prior to the publication of REACH2, there was no established standard of care for the treatment of steroid-refractory aGvHD in Australia and New Zealand, and treatment included a broad range of immunosuppressive therapies.2
Ruxolitinib is now generally the preferred agent because of its superior efficacy and toxicity profile as demonstrated in the REACH2 study.2 The patient population included in REACH2 is summarised in Table 2.
| Acute GvHD (REACH2) | Chronic GvHD (REACH3) |
|---|---|
| Inclusion criteria | |
| Age 12 years or older | Age 12 years or older |
| Grades II–IV glucocorticoid-refractory acute GvHD that involved use of systemic immunosuppressive therapy | Moderate or severe glucocorticoid-refractory or –dependent chronic GvHD, according to NIH consensus criteria27 |
| Myeloid and platelet engraftment, defined as an absolute neutrophil count of >1.0 × 109/L and a platelet count of ≥20 × 109/L | Previous JAK inhibitor treatment for aGvHD if it resulted in CR or PR and if JAK inhibitor was stopped ≥8 weeks before |
| Exclusion criteria | |
| Tumour relapse after allogeneic SCT in previous 6 months | Graft loss within 6 months before treatment initiation |
| Relapsed primary cancer after undergoing allogeneic SCT | Relapse of the primary cancer |
| More than one previous treatment for steroid-refractory acute GvHD | Patients treated previously with two or more systemic therapies for cGvHD in addition to glucocorticoids with or without calcineurin inhibitors |
| Active, uncontrolled infection | Active, uncontrolled infection |
| JAK inhibitor therapy for any indication after initiation of allogeneic SCT conditioning | |
| Response assessment | |
| Overall response at day 28 – proportion of patients who had a complete response or partial response as compared with baseline organ staging without the use of additional systemic therapy for acute GvHD | Overall response at week 24 – complete or partial response according to 2014 NIH consensus criteria26 |
| Durable overall response at day 56 – proportion of patients who had response at day 28 that was maintained at day 56 | Response on the modified Lee Symptom Scale36, 37 (≥7-point reduction from baseline in total symptom score) at week 24 |
- aGvHD, Acute GvHD; cGvHD, chronic GvHD; CR, complete response; GvHD, graft-versus-host disease; JAK, Janus kinase; NIH, National Institutes of Health; PR, partial response; SCT, stem cell transplant.
Doses of ruxolitinib should be individualised based on safety and efficacy; however, a starting dose of 10 mg orally twice daily with or without food is recommended.4 Ruxolitinib is well absorbed, even in patients with gastrointestinal aGvHD, and can be given through a nasogastric tube (Box 1).
Box 1. Summary recommendations: aGvHD
- Initiate ruxolitinib for patients with corticosteroid-refractory aGvHD, along with concomitant optimisation of antimicrobial prophylaxis (level II, grade A).
- A starting ruxolitinib dose of 10 mg BD is recommended (level II, grade A).
- Monitor patients carefully for drug interactions and cytopenia (level III-2, grade B).
-
Assess response to ruxolitinib according to MAGIC criteria (level III-2, grade B).
- In patients who respond, taper other immunosuppressive agents for aGvHD.
- In patients who achieve CR, consider tapering ruxolitinib after day 56; if signs or symptoms of aGvHD re-occur, re-escalate to therapeutic dose of ruxolitinib.
- Stop ruxolitinib treatment if there is no response after 28 days or progression prior to this time.
Of note, the REACH2 study included patients who had achieved engraftment, with absolute neutrophil counts of >1.0 × 109/L and platelets ≥20 × 109/L. However, growth factor and transfusion support were permitted. Reduced doses of ruxolitinib can be considered in patients with lower counts, but given the poor prognosis associated with corticosteroid-refractory aGvHD, this should be balanced against the severity of aGVHD and the ability to support counts with growth factors and/or transfusion.
Antiviral and antifungal prophylaxis in patients with acute GvHD treated with ruxolitinib
There is an increased risk of infection in all patients with aGvHD. In REACH2, infection of grade 3 or greater severity up to day 28 occurred in 22% of patients who received ruxolitinib and in 19% of patients who received control therapy.2 Prophylactic antibacterial, antifungal and antiviral agents should be instituted as per institutional guidelines and protocols.
Guidelines for antifungal prophylaxis in HSCT16 recommend the use of first-line posaconazole in patients with extensive or severe GvHD. Alternative agents include voriconazole, itraconazole, micafungin, liposomal amphotericin and isavuconazole.16
Routine prophylaxis against varicella-zoster virus and pneumocystis pneumonia (PCP) should be continued and/or extended after a diagnosis of aGvHD is made. Commonly used agents include valaciclovir for antiviral prophylaxis and co-trimoxazole for PCP and toxoplasmosis prevention.17 CMV viraemia monitoring and pre-emptive therapy should follow institutional practice.
Avoiding or minimising drug interactions with ruxolitinib in patients with aGvHD
Ruxolitinib is metabolised predominantly through the cytochrome P450 isozyme CYP3A4 pathway and, to a lesser degree, the CYP2C9 pathway. For patients with GvHD, ruxolitinib dose reductions of up to 50% (i.e. 5 mg twice a day) should be considered in those administered products that are dual inhibitors of CYP2C9 and CYP3A4 enzymes (e.g. fluconazole).2 Concomitant use of ruxolitinib with fluconazole doses greater than 200 mg is not recommended.2 Ruxolitinib dose reductions are not needed with concomitant use of posaconazole or voriconazole, but close monitoring is recommended.
Management of cytopenia in patients taking ruxolitinib for aGvHD
Cytopenias in the post-transplant setting can be because of several factors, including medications, GvHD, poor graft function, and many other causes. These need to be ruled out prior to ruxolitinib dose adjustments for corticosteroid-refractory GvHD.
Ruxolitinib-related cytopenia and dose adjustments
It is important to monitor patients for ruxolitinib-related cytopenia and to mitigate the risk of this adverse event. Depending on the clinical situation, consider reducing the dose to 5 mg twice daily or temporarily stopping ruxolitinib in patients who develop severe worsening of cytopenia, in the setting of addressing other possible causes as much as possible (Table 3).
| Laboratory parameter | Dosing recommendation |
|---|---|
| Platelet count <20 × 109/L | Reduce ruxolitinib by one dose level. If platelet count ≥20 × 109/L within seven days, dose may be increased to initial dose level; otherwise, maintain reduced dose. |
| Platelet count <15 × 109/L | Hold ruxolitinib until platelet count ≥20 × 109/L, then resume at one lower dose level. |
| Absolute neutrophil count (ANC) ≥0.5 × 109/L to <0.75 × 109/L | Reduce ruxolitinib by one dose level. Resume at initial dose level if ANC >1 × 109/L. |
| Absolute neutrophil count <0.5 × 109/L | Hold ruxolitinib until ANC >0.5 × 109/L, then resume at one lower dose level. If ANC >1 × 109/L, dosing may resume at initial dose level. |
| Total bilirubin elevation, no liver GvHD | 3.0 to 5.0 × ULN: Continue ruxolitinib at one lower dose level until ≤3.0 × ULN. |
| >5.0–10.0 × ULN: Hold ruxolitinib up to 14 days until total bilirubin ≤3.0 × ULN. If total bilirubin ≤3.0 × ULN dosing may resume at current dose. If not ≤3.0 × ULN after 14 days, resume at one lower dose level. | |
| >10.0 × ULN: Hold ruxolitinib until total bilirubin ≤3.0 × ULN, then resume at one lower dose level. | |
| Total bilirubin elevation, liver GvHD | >3.0 × ULN: Continue ruxolitinib at one lower dose level until total bilirubin ≤3.0 × ULN. |
| Other adverse reactions: Grade 3 | Continue ruxolitinib at one dose level lower until recovery. |
| Other adverse reactions: Grade 4 | Discontinue ruxolitinib. |
- GvHD, graft-versus-host disease; ULN, upper limit of normal.
- Adapted from Australian Product Information – Jakavi® (Ruxolitinib), Novartis Pharmaceuticals Australia Pty Limited (14 October 2022).4
Ruxolitinib should be tapered gradually, because a ‘withdrawal syndrome’ that resembles systemic inflammatory response syndrome (SIRS) can occur when it is discontinued abruptly or tapered too quickly.18 Patients should be monitored for a withdrawal-like syndrome and ruxolitinib should be restarted in patients with significant withdrawal symptoms or flare of aGvHD.
If ruxolitinib is interrupted, consider increasing the corticosteroid dose to ≥0.4 mg/kg/day methylprednisolone (or equivalent prednisone to ≥0.5 mg/kg/day) for a minimum of 7 days to avoid a significant aGvHD flare.2
Ruxolitinib dosing can be restarted or increased following recovery of the haematologic parameter(s) to acceptable levels. The objective for restarting or escalating after a reduction for haematologic safety is to find the highest tolerable dose regimen of ruxolitinib for each patient, that is necessary to obtain a clinical response. Ruxolitinib doses should not be increased by more than 5 mg twice daily and not more often than every 2 weeks.2
For guidance on dose modifications because of cytopenia, refer to Table 3.
CMV reactivations and treatment
CMV reactivations and their treatment using (val)ganciclovir are common in patients with aGvHD, and both also contribute to cytopenia. Clinical acumen is necessary to balance competing priorities. If ruxolitinib is used with (val)ganciclovir, monitor full blood count carefully and monitor viral loads frequently (e.g. twice weekly). Alternative therapies for prophylaxis and treatment with fewer adverse effects on blood counts should be considered if available (e.g. letermovir, viral-specific T cells and foscarnet). Growth factor support should be considered as clinically indicated in this setting.
How to taper or discontinue immunosuppression and ruxolitinib in patients with aGvHD
It is important to assess responses to systemic treatment using MAGIC criteria10 daily during hospital admission and regularly in the outpatient setting.
Discontinuing immunosuppressants
One of the advantages of ruxolitinib treatment is the ability to reduce steroid exposure. In patients with a complete response (CR) or PR to ruxolitinib, cessation of other immunosuppressive agents for aGvHD should be considered prior to tapering of ruxolitinib. Corticosteroids should be tapered first. The REACH2 study recommended a taper no earlier than day 7 of ruxolitinib therapy and under regular observation with the aim of cessation within 5–7 weeks (e.g. by 10% every 5 days). Calcineurin inhibitors can be tapered as per institutional practice (e.g. 25% dose reduction per month).
Discontinuing ruxolitinib
In patients who achieve a CR, consider tapering ruxolitinib after day 56 (Table 2).2 Ruxolitinib should be slowly tapered by reducing the dose by 50% every 2 months. If signs or symptoms of GvHD reoccur during or following ruxolitinib tapering, re-escalation back to a therapeutic dose is indicated.4 If disease stabilises, another taper should not be attempted before 6 months.
In patients who achieve a PR, consider continuation of therapy until evidence of progression or toxicity, and reassess at 6 months.
- signs of progression of GvHD after at least 28 days of treatment with ruxolitinib, based either on objective increase in stage/grade or new organ involvement;
- lack of improvement in GvHD (to PR or better) compared with baseline after at least 28 days of treatment with ruxolitinib; or
- signs of loss of response on maximal doses of ruxolitinib, defined as objective worsening of GvHD, determined by increase in stage, grade or new organ involvement at any time after initial improvement.
Chronic graft-versus-host disease
cGvHD can affect patients with or without a history of aGvHD, although aGvHD increases the risk of cGvHD.9 Routine screening for cGvHD should be undertaken at least 3-monthly post-HSCT, including screening for lung cGvHD.20 Lung cGvHD can be very subtle, with forced expiratory volume in 1 s (FEV1) decline preceding any symptoms.
cGvHD manifests heterogenously and can involve almost any organ21, 22; for this reason, the NIH developed criteria for diagnosis. The diagnosis requires distinction from aGvHD, the presence of at least one diagnostic clinical sign or one distinctive manifestation of cGvHD (confirmed by testing) and exclusion of other possible diagnoses.9
Screening for lung cGvHD with lung function tests or spirometry is important when there is evidence of cGvHD in any organ or if there is evidence of cGvHD progression in any organ.
Severity scoring for cGvHD was published in 2005 and updated in 20149, 23 and is currently under revision. As with aGvHD, tools such as the eGvHD app13 are important in facilitating diagnostic and severity assessments.
cGvHD has significant impacts on quality of life (QoL), and the decision to start systemic therapy for cGvHD is based on severity (mild, moderate or severe according to NIH classifications).11
Localised therapy, where applicable, is generally suggested for mild cGvHD9 affecting the skin, mouth or eyes and should be continued until local changes resolve.24 For moderate to severe cGvHD, in addition to local therapies, oral prednisone, at an initial dose of 1 mg/kg/day, remains the standard first-line systemic treatment.
Special mention should be made in the context of lung cGvHD, where combination therapy with inhaled fluticasone, azithromycin and montelukast (FAM) is also recommended.25 This consists of prednisone 1 mg/kg/day for 2 weeks then tapered by 0.25 mg/kg/day per week, aiming for 0.25 mg/kg/day by 5 weeks; plus inhaled fluticasone 440 mcg twice daily (equivalent) ± long-acting beta-agonist; plus azithromycin 250 mg 3 days per week; and montelukast 10 mg daily.25 The rapid taper in the FAM protocol is intended to avoid long-term steroid exposure but can only be followed stringently if no other organs are involved or the patient demonstrates responses amenable to such a rapid steroid taper.
Responses to corticosteroid therapy are assessed monthly using NIH criteria,26 with age-appropriate modifications used in children.
Half of all patients with cGvHD become corticosteroid-refractory or corticosteroid-dependent, increasing the likelihood of poor outcomes.1 The REACH3 study defined steroid-refractory cGvHD during first-line treatment as disease progression despite the use of prednisone at ≥1 mg/kg/day for ≥1 week or disease persistence without improvement despite continued treatment with prednisone at ≥0.5 mg/kg/day or 1 mg/kg every other day for at least 4 weeks, or an increase to prednisolone dose to >0.25 mg/kg/day after two unsuccessful attempts to taper the dose.27 An unsatisfactory response to corticosteroids prompts the need for second-line treatment (Fig. 2).
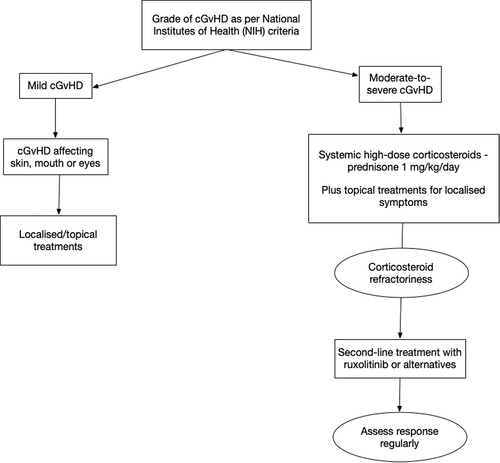
Treatment of steroid-refractory cGvHD
In patients with steroid-refractory or -dependent cGvHD, ruxolitinib is considered the standard of care. In the REACH3 study, ruxolitinib was shown to be superior to the best available therapy, including ibrutinib and extracorporeal photopheresis, for treating corticosteroid-refractory or -dependent cGvHD.1 Inclusion and exclusion criteria used in REACH3, as well as assessment of response is summarised in Table 2. The recommended starting dose of ruxolitinib for cGvHD is 10 mg orally twice daily, with or without food (Box 2).4
Box 2. Summary recommendations: cGvHD
- Initiate ruxolitinib for patients with corticosteroid-refractory or corticosteroid-dependent cGvHD, along with concomitant optimisation of antimicrobial prophylaxis (level II, grade A).
- A starting ruxolitinib dose of 10 mg BD is recommended (level II, grade A).
- Monitor patients carefully for drug interactions and cytopenias (level III-2, grade B).
- If patients achieve PR/CR with ruxolitinib: taper corticosteroids; once corticosteroids have been stopped and CR is achieved, taper ruxolitinib (level II, grade A).
Managing antiviral and antifungal prophylaxis in patients treated with ruxolitinib for cGvHD
Antiviral and antifungal prophylaxis depends on the level of patient immunosuppression; patients treated with high-dose corticosteroids require infection prophylaxis. In patients with cGvHD being treated with ruxolitinib, consider initiating antiviral and antifungal prophylaxis if the patient is not already on these.28, 29
In REACH3, infections of any type occurred in 63.6% of patients who received ruxolitinib as compared with 56.3% who received control therapy – viral infections were the most common, followed by bacterial and fungal infections.1 Similar to aGvHD, guidelines recommend first-line posaconazole in patients with extensive or severe cGvHD.16 Ruxolitinib dose reduction is not needed with concomitant use of posaconazole or voriconazole, but close monitoring is recommended. Ruxolitinib dose reduction of up to 50% (to 5 mg BD) is recommended when used concomitantly with fluconazole,4 and the fluconazole dose should not exceed 200 mg daily.4 If available, monitoring of azole levels is recommended to ensure therapeutic levels are maintained.
Additional strategies to protect against infection, including vaccinations, COVID-19 preventive strategies30, 31 and intravenous immunoglobulin, should be as per institutional guidelines.
Management of cytopenia in patients taking ruxolitinib for cGvHD
As with aGvHD, cytopenia in patients with cGvHD can be multifactorial, and drug-related cytopenia should be considered when other causes (such as viral reactivation) have been managed or excluded. Ruxolitinib dose reduction or interruption, along with supportive care strategies (e.g. growth factors), may be needed in patients with worsening cytopenias attributed to ruxolitinib1 (Table 3). Ruxolitinib should be tapered gradually to avoid a SIRS-like syndrome or flare of GvHD, but in uncommon cases where abrupt cessation is needed, the corticosteroid dose should generally be maintained or increased to >0.4 mg/kg/day methylprednisolone (or equivalent prednisone to >0.5 mg/kg/day) for a minimum of 7 days to avoid a significant cGvHD flare.1 Ruxolitinib dosing may be restarted or increased following recovery of the haematologic parameter(s) to acceptable levels.
Tapering or ceasing corticosteroids and ruxolitinib in patients with cGvHD
Assessing response to systemic therapy for cGvHD should be performed at least monthly using the 2014 response criteria.26 Maximal response to ruxolitinib should be reviewed at 24 weeks (Table 2).1 Long-term steroid use is associated with significant adverse events, so systemic corticosteroids should be tapered in patients taking ruxolitinib who achieve a CR or PR. If a flare occurs during the corticosteroid taper, the dose should be increased to the previous dose level and continued for at least 3 months before attempting to resume the steroid taper.
Once systemic corticosteroids have been stopped and the patient has achieved CR, a 50% ruxolitinib dose reduction every 2 months can be initiated. The initial dose reduction is to 5 mg orally twice daily.4 If sustained cGvHD response is observed (i.e. no worsening of cGvHD signs and symptoms), the dose can be further tapered by a second 50% dosage reduction to 5 mg orally daily for an additional 2 months, prior to ruxolitinib cessation.1 If signs or symptoms of cGvHD re-occur during or following ruxolitinib tapering, re-escalate4 to the therapeutic dose.
In patients who do not respond to ruxolitinib by 6 months, treatment cessation and alternative agents can be considered. Clinical trials, if available, are highly recommended in this context.
Quality-of-life considerations in patients with cGvHD
Patients with cGvHD have impaired physical, social, psychological and general QoL, which worsen with disease severity.1 Those in middle age appear to have worse QoL, despite lower symptom burden compared to younger or older age.32 Factors that can negatively impact QoL include specific gastrointestinal and joint and fascia manifestations, as well as poorer functional status and exercise intolerance.32
The collection of valid and concise QoL data is essential in developing treatment strategies for cGvHD.32 The use of patient-reported outcome measures, based on NIH criteria, is encouraged to assess QoL and understand treatment challenges from the patient perspective. Patient-reported signs, symptoms and outcomes are part of regular NIH response assessments.26 These measures can be supplemented with patient-reported QoL measures, using tools such as Functional Assessment of Cancer Therapy – Bone Marrow Transplantation, the 36-Item Short Form Health Survey and EuroQol-5 Dimension.
Other novel agents for the treatment of GvHD
As the prophylaxis and treatment landscape for GvHD continues to evolve, the clinical outcomes of HSCT will further improve. Treatments with promising evidence include belumosudil (an oral inhibitor of Rho-associated coiled-coil-containing protein kinase 2) and ibrutinib (an inhibitor of Bruton tyrosine kinase), both of which have activity against cGvHD.33-35 There is a need for ongoing clinical research to further improve and expand the treatment options for GvHD.
Conclusions
The development of second-line treatments for patients with steroid-refractory acute and chronic GvHD is vital to improving outcomes for patients following allogeneic stem cell transplants. Ruxolitinib is a second-line treatment option that is generally preferred, when available, for this indication, and this position statement has outlined practical considerations for its use in clinical practice in Australia.
Acknowledgements
The authors thank Anne Dyson, MBBS, of WriteSource Medical Pty Ltd., Sydney, Australia, for providing medical writing support. The medical writing support was funded by Novartis Pharmaceuticals Australia Pty Limited, in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022). Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.




