It feels like lupus: accessible science for the low-vision community on immune system dysfunction in lupus
Abstract
Science communication is often confined to spoken, written or graphical form, neglecting the integration of other tools that would open inclusive scientific dialog to the low-vision community. To address this barrier, members from the Monash Rheumatology clinical and laboratory research groups formed a Lupus Sensory Science team to create a breakout room at the 2023 Monash Sensory Science Exhibit on Autoimmunity. Our goal was to develop multimodal displays and artworks to engage participants with blindness and low vision with the immunological underpinnings of systemic lupus erythematosus (SLE). Here I describe how we created several stations using a combination of tactile posters and models to communicate disease manifestations and immune system dysregulation in SLE. I reflect on how participants keenly engaged with our artworks, asking thoughtful questions that stimulated interesting discussions about treatment options in SLE. In addition, I analyze how our exhibit could be improved to further increase accessibility for the low-vision community. Overall, we learned a lot about how to be inclusive in scientific communication methods and we will strive to continue to engage all members of our community in scientific discussion.
SCIENCE COMMUNICATION IS MORE THAN THE SPOKEN OR WRITTEN WORD
I can see the need for accessible science communication when I go to the weekly lupus clinic at Monash Health, where I talk to patients about collecting their blood for studies run by our research group. I must ensure that I avoid the jargon we use in the laboratory. However, my communication is almost completely limited to the spoken word, disregarding tools that would provide accessible scientific information to the blind and low-vision community, which includes some patients with lupus. In other settings, such as in mainstream schools, students with vision impairment report challenges learning scientific concepts because they are usually presented two-dimensionally.1 Contrastingly, in specific school settings for students with low vision where three-dimensional and tactile models were provided, students were found to master scientific concepts just as well as fully sighted students.2
Our team was therefore humbled by the invitation to participate in the Monash Sensory Science Exhibit by creating a breakout room on the topic of lupus, with the aim of developing creative and informative multimodal models to effectively communicate lupus and the underpinning immunological processes to participants with blindness and low vision. Our lupus sensory science group combined expertise from “bench to bedside” with members from our clinical team and research team. We participated in a briefing from Vision Australia before the event where we received training on the causes and effects of vision loss, communication tips, appropriate guiding and adapting the environment suitably for people with low vision.
LUPUS DISEASE MANIFESTATIONS
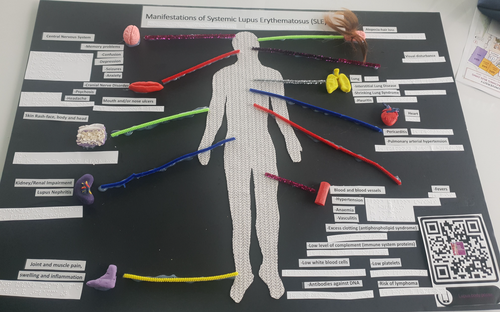
First, we set out to introduce the audience to the disease manifestations present in systemic lupus erythematosus (SLE). SLE is a serious, multisystem chronic autoimmune disease that ranges from mild disease (with involvement from systems such as the skin) to serious organ damage such as end-stage kidney disease.3 As the participants walked into our breakout room, one of the first artworks that they could interact with was a tactile poster (Figure 1). This had the shape of a human body in the center, cut from a fabric that had a distinct texture from the rest of the poster board. From the relevant areas of the human body, a pipe cleaner pointed outward to a small model of the area affected sculpted from play dough together with a braille description. Participants could also scan a QR (quick response) code on the poster which would take them to a recorded version that they could listen to. Three separate push buttons accompanied the tactile poster with sounds of (1) fine crackles of interstitial lung disease (fibrosis/scarring), (2) pleural rub (pleuritis) and (3) pericardial rub (pericarditis) which results from inflammation in the lung and heart.4, 5 From this poster, our goal was to convey that SLE can affect all areas of the body.
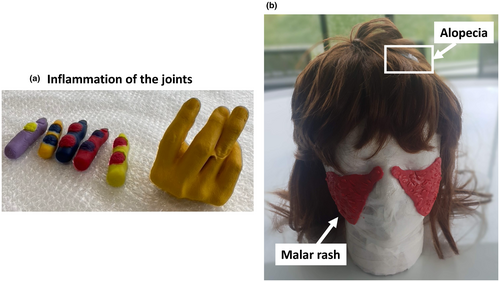
Next, we depicted the more common manifestations in SLE such as musculoskeletal involvement, and cutaneous manifestations including malar rash (also known as butterfly rash) and alopecia. Musculoskeletal involvement leads to patients often presenting in the clinic with joint pain. Indeed, up to 95% of patients with SLE have been observed to develop arthritis, commonly in the small joints of the hand and wrist.6 We demonstrated this to participants by creating a model of inflamed joints that they were able to feel (Figure 2a). This was developed by creating a cast from the hand of a patient with lupus presenting with inflamed joints, who generously gave us 10 min of their time to cast their hand in a bucket. Cutaneous manifestations are also highly common in SLE and include malar rash, which consists of an erythematous rash spanning the bridge of the nose and cheeks.6 Malar rash was modeled using play dough on a foam mannequin head (Figure 2b). We also demonstrated alopecia, another cutaneous feature of SLE characterized by patches of hair loss using a wig on the mannequin head (Figure 2b).
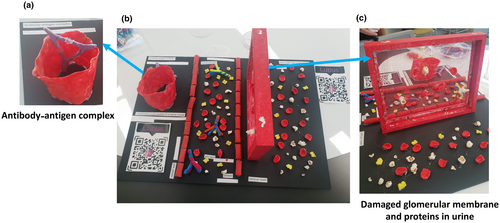
Kidney involvement in SLE is called lupus nephritis. Proteinuria is one of the most common clinical presentations of renal involvement, characterized by elevated proteins in the urine, which serves as a biomarker for the severity of kidney disease.6 Now that we had established the key clinical presentations of SLE with our participants, we sought to link a key clinical presentation such as kidney involvement and proteinuria with immunological involvement to introduce this next concept: immune dysregulation underlies SLE. To do this, we designed a tactile poster that introduced an antibody–antigen complex, deposited in a blood vessel (Figure 3a). Autoantibody immune complexes activate the complement cascade and recruit innate immune cells, thus propelling inflammation in the kidney, creating tissue damage.6 Next, participants could feel inside a renal blood vessel where there are erythrocytes (red orecchiette), proteins (popcorn), uric acid (yellow braid) and antibodies (twisted pipe cleaners; Figure 3b). Red rigatoni pasta represented the blood vessel walls, which these molecules are usually constrained within. A vertical mesh was upholstered within an empty photo frame to represent the glomerular membrane. In a healthy individual, this membrane is intact. However, membrane damage occurs in SLE when antibody complex deposition triggers inflammation in blood vessels, which was demonstrated by creating holes of various sizes in the mesh (Figure 3c). This damage allows erythrocytes and proteins to pass through the membrane, which is then excreted from the body in urine.
IMMUNOLOGICAL PROCESSES THAT UNDERPIN SLE
By interacting with these artworks and models, participants now had a clear understanding of SLE manifestations at a gross level and had started to appreciate how these were connected to dysregulated immune processes. It was now time to delve even deeper into the immunological programs that underly disease. To address these topics, we designed a tactile poster using a combination of sensory tools (Figure 4), including push buttons with a brief explanation of each process. The immunopathology underlying SLE is complex, with many components of the immune system driving autoimmunity. Therefore, we aimed to communicate how they act in concert to propel inflammation.
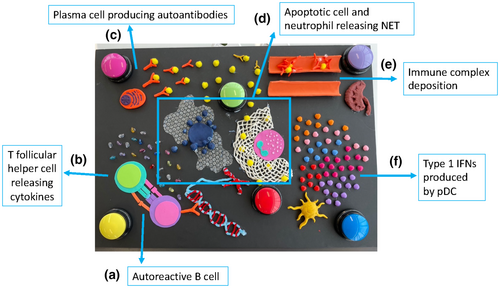
One of the key immunological programs that drives disease manifestations in SLE is that of B-cell activation and differentiation into autoantibody-producing plasma cells. We introduced a B cell interacting with a T follicular helper cell using foam paper and highlighted key aspects of that interaction. For instance, the B-cell receptor binding to nuclear autoantigens and cytokines (represented as beads) such as interleukin-21 released by the T follicular helper cells, which promotes B-cell proliferation and the generation of high-affinity autoantibodies3 (Figure 4a, b).
Play dough was used to model a plasma cell and the abundance of endoplasmic reticulum contained within, producing a high concentration of autoantibodies bound to autoantigen (also modeled using play dough; Figure 4c). Increased rates of apoptosis in SLE, coupled with defective clearance of apoptotic debris, results in further release of autoantigens which are recognized by antibodies.3 An apoptotic cell was modeled using play dough and bubble wrap to create ‘blebbing’ (Figure 4d). Nearby, a neutrophil releasing a neutrophil extracellular trap (NET) was modeled by crocheting a net (Figure 4d). Indeed, in SLE increased formation of NETs is another source of autoantigen exposure.3 As participants had learned from the kidney station, immune complex deposition in blood vessels is a key process underpinning renal pathology in SLE, and they could trace their hands over our poster to explore how the cascade of inflammation ultimately resulted in immune complex deposition in a blood vessel (Figure 4e). This was compared with a smooth, healthy blood vessel that they could run their hands through facing no obstructions.
Type 1 interferons (IFNs) are another key component to pathogenesis in SLE. This is reflected by the increased expression of interferon-stimulated genes, forming a type 1 IFN signature that is observed in 60–80% of patients.7 Type 1 IFNs are produced by plasmacytoid dendritic cells in high concentrations and act on many facets of the immune system, such as B cells to promote the production of autoantibodies.7 We modeled a plasmacytoid dendritic cell out of play dough and demonstrated the increased production of type 1 IFN by a spread of beads that participants could run their hands over (Figure 4f). Importantly, the type 1 IFN beads were a different shape to the other cytokine beads placed on the other side of the poster. The purpose of this was to convey that they are a different kind of cytokine but that fundamentally, cytokines are signaling molecules.
MULTI-MODAL SCIENTIFIC COMMUNICATION
Participants keenly engaged with our displays and models with a particular interest in the complex interplay of several arms of the immune system driving pathology in SLE. A common question was “How do you treat SLE when there are so many things going on”? This stimulated some interesting conversation, where we talked about this being an important challenge in SLE treatment and research. Indeed, SLE has suffered from an astonishing lack of new treatments, with glucocorticoids (steroids) remaining the mainstay treatment because they broadly dampen inflammation, despite a long list of dose-dependent adverse side effects they bring, such as obesity and osteoporosis.8 Newer treatments have been few and far between and often unsuccessful because they are targeting only one facet of inflammation.
Overall, designing and presenting the lupus breakout room opened our eyes to the many ways that we can communicate science to include all members of our community to engage everybody in scientific discussion and invention. While we included many forms of accessibility, such as recordings on push buttons, we found that participants preferred to sit at the poster and be guided through the display conversationally. We were very happy to do this and share our passion for science. In addition, we included braille labels on some of our displays, but found that this had low uptake which was likely a reflection of low braille literacy. While braille usage in Australia is unclear, only ~10% of children with low vision in the United States learn to read braille.9 Instead, we found that tactile interaction with our models was extremely useful in explaining scientific concepts, such as neutrophil NETs and immune complex deposition in blood vessels.
One barrier that has been reported by people with low vision in mainstream scientific or mathematic settings, even when accessible teaching strategies are included, is negative attitudes held by teachers toward teaching these concepts to students with low vision.10, 11 This, in turn, contributes toward students losing confidence in their ability to learn the material.10, 11 We sincerely hope that the enthusiasm and detailed preparation of the lupus sensory science team and the wider Monash Sensory Science Exhibit on Autoimmunity made participants feel included and valued in the scientific community. Importantly, we must continue to execute these learnings to improve scientific accessibility and inclusion in meaningful ways for the low-vision community.
ACKNOWLEDGMENTS
The Lupus Sensory Science Team consisted of Kathleen Elford (Lupus sensory science team leader) and Anita Cummins, who coordinate the clinical trials in the Rheumatology Department at Monash Health; Celine Shi (Research Assistant for the Australian Lupus Registry and Biobank); Dr Raychel Barallon (Rheumatologist at Monash Health); Dr Taylah Bennett (Immunologist/Postdoctoral Researcher in the Rheumatology Research Lab); Darcey Kennedy, Tammi Tang and Wing Ki Liu (2023 Honors students, Rheumatology Research Lab). The whole team contributed to designing and creating the models. K Elford, A Cummins, C Shi, TJ Bennett and D Kennedy presented the tactile models in person at the exhibit. Dr Laura Ciacchi and Dr Lisa Ciacchi created the transverse cross section of a blood vessel and the antigen–antibody complex on the kidney poster. Dr Erica Tandori provided guidance and assistance to develop the models. We thank everyone involved in organizing the Sensory Science Exhibit, and for inviting our team to take part. Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
The author declares no conflict of interest.