Liposomal delivery of self-peptide and calcitriol as tolerogenic immunotherapy in rheumatoid arthritis: an exploration using sensory science
Abstract
Immunology research holds significant potential for enhanced inclusivity at the beginning of the science literacy journey, but persistent challenges stem from limited awareness that improvement is needed in this field. At the 2023 Monash Sensory Science Exhibition, we had the opportunity to present several tactile posters, using simple materials, for visually impaired participants to showcase our research on the pathogenesis of rheumatoid arthritis as a result of immune tolerance breakdown and liposome-based tolerogenic immunotherapy. The posters stimulated lively discussions about autoimmune arthritic diseases and our research. With consideration of the diversity of the participants, the efforts of scientists in promoting science literacy for the community can promote a more inclusive environment and engage and inspire a broader audience.
“Strength lies in differences, not in similarities” — Stephen R Covey
MAKING SCIENCE ACCESSIBLE
The potential for academic and scientific activities to further enhance inclusivity and embrace individuals with diverse needs, as well as those at the beginning of their science literacy journey, regardless of age, is substantial. However, in academia, challenges in achieving this inclusivity persist, in part, as a result of limited awareness.
To bring awareness to the needs of individuals with diverse requirements, the Monash Sensory Science Exhibition was designed for all ages and levels of science literacy, particularly for visual and/or hearing-impaired audiences. The aim was to employ a sensory science approach, where tactile models are used, to better convey the underlying principles of autoimmunity.
As immunology and rheumatology researchers, we had the opportunity to participate in this year's exhibition to demonstrate a common autoimmune disorder, rheumatoid arthritis (RA), using a mixture of simple tactile posters and models. These tactile representations were crafted using a range of easily recognizable materials. Each offered distinct textures to ensure that the information presented using these familiar materials, and the immersive experience, would leave a lasting impression on the participants. We displayed the clinical features and pathogenesis of RA, and the mechanisms of action of liposomes encapsulating self-peptides and calcitriol as a potential tolerizing immunotherapy for RA.1
A TOUCH OF PATHOGENESIS AND SYMPTOMS OF RA
RA is a chronic, systemic inflammatory autoimmune disease that involves articular and extra-articular regions in the body. It affects an estimated 20 million patients worldwide, and is common in females.1, 2 Typically, the immune system defends the individual against harmful invaders such as microbes and tumors. However, in RA, the body mistakenly attacks healthy tissues, particularly in the joints. This immune attack leads to inflammation, resulting in painful, stiff and swollen joints, usually affecting both sides of the body. However, RA does not stop at the joints and can also affect other regions of the body such as the lungs, skin, intestines and heart due to systemic inflammation or drug adverse reactions. Different types of immune cells are involved in this disease process, including antigen-presenting dendritic cells (DCs), B cells, autoreactive and regulatory T cells. Over time, without proper management, RA can cause joint damage and deformities. Daily activities are difficult for patients with RA with uncontrolled disease, impacting work and leisure participation and quality of life.
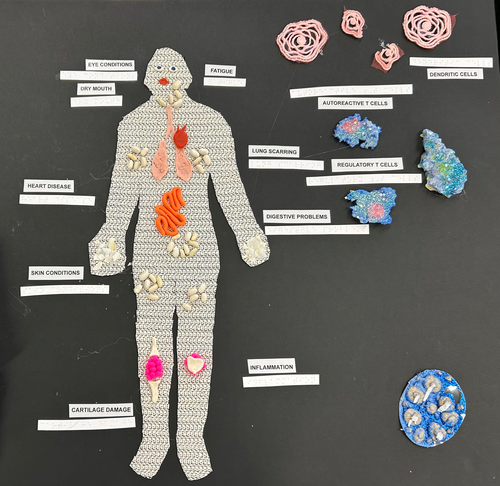
To showcase the systemic impact of RA, we created the first tactile poster to demonstrate the various clinical symptoms (Figure 1). Using a textured cloth to outline the shape of an entire human figure on the left-hand side of the board, various materials such as seeds, plasticine and small balls of yarn were used to represent affected organs such as the eyes, heart, lungs, intestinal tract and joints (Figure 1). Systemic issues associated with RA, such as fatigue and inflammation, were also indicated. On the right-hand side of the board, cells involved in the pathogenesis of RA, such as DCs, autoreactive T cells and regulatory T cells, were represented using textured cloths and cut-outs from cardboard illuminated with colored glitter. To aid interpretation for the visually impaired, braille translations of each component were provided.
As RA is an autoinflammatory disease of the joints, we used sensory methods to showcase the cardinal signs of inflammation (Figure 2). To provide demonstrable differences between a diseased and healthy knee joint in terms of tumor (swelling) and functio laesa (loss of function), salt granules were affixed to the top of the synovial tissue models to represent the deterioration and deformity in the knee joint. Participants were also guided to the model representing a healthy knee joint, where they could feel the smooth layers of synovial tissues on both sides of the joint, replicating the natural and normal state of a knee joint. The different joint components in the diseased model were also intentionally left unattached to facilitate the insertion of a heating pad between the affected joints, simulating the calor (warmth) associated with articular inflammation in RA. Although not explicitly replicated in the models, participants were verbally informed about dolor (pain) and rubor (redness) that can occur in inflamed joints.

FEELING THE ANTIGEN-SPECIFIC TOLERIZING IMMUNOTHERAPY FOR RA
The current treatment for RA encompasses lifestyle modifications, physical therapy, inflammation control and pain alleviation. I was found that over a period of 12 months, fewer than 80% of patients who started in remission remained in remission when their RA medications were stopped.3 Although this underscores the efficacy of current drugs in managing symptoms, it is clear that current therapeutic modalities do not reverse the underlying immune mechanisms. Furthermore, drug-induced remission does not signify that the underlying inflammation is cured, RA usually relapses when current drugs are stopped. Consequently, there is a need for a treatment with more lasting effects. At present, there remains no cure for RA.
Antigen-specific immunological tolerizing strategies aim to directly deal with the underlying specific causes of autoimmune diseases. In RA, antigen presentation by DCs and other antigen-presenting cells to pathogenic T cells initiates the inflammatory cascade; however, it was discovered in our laboratory that if the nuclear factor-kappa B (NF-κB) signaling pathway is attenuated in DCs, self-antigen-specific regulatory T cells can be induced, with disease-suppressive capacity.4 Our laboratory has developed and trialed a nanoparticulate liposome formulation that encapsulates an arthritogenic self-peptide collagen II (259–273), which is presented by HLA-DRB1*04:01 or *01:01 RA-associated molecules, in addition to the NF-κB inhibitor 1,25-dihydroxycholecalciferol (calcitriol) within the phospholipid bilayer.5 The primary targets of these liposomes are the DCs residing in lymph nodes draining the skin (dLNs), which actively uptake the liposomes after subcutaneous administration. Subsequently, the induced CD4+ antigen-specific regulatory cells migrate to the joint and joint dLN. Through cognate interactions these T cells regulate DCs within the joint dLN, resulting in the bystander regulation of autoreactive CD4 cells of other specificities, such as citrullinated-vimentin and anti-citrullinated protein antibodies, autoreactive B cells and CD8 cytotoxic T lymphocytes. The goal of this process is to induce long-lasting immunoregulation of autoimmunity, with improved outcomes and fewer adverse events than are associated with conventional therapeutics, by specifically targeting and regulating the immune response in the joints.
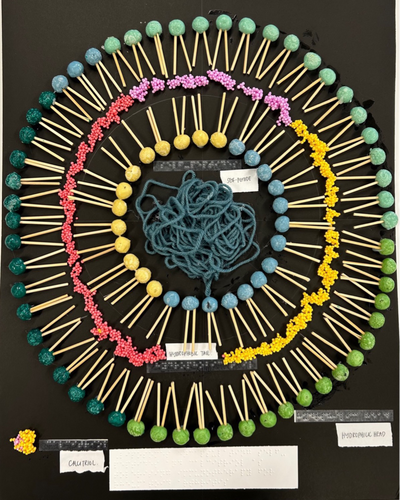
We showcased the nanoparticulate liposome formulation developed in our laboratory using a combination of playdough, bamboo skewers, slime balls and loose yarn (Figure 3). To mimic a phospholipid bilayer, we inserted skewer sticks, symbolizing hydrophobic tails, into playdough balls representing hydrophilic heads. This bilayer structure was repeated to emulate the structure of a liposome. Within this liposome model, we positioned slime balls to represent the hydrophobic calcitriol in our formulation between the phospholipid layers, while loose yarn, representing the hydrophilic self-peptide in our formulation, was placed in the aqueous liposome core. As in the first tactile poster, the braille translation of each component was placed adjacently.
With our guidance, participants had the opportunity to touch and feel the liposome model in different textures designed to represent the components in our nanoparticulate liposome formulation (Figure 4). We also explained how the liposome formulation induces tolerance in DCs and aims to achieve long-term control of RA.

AUDIENCE FEEDBACK, QUESTIONS AND INSIGHTS
Apart from providing feedback that the demonstration was informative and engaging, participants mentioned that the exhibition presented them with a never-before opportunity to freely discuss their thoughts and beliefs in relation to RA. As a result, we answered many questions, particularly on nutrition, such as on the consumption of naturopathic supplements (e.g. turmeric capsules) and the reasons for the female preponderance to RA. Throughout these exchanges, we shared lifestyle interventions to prevent inflammation with the audience, which included exercising as tolerated, maintaining good oral health and adopting a Mediterranean diet. Discussion about the higher risk of RA in females led to a lively participant discussion about the impact of different gender identities and treatment with female hormones on the risk of RA. Overall, this station was well-received among the participants.
CONCLUDING REMARKS
Over the course of the exhibition, we had the privilege to engage with the participants from the community to showcase our research discoveries. Through tactile posters, vivid descriptions and interactive displays, we have strived to make the complexities of the research accessible to all. As we dismantle the silos between science and the public, we hope our efforts inspire a more inclusive and informed world, where everyone, regardless of their abilities and exposure to formal science teaching, can engage with and benefit from scientific advancements.
ACKNOWLEDGMENTS
This article arises from the 2023 Monash Sensory Science Exhibition. The authors thank Professor Jamie Rossjohn, Drs Lisa and Laura Ciacchi, Jennifer Huynh and everyone involved in the Monash Sensory Science Exhibition for the opportunity to present and share our work, Dr Erica Tandori and her team who assisted the authors with the designing and construction of the tactile models and Thais Aragao-Horoiwa who provided insights into designing the liposome model. We also acknowledge Gerard Hynes from Hynesite Photography for providing the in-action photo at the exhibition used in Figure 4. Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
AUTHOR CONTRIBUTIONS
Jia Yi Hee: Conceptualization; writing – original draft. Benjamin Cai: Conceptualization; writing – original draft. Ranjeny Thomas: Conceptualization; writing – review and editing.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.