Bridging science and accessibility: a tactile journey from gluten through to coeliac disease
Abstract
As part of the Monash Sensory Science Exhibition, our team guided participants through a multisensory journey unraveling coeliac disease development and pathology. Through tactile and sensory exhibits, we showed how benign dietary gluten can be transformed into a harmful entity for the 1 in 70 Australians with this illness. In contrast to the common misconception of coeliac disease as a food allergy, our exhibits revealed its closer association with autoimmune diseases such as type 1 diabetes, involving genetic susceptibility linked to specific human leukocyte antigens, crucial antigen-specific T- and B-cell responses and autoantibody production. Tactile models underscored the severe consequences of the proinflammatory immune response to gluten on patient health and quality of life. This educational event affirmed to us the value and importance of fostering inclusivity in science education.
“Science knows no country because knowledge belongs to humanity, and is the torch which illuminates the world.” - Louis Pasteur
As part of the Monash Sensory Science Exhibition, we had the privilege of taking attendees on a multisensory journey through the development and pathology of coeliac disease. Our diverse audience comprised people from the community who live with sensory impairment, with most attendees experiencing impaired vision to varying degrees. They ranged from children right through to senior citizens with diverse needs and scientific literacy brought together by a mutual interest in immunology. Our multidisciplinary team, made up of immunologists, structural biologists and coeliac clinicians, used tactile and other sensory tools to explain how gluten, a common and usually innocent dietary protein, can become toxic to the 1 in 70 Australians with this food-triggered illness. The exhibits demonstrated how consuming gluten in coeliac disease can lead to the activation of a proinflammatory T-cell response and, ultimately, the consequences of this on the small intestine. While many people assume coeliac disease is a food allergy or sensitivity, the exhibits demonstrated how coeliac disease pathology shares more in common with classical autoimmune diseases such as type 1 diabetes.1, 2 This includes features such as genetic susceptibility linked to specific human leukocyte antigens (HLAs), a central role for antigen-specific T- and B-cell responses, autoantibody production and environmental factors essential for triggering disease development. The use of tactile models allowed us to highlight the serious negative consequences of the damaging immune response on patient health and quality of life.
ACTIVATING THE GLUTEN-SPECIFIC T-CELL RESPONSE
Our initial series of exhibits showed attendees how gluten can trigger the inflammatory response typical of coeliac disease. Visitors were invited to touch and feel the exhibits. Several examples of gluten-containing cereals, such as wheat, rye and barley, and gluten-free cereals, such as rice, corn and quinoa, were available for participants to feel. The word “gluten” is derived from the Latin word for glue, and chemically it consists of two sets of proteins, gliadins and glutenins, that together form a strong viscoelastic network that confers many of the desirable food properties of gluten, such as allowing dough to be kneaded and form fluffy bread. The gliadins also contain most of the peptides that trigger T cells in coeliac disease. Because of their high glutamine and proline content, these peptides resist gastrointestinal degradation.3 Next, two tactile displays outlined the events from the time gluten is ingested through to it activating a gluten-specific T-cell response (Figure 1). The first showed how gluten (the polypeptide structure represented by a ball of wool) is modified by an enzyme, tissue transglutaminase, which introduces site-selective peptide modifications, converting specific glutamine residues to glutamate (shown by a different-sized bead, which could be felt by attendees; Figure 1a). This post-translational modification is crucial, as it allows the gluten to more efficiently bind to the antigen-presenting cells expressing the disease-associated HLA molecules, HLA-DQ2.5, 2.2 and/or DQ8. In the late 1980s, researchers reported on the exceptionally strong association of these specific HLA types with coeliac disease, with nearly all patients with coeliac disease expressing at least one of these HLA genotypes.4 This tells us that a particular type of T cell called a “CD4” T cell is crucial in coeliac disease, and that they are activated by a selection of peptides able to bind to the disease-associated HLA-DQ2.5, 2.2 and/or DQ8. Considerable efforts have gone into defining the specific gluten peptides that trigger each HLA “variety” of coeliac disease.5-7 So, what happens when these gluten-specific T cells become activated?
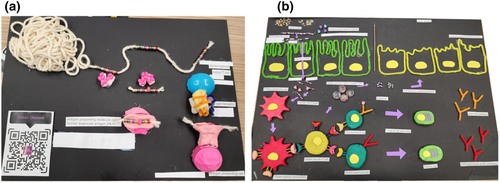
Activation of gluten-specific T cells spells bad news for the person with coeliac disease, as shown in the next display (Figure 1b). The nutrient uptake of the gluten-containing food (shown by grains) occurs across the small intestinal villi (represented by green finger-like projections; Figure 1b). Specific gluten peptides undergo post-translational modification and can now be presented on the surface of antigen-presenting cells (shown by red plasticine) expressing HLA-DQ2.5, 2.2 and/or DQ8. This in turn can now effectively activate the gluten-specific T cells (yellow plasticine). This complex of HLA on the antigen-presenting cell, gluten peptide and T-cell receptor was shown in fine detail by plasticine and beads. Pivotal work contributing to the field's understanding of these interactions has been made by the Monash structural biology team and collaborators.6, 8-12
Activated T cells secrete a range of proinflammatory cytokines such as interferon-gamma, interleukin-2 and interleukin-21.5, 13 The net effect of these chemical signals is to orchestrate a series of inflammatory cascades that ultimately damage the small intestinal lining (mucosa, shown by yellow strings; Figure 1b). This involves activating components of the primitive innate immune system which contribute to mucosal damage. Furthermore, antibody-producing B cells (green plasticine) can present gluten complexed to transglutaminase to T cells. In turn, they receive help from T cells to produce antibodies to transglutaminase and gluten peptides (Y-shaped pipe cleaners; Figure 1b). In the clinic, these antibody tests are used to screen for coeliac disease as they reflect the T-cell activation to gluten that only occurs in people with the illness. When people with coeliac disease commence a gluten-free diet, these antibody tests eventually return to normal, reflecting the reduction in T-cell activation by gluten. Given the pivotal role of gluten-specific T cells in the pathogenesis of coeliac disease, along with their absence in individuals without the illness, innovative and highly sensitive blood tests capable of identifying the presence of gluten-specific T cells offer an appealing potential diagnostic strategy in the future.14, 15
CONSEQUENCES FOR THE PATIENT WITH COELIAC DISEASE
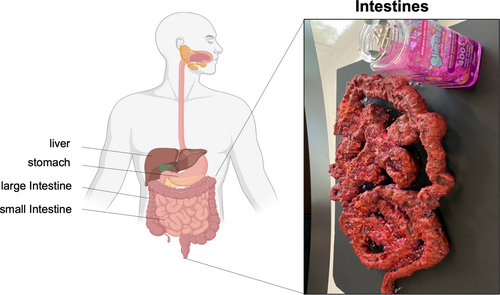
Small intestinal mucosal damage driven by the T-cell–mediated inflammatory cascade is a defining feature of coeliac disease. The normal small intestine is a long, coiled structure approximately 6–7 m long. Its surface is made up of tiny structures called villi that dramatically increase the surface area of the small intestine to absorb nutrients, which is its key role. Contemporary estimates of the surface area indicate the size of half a badminton court. Along the inner surface is a single layer of sticky mucous that provides protection for the gastrointestinal tract and a first line of defense against bacteria. An exhibit revealing this remarkable feature of the small intestine was created using expanding spray foams and paint, with slimy water beads used to mimic the slippering mucosal surface (Figure 2). The model not only looked like a small intestine, but felt like one too, enabling participants to trace its convoluted path with their fingers and understand the importance of the mucous layer and how nutrients are absorbed across the intestine.
A direct result of the immune activation triggered by gluten is an influx of T cells into the intestinal lining and structural changes characterized by flattening (atrophy) of the villi. So dramatic can this change be in coeliac disease that the surface area can fall from half a badminton court to that of an A4 sheet of paper. To illustrate this dramatic structural deterioration, the next exhibit featured a carpet rolled into the shape of the small intestine (Figure 3). Two versions were made: the normal healthy small intestine shown by lush, shagpile carpet representing the long, healthy finger-like villi and active coeliac disease shown by flat, featureless carpet, representing villous atrophy. The tactile experience allowed visitors to run their fingers and hands along each of the carpets to get a powerful demonstration of the differences between healthy bowel and active coeliac disease. It was a stark reminder of how destructive the immune system can be in an autoimmune response, and that coeliac disease is far from a “lifestyle choice” or simple food sensitivity. The good news, participants were reminded, was that a strict gluten-free diet can resolve the intestinal damage by removing the antigenic trigger for the gluten-specific T cell. Of course, the gluten-free diet needs to be lifelong, as gluten exposure at any stage will reactivate these T cells.
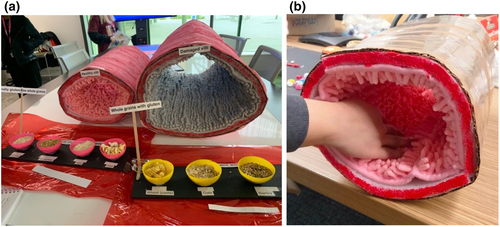
While the small intestine is a key target of the inflammatory response, it is now appreciated that this effect can be systemic, and adversely affect many bodily systems, such as reducing bone density and increasing the risk of infections and some types of cancer such as lymphoma.16 This powerful tactile exhibit not only engaged the senses but also conveyed the important clinical message about the importance of early diagnosis and effective management of coeliac disease.
A LEARNING EXPERIENCE FOR ALL
Throughout the exhibition, our team consisting of scientists with expertise in coeliac disease and a coeliac nurse coordinator and educator were on hand to take participants through the exhibits, answer questions and share their knowledge about coeliac disease. This merging of basic science and clinical expertise allowed us to illuminate the foundational principles of the science while also offering participants a glimpse into the tangible impact of coeliac disease on patients’ lives. The tactile exhibits were well received, with the gut lumen model made up of contrasting carpet textures the most immersive and the most popular. Combining these tactile models with a range of media formats including written text, braille and QR (quick response) code linked to pre-recorded audio descriptions ensured attendees of all sensory capabilities were catered for. While the multimedia exhibits were helpful tools, it was our team members who played the most crucial role by offering tailored explanations to each attendee, ensuring the scientific content was clear and accurately communicated. While we endeavored to ensure that attendees found this exhibition both engaging and informative, for our team, this event proved to be an empowering and enlightening experience. It strongly reaffirmed the significance of fostering inclusivity in science education and ensuring that the wonders of immunology are accessible to everyone.
Coeliac disease, although common, remains largely undiagnosed. Its treatment with a strict and lifelong gluten-free diet is onerous and often fails to resolve patient symptoms and intestinal damage. Our overarching research goal is to address these unmet needs by understanding the molecular events underpinning coeliac disease pathogenesis. This understanding, in turn, will steer the development of innovative diagnostic and treatment strategies aimed at enhancing patient health and quality of life. Raising awareness of this under-researched illness is important, and we extend our gratitude to the Monash Sensory Science program that provided us with this very special opportunity to cast a spotlight on coeliac disease.
ACKNOWLEDGMENTS
We greatly appreciate the efforts of Dr Erica Tandori and Dr Stuart Favilla who assisted with the design, creation and presentation of the tactile models. We are grateful to the Monash Sensory Science Exhibition team with special thanks to curator Dr Erica Tandori for organizing this event.
AUTHOR CONTRIBUTIONS
Lee M Henneken: Methodology; writing – review and editing. Tiing Jen Loh: Conceptualization; methodology; project administration; writing – review and editing. Laura Ciacchi: Conceptualization; methodology; project administration; writing – review and editing. Lisa Ciacchi: Conceptualization; methodology; project administration; writing – review and editing. Jia-Jia Lim: Methodology; writing – review and editing. Hugh H Reid: Supervision; writing – review and editing. Jason A Tye-Din: Supervision; writing – original draft.
CONFLICTS OF INTEREST
JT-D has privately or via his institute been a consultant or advisory board member for Anatara, Anokion, Codexis, Chugai, EVOQ Therapeutics, Equillium, IM Therapeutics, Janssen, Kallyope, Mozart Therapeutics, Teva, Topas, Tillotts and Vaccitech; has received honoraria from Takeda and research funding from Chugai, Codexis, ImmusanT Inc., Novoviah and Tillotts Pharmaceuticals. He is an inventor on patents relating to the use of gluten peptides in coeliac disease diagnosis and treatment. All other authors declare no conflict of interest.