The Role of Serum Testosterone in the Pathogenesis and Treatment of Prostate Cancer: A Review Based on the Clinical Evidence
Funding: This study was supported by Grant-in-Aid for Scientific Research (C) (20K09555 to Shinichi Sakamoto), Grants-in-Aid for Scientific Research (24K02575 to Shinichi Sakamoto), the Japan China Sasakawa Medical Fellowship (to Xue Zhao), and JST SPRING (Grant Number JPMJSP2109 to Xue Zhao).
ABSTRACT
Serum testosterone plays a pivotal role in the pathogenesis and treatment of prostate cancer, influencing tumor growth and progression. This review synthesizes current clinical evidence on the dual role of serum testosterone as both a biomarker of carcinogenesis and a target for therapeutic intervention. We discuss the mechanisms linking androgen signaling to prostate cancer development, emphasizing the role of testosterone in androgen receptor activation and cellular proliferation. Furthermore, we explore the clinical implications of testosterone suppression strategies, including androgen deprivation therapy (ADT) and bipolar androgen therapy (BAT), highlighting their impact on patient outcomes. Emerging evidence on the prognostic significance of nadir testosterone levels, testosterone rebound, and treatment resistance is also analyzed. Finally, we address the challenges and opportunities in testosterone monitoring, aiming to enhance precision medicine approaches for managing prostate cancer. This review underscores the importance of personalized testosterone-based strategies to optimize therapeutic outcomes and improve patient quality of life.
Abbreviations
-
- ADT
-
- androgen deprivation therapy
-
- AR
-
- androgen receptor
-
- BAT
-
- bipolar androgen therapy
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- CRPC
-
- castration-resistant prostate cancer
-
- CSS
-
- cancer-specific survival
-
- DHT
-
- dihydrotestosterone
-
- GS
-
- Gleason scores
-
- HR
-
- hazard ratio
-
- HSPC
-
- hormone-sensitive prostate cancer
-
- IM
-
- intramuscular
-
- LH-RH
-
- luteinizing hormone-releasing hormone
-
- mCRPC
-
- metastatic castration-resistant prostate cancer
-
- mHSPC
-
- metastatic hormone-sensitive prostate cancer
-
- OR
-
- odds ratio
-
- ORC
-
- replication origin complex
-
- ORS
-
- origin replication sites
-
- OS
-
- overall survival
-
- PARP
-
- poly (ADP-ribose) polymerase
-
- PCa
-
- prostate cancer
-
- PD-L1
-
- programmed death-ligand 1
-
- PFS
-
- progression-free survival
-
- PSA
-
- prostate-specific antigen
-
- PSMA
-
- prostate-specific membrane antigen
-
- pT
-
- pathological tumor stage
-
- QoL
-
- quality of life
-
- RP
-
- radical prostatectomy
-
- SHBG
-
- sex hormone-binding globulin
-
- TCR
-
- time to castration resistance
-
- TD
-
- testosterone deficiency
-
- TRT
-
- testosterone replacement therapy
-
- TST
-
- testosterone
1 Background
Testosterone (TST) is the primary male sex hormone and plays a crucial role in male health. Primarily secreted by the testes, serum TST levels rise sharply during puberty, promoting the development of male reproductive organs and secondary sexual characteristics, including muscle and bone growth, voice deepening, and hair distribution [1, 2]. Additionally, TST is essential for maintaining sexual function, mood, energy levels, cognitive function, and cardiovascular health. Low serum TST levels may lead to issues, such as decreased libido, osteoporosis, obesity, and depression, and are also associated with an increased risk of cardiovascular disease [3, 4]. Therefore, maintaining normal TST levels has a significant impact on men's overall health, quality of life (QoL), and longevity.
Many studies aimed to clarify serum TST's role in prostate cancer (PCa). Androgens (TST) are widely recognized to influence proliferation, apoptosis, angiogenesis, metastasis, and differentiation of PCa in complex ways. Prostate growth is androgen-dependent; without androgens, prostate development is hindered, and androgen deprivation leads to prostate atrophy. Historically, the link between TST and PCa centered on the concept of TST as “fuel” for cancer cells. However, this traditional view has been challenged by some negative findings. Multiple TST trials lasting up to 36 months have failed to reveal any significant increase in PCa incidence, and at least 16 longitudinal studies involving hundreds of thousands of men have consistently found no long-term risk of PCa development [5, 6].
Currently, the serum TST shows a complex and contradictory association with PCa that warrants further investigation. This review aims to reassess and clarify the relationship between serum TST and PCa based on recent clinical evidence, further exploring the role of serum TST levels in the progression, diagnosis, treatment, and prognosis of PCa patients.
2 Serum TST and PCa Risk
Prostatic growth and development are largely dependent on androgens. The link between serum TST levels and PCa was first suggested by Huggins [7], but epidemiological studies since then have shown inconsistent associations between circulating TST levels and PCa risk. Although some studies report a slightly increased risk of PCa with higher TST levels [8-12], others suggest a mildly decreased risk or no significant association [13-15]. Gann et al. [10] identified a higher risk of PCa in men with the highest quartile of serum TST levels. Pierorazio et al. [16] found an association between calculated free TST and the risk of high-grade PCa. Yano et al. [17] observed an increased risk of PCa in men with increased TST levels and prostate-specific antigen (PSA) < 10 μg/L. Muller et al. [15] did not find any association between TST or dihydrotestosterone (DHT) levels and PCa incidence or Gleason grade in the placebo arm of the REDUCE trial. Roddam et al. [6] conducted a meta-analysis that did not find a significant association between TST levels and the risk of PCa.
No significant correlation has been found between prostate volume, PSA levels, endogenous TST, or PCa incidence [6, 15, 18, 19], although some clinical researchers have proposed that the ratio of TST levels to prostate volume may predict tumor progression in low-risk PCa [20]. Population studies also show that natural variations in TST levels do not uniformly correlate with PCa [6]. Methodological limitations, including low statistical power, small sample sizes, minimal differences in hormone levels between cases and controls, and laboratory variability, have hindered definitive conclusions [21]. Moreover, confounding factors, such as body size, physical activity, diabetes, and benign prostatic hyperplasia are not consistently controlled for, adding further complexity.
Recent studies, however, have highlighted a potential link between low free-TST levels and PCa. But in three studies that directly measured free-TST, no significant association was observed [9, 13, 22]. A meta-analysis by Eaton et al. [23] that reviewed eight prospective studies (817 cases, 2107 controls) found no overall association between TST levels and PCa risk. Yet some evidence suggests that bioavailable TST, when adjusted for sex hormone-binding globulin (SHBG), may correlate with PCa risk [10], as SHBG itself has shown associations with PCa and is inversely correlated with obesity [24]. But the lack of a protective effect of obesity on PCa suggests that circulating TST is unlikely to be a major risk factor. Associations between sex steroid hormones and PCa appear stronger in leaner men than in overweight or obese men, likely due to disrupted insulin metabolism and altered sex steroid balance in the latter, which obscures androgen and estrogen associations [25, 26]. Such findings complicate the androgen-PCa relationship, as SHBG and TST levels interact with multiple metabolic factors. Geographic and racial differences in androgen levels and PCa risk may further complicate these associations, possibly due to variations in the intraprostatic conversion of TST to DHT linked to 5α-reductase enzymatic activity [27, 28]. Some studies observed a modest decrease in PCa risk with higher total TST levels, suggesting that low TST might serve as a marker for more aggressive PCa [5, 29, 30]. Notably, research by Morgentaler et al. [31] indicates that hypogonadal men with lower TST levels have higher positive biopsy rates, with heightened risk particularly in men with TST < 250 ng/dL and a PSA level of 2.0–4.0 ng/mL. Men with TST levels < 250 ng/dL had a 21% cancer rate, compared to 12% for those with TST levels > 250 ng/dL. Cancer probability more than doubled for men in the lowest tertile compared to those in the highest tertile for both total and free TST. Particularly concerning was the combination of low TST and a PSA of 2.0–4.0 ng/mL, yielding a 30% cancer rate [31].
These observations imply that low TST does little to prevent PCa. Conversely, PCa is very common in men with TST deficiency, and low TST levels increase the risk of higher Gleason scores (GS) and poor outcomes in PCa [32-35].
Nonetheless, whether low TST contributes causatively to PCa or results from PCa progression remains unresolved. Some studies report an increase in TST levels following radical prostatectomy (RP), suggesting that PCa itself may suppress TST production [36, 37]. Further investigation is warranted to clarify the timing and mechanisms of TST suppression in PCa's natural history and to compare pre- and post-diagnostic serum TST levels to better understand the impact of PCa on androgen biosynthesis. Although experimental data indicate that androgens promote the development of PCa in experimental systems, there is no clear clinical evidence supporting the notion that elevated endogenous TST levels drive the progression of PCa in humans [38].
3 Testosterone Replacement Therapy (TRT) and PCa
Testosterone deficiency (TD) is associated with various health problems, such as sexual dysfunction, cardiovascular disease, and psychological problems [39]. TRT is the preferred treatment for TD and has been shown to alleviate or reverse these symptoms [40, 41]. However, its use in PCa patients raises ethical and medical concerns despite its potential benefits.
3.1 Androgen Saturation Model
The androgen saturation model explains the paradoxical relationship between TST and PCa. Studies suggest that the maximum (saturation) level of TST binding to androgen receptors (AR) occurs at relatively low concentrations [42, 43]. Once ARs are fully occupied, excess TST cannot further stimulate cell growth, limiting its impact on PCa progression. Prostate tissue is sensitive to low TST concentrations but shows minimal response to higher levels [44, 45]. Research indicates that young and elderly men with elevated serum TST do not experience increased PSA levels or prostate volume, supporting this model [46-48].
3.2 Safety and Benefits of TRT in PCa Patients
- No increased risk of PCa: TRT does not elevate the risk of developing PCa in healthy individuals [49-51]. A recent double-blind, placebo-controlled randomized trial evaluated the prostate safety of TRT in 5246 hypogonadal men aged 45–80 years with cardiovascular risk. Over a mean treatment duration of ~22 months, TRT showed no significant difference in the incidence of high-grade PCa, any PCa, or other adverse prostate events compared to placebo. Although PSA levels increased in the TRT group, changes in prostate symptoms (International Prostate Symptom Score) were comparable between groups. These findings suggest that TRT does not significantly elevate PCa risk in carefully selected men, providing valuable insights into its safety profile [52].
- No promotion of recurrence or progression: In patients treated with TRT following RP or radiotherapy, no biochemical or clinical recurrence of PCa has been observed [53-57].
- Lower recurrence rates: Men with PCa receiving TRT exhibit a lower rate of biochemical recurrence compared to control groups, suggesting that increased androgen levels may have a protective effect on PCa recurrence [58-60].
Elevated or normal TST levels may maintain prostate and early PCa cells in a well-differentiated state. In contrast, declining TST levels due to aging or disease progression may lead to dedifferentiation and greater malignancy in PCa cells [61]. Additionally, men with PCa and TD are at higher risk for aggressive disease [62], though low TST levels do not independently predict bone metastasis [63].
These findings highlight the complex relationship between serum TST and PCa, offering a new perspective on treatment strategies, such as bipolar androgen therapy (BAT). Maintaining high physiological TST levels may have a protective role against PCa. Moreover, evidence indicates that TST supplementation does not promote the progression or recurrence of PCa, providing a promising avenue for future research.
4 Whether Low Serum TST Promotes PCa and Guides PCa Diagnosis?
Multiple studies indicate that lower serum total TST and free TST levels are associated with more aggressive PCa and poorer prognosis [32-35]. These findings suggest that the relationship between TST and PCa is not linear, and that a threshold may exist for the onset and progression of cancer at different stages.
Several studies have linked low TST levels with higher GS in PCa [18, 44], though findings are inconsistent. Zhang et al. [36] found that patients with high-grade tumors had lower total TST levels than those with moderate-grade tumors or without PCa. Similarly, Schatzl et al. [64] reported a higher mean GS in patients with partial androgen deficiency (TST < 300 ng/dL) compared to those with normal TST levels, suggesting lower TST levels correlate with higher GS in PCa. Hoffman et al. [65] demonstrated that patients with low TST were more likely to have a GS ≥ 8 and a higher percentage of positive cores on biopsy, proposing that low serum free TST may serve as a marker for more aggressive disease, whereas total TST levels did not show a similar association. Studies also link lower TST levels with advanced disease characteristics [66-69]. For instance, Teloken et al. [68] associated low preoperative TST with positive surgical margins in RP, whereas Massengill et al. [69] observed significantly lower preoperative TST levels in nonorgan-confined PCa cases. These findings, confirmed across ethnic backgrounds, suggest that low TST may predict extra-prostatic disease.
In contrast, other studies found no association between TST levels and GS in localized PCa [70]. Baseline serum TST levels do not predict prognosis in men with clinically localized high-risk PCa treated with RP or neoadjuvant chemohormone therapy and RP alone [71]. High pretreatment TST, however, has been associated with organ-confined disease [72]. Limitations in these studies include only in localized disease, retrospective designs, unmeasured variables like body mass index (BMI) and SHBG, and reliance on single hormone measurements, despite Platz et al.'s finding that single measures are reasonably representative over several years [18].
The connection between low TST and higher PCa risk remains speculative. Hypotheses include TST suppression due to advanced disease [73], a feedback mechanism involving PSA or DHT [36, 37], and altered hormonal environments promoting androgen-independent PCa cells [69, 74]. One theory posits that PCa cells produce inhibin, which suppresses TST via the hypothalamic–pituitary axis [75]. Supporting this, Miller et al. [37] reported elevated TST and gonadotropins after RP, unlike after benign procedures. In animal studies, inhibin inhibits pituitary gonadotropin production, with similar effects noted in human studies where increased inhibin correlates with higher PSA failure rates [76-78]. These results collectively indicate that factors from malignant prostate tissue might modulate the hypothalamic–pituitary axis, potentially through inhibin, as reflected in endocrine changes observed post-RP. This also explains the elevated TST after RP mentioned in the previous section.
At the molecular level, low testosterone levels may impose strong selective pressure on prostate cancer cells, compelling them to enhance AR signaling in order to sustain growth. In other words, a low testosterone milieu can drive tumor progression by promoting adaptive changes in the AR signaling pathway—such as gene amplification, splice variant formation, and point mutations. These adaptive changes can be summarized as follows:
4.1 AR Gene Amplification
In a low androgen environment, cancer cells often amplify the AR gene to capture trace levels of androgens, thereby increasing AR protein expression and sensitivity. Multiple studies have shown that approximately 20%–30% of prostate cancers that recur after androgen deprivation therapy (ADT) exhibit AR gene amplification [79, 80]. This phenomenon helps maintain signal transduction even at extremely low androgen concentrations, thus promoting tumor cell proliferation and invasion.
4.2 Pathogenic AR Mutations and Splice Variants
In addition to gene amplification, a low testosterone environment may promote the occurrence of pathogenic point mutations in the AR gene [81] or the generation of constitutively active splice variants (e.g., AR-V7) [82]. These alterations enable the AR to continuously activate downstream signaling pathways in the absence of, or in response to, only minimal levels of androgens, thereby contributing to therapy resistance and tumor progression. Although pathogenic AR variants are relatively rare in treatment-naïve prostate cancer, their frequency significantly increases in resistant tumors following prolonged ADT. Notably, splice variants, such as AR-V7, which lack the ligand-binding domain, are constitutively active and are commonly observed in CRPC and low testosterone conditions [83, 84].
4.3 Point Mutations in AR
Certain AR mutations (e.g., T878A, H875Y) expand the ligand-binding spectrum, permitting activation of the receptor by other steroid hormones (such as glucocorticoids), which further promotes castration resistance [85].
Although low serum TST is associated with more aggressive PCa and might serve as a marker for advanced disease, current evidence does not conclusively show that it promotes PCa. Nonetheless, low TST may have prognostic value and aid in diagnosing aggressive PCa, particularly when combined with other markers like PSA.
By combining the contents of the previous sections, we summarize Table 1. Table 1 provides an overview of how pretreatment serum TST levels relate to the GS or stage of PCa based on various studies. The studies included in this table present different methodologies and outcomes, reflecting the complexity and variability in the research on TST and PCa.
| TST cutoff or specific values (both sets of units ng/dL and nmol/L will be listed. The molecular weight of testosterone is about 288.42 g/mol) | Nationality of subjects | Results | GS or risk-level | Comments | References | |
|---|---|---|---|---|---|---|
| Highest quintile of TST | Finland, Norway and Sweden | No association found between high TST and PCa development and growth | Pooled prospective study | [5] | ||
| Highest quartile of TST | The United States | Men with the highest quartile of TST had a PCa, OR of 2.6 (1.34–5.02) | Prospective, nested case–control. Non-morning serum TST | [10] | ||
| Highest quartile of TST | The United States | No association found between total TST or other sex hormones and overall PCa | Stored blood samples, calibrated by SHBG | [18] | ||
| High free TST | The United States | High free TST associated with high-risk PCa, HR 1.61 (1.18–2.20) | High-risk level PCa | Prospective, population-based. Long-term serum storage, non-morning serum TST | [16] | |
| Increased TST and PSA < 10 μg/L | Japan | Increased TST associated with increased PCa risk HR 1.31 (1.03–1.65) in men with PSA < 10 μg/L | Retrospective cohort | [17] | ||
| Not specified | REDUCE trial (subjects from North America (e.g., the United States, Canada), Europe (e.g., the United Kingdom, Germany, Italy, France, and so forth), Asia (mainly Japan, but also some other Asian countries), and Latin America, Africa, and Oceania (e.g., Australia, New Zealand) | No association found between TST or DHT and PCa incidence or Gleason grade | No association with GS | Prospective cohort. Placebo arm of the REDUCE trial | [15] | |
| Not specified | Global data from the Endogenous Hormones and Prostate Cancer Collaborative Group | No association found between TST and PCa risk | Meta-analysis. Pooled data from 18 prospective studies | [6] | ||
| Low total or free TST levels | The United States | With an overall PCa incidence of 14%, no significant difference between cancer group and benign group | GS of 6 or 7 for all cancers | Retrospective cohort | [30] | |
| < 3.0 ng/mL (300 ng/dL) | < 10.41 nmol/L | Austria | Average GS were higher (7.4 vs. 6.2) and PSA levels were lower (25.3 vs. 31.9 ng/mL) | GS ≥ 8 (TST was 2.8 ± 2.7 ng/mL) | [64] | |
| < 240 ng/dL | < 8.3 nmol/L | Italy | Low TST associated with PCa OR 0.70 (0.55–0.89), especially in patients with Total TST < 8.3 nmol/L (240 ng/dL) | Retrospective cohort. Compared with BPH cohort | [35] | |
| Free TST < 1.5 ng/dL | < 0.052 nmol/L | The United States | PCa incidence increased (43% ves 22%), and with higher GS | GS ≥ 8 | Retrospective cohort | [65] |
| < 3.85 ng/mL (385 ng/dL) | < 13.36 nmol/L | Korea | The low TST group had a significantly higher incidence of PCa (38.9% vs. 29.5%) | No association with increased risk of high-grade PCa (GS ≥ 7) | Only in the Korean men | [32] |
| Free TST < 6.9 ng/dL | < 0.239 nmol/L | China | Low free TST was associated with higher risk of pT3 and higher Gleason score | GS ≥ 8 | Retrospective cohort. Total and free TST were measured | [33] |
| Low total TST levels | The United States | Associated with high pathological stage. Pretreatment TST was independent predictor of extrprostatic disease | pT3–pT4 | Retrospective cohort | [69, 70] | |
| Japan | ||||||
| < 250 ng/dL | < 8.68 nmol/L | The United States | Overall incidence of PCa was 21% | Total and free TST were measured | [31] | |
| > 250 ng/dL | > 8.68 nmol/L | Overall incidence of PCa was 12% | ||||
| < 270 ng/dL | < 9.37 nmol/L | Brazil | The positive rate of surgical margin was higher in patients with low total TST | No association with GS or stage. | Retrospective cohort. Localized PCa | [68] |
| < 385 ng/dL | < 13.36 nmol/L | The United States | Associated with high pathological stage | ≥ pT3 and GS ≥ 8 | Retrospective cohort | [72] |
- Abbreviations: CI, confidence interval; GS, Gleason score; HR, hazard ratio; OR, odds ratio; PCa, prostate cancer; PSA, prostate-specific antigen; pT, pathological tumor stage; TST, testosterone.
5 Serum TST and ADT
Dr. Charles Huggins [86] discovered that ADT offered significant palliative benefits for advanced PCa, establishing it as the preferred treatment. Lower TST levels achieved through ADT have previously been linked to longer treatment responses [87, 88]. Current guidelines recommend maintaining TST levels below 50 ng/dL during ADT, although this target is debated as more precise assays have emerged [89]. The classical castration level of serum TST (< 50 ng/dL) was established over 40 years ago with limited testing methods. Modern chemiluminescence assays show a mean postsurgical TST level of 15 ng/dL [90]. There is ongoing debate about redefining the castration threshold to < 20 ng/dL.
The clinical importance of achieving lower TST levels in ADT is well-documented. Morote was the first to report that TST breakthroughs at 20 and 50 ng/dL were linked to poorer outcomes, suggesting that avoiding such breakthroughs is a strong predictor of survival during androgen-independent progression [88]. Perachino observed that a TST of 40 ng/dL at 6 months was directly associated with an increased risk of death [91]. Bertaglia similarly concluded that a TST level below 30 ng/dL after 6 months was a positive prognostic factor, although outcomes for TST levels under 20 ng/dL could not be fully evaluated due to low mortality in this group [87]. The median nadir TST in their study was 39 ng/dL, significantly higher than our team's median of 13 ng/dL, possibly due to differences in ADT protocols and patient characteristics, such as ethnicity, cancer stage, and initial treatments.
Our team retrospectively analyzed data from Japanese patients who received ADT as their initial PCa treatment [92, 93]. We found that a nadir TST below 20 ng/dL and a reduction of over 480 ng/dL were significant prognostic factors [92]. Patients were grouped by whether they reached this nadir before or after 6 months; however, no difference in overall survival (OS) was observed between the groups. This suggests that achieving a nadir TST below 20 ng/dL may be more crucial for prognosis than the rate of decline [93, 94].
Clinical evidence highlights the prognostic value of serum TST levels in patients undergoing ADT. Significant differences in time to castration resistance (TCR) were observed among three serum TST groups (< 20, 20–50, and ≥ 50 ng/dL) [95]. A TST level < 30 ng/dL was linked to a significantly lower risk of death [87], whereas a level of 32 ng/dL was associated with progression-free survival (PFS) in androgen-independent cases [88].
6 Serum TST as a Central Determinant in CRPC Therapy and Emerging TST-Centered Strategies in PCa
Recent advancements in PCa treatment underscore the critical role of serum TST not only as a therapeutic target but also as a biomarker guiding personalized therapy. A multicenter retrospective study involving 258 metastatic hormone-sensitive prostate cancer (mHSPC) patients demonstrated that baseline TST levels significantly influence TCR and survival outcomes. Patients were stratified into high and low TST groups using a 12 nmol/L cutoff. Results indicated that lower baseline TST was associated with a shorter TCR (19.0 vs. 22.4 months, p = 0.031) and poorer cancer-specific OS, particularly in patients not receiving upfront combination therapy. These findings suggest that patients with low baseline TST may benefit from combination therapy to delay progression to CRPC and improve outcomes [96].
Serum TST levels also correlate with responses to androgen receptor (AR)-targeted therapies, offering a potential framework for optimizing CRPC management [97]. Higher TST levels (≥ 13 ng/dL) were linked to improved outcomes with novel AR inhibitors such as enzalutamide and abiraterone, whereas patients with TST < 13 ng/dL responded better to chemotherapies like docetaxel and cabazitaxel. These findings highlight the importance of precision medicine in PCa, with TST serving as a predictive biomarker for treatment selection [98]. Moreover, BAT, involving high-dose TST supplementation, has shown promise in re-sensitizing CRPC tumors to AR inhibitors or chemotherapy through mechanisms such as DNA damage induction and AR activity inhibition [97, 98].
The transition to a TST-centered approach has further evolved with clinical evidence from trials like SPARTAN and PROSPER, which validated the efficacy of modern AR drugs and chemotherapies [99]. For patients with TST levels ≥ 13 ng/dL, AR-targeting agents, such as enzalutamide demonstrate superior efficacy, whereas those with TST < 13 ng/dL benefit more from chemotherapy, confirming a differential response pattern based on TST levels [100, 101]. Additionally, pretreatment TST levels are associated with prognosis and QoL, with higher TST predicting fewer side effects and better outcomes [102, 103].
ADT remains a cornerstone of PCa treatment, but its effects on TST recovery vary based on factors, such as treatment duration, baseline TST levels, and patient comorbidities. Studies indicate that longer ADT duration significantly delays TST recovery, with approximately 50% of patients undergoing ADT for over 2 years remaining castrated for more than 1 year post-discontinuation [104-106]. Luteinizing hormone-releasing hormone (LH-RH) antagonists enable faster recovery than agonists, and regular monitoring of TST levels post-ADT is recommended for informed clinical decision-making [107-109]. Notably, nadir TST levels below 20 ng/dL are associated with improved OS, whereas patients experiencing a “TST bounce” (nadir TST < 20 ng/dL and max TST ≥ 20 ng/dL) demonstrate enhanced OS and cancer-specific survival (CSS) [93, 110-112]. These findings emphasize the need to tailor ADT strategies to optimize TST recovery and patient outcomes.
The integration of traditional castration therapy with innovative TST supplementation strategies marks a paradigm shift in PCa management. This TST-centered approach not only redefines the role of TST from suppression to dynamic modulation but also serves as a foundation for precision medicine, paving the way for transformative treatments and improved outcomes in PCa patients.
In Table 2, we summarized the guiding effect of serum TST cutoffs on relevant treatment.
| Serum TST (both sets of units ng/dL and nmol/L will be listed. The molecular weight of testosterone is about 288.42 g/mol) | Nationality of subjects | Therapeutic approach | Effects and impact of serum TST on the therapy | References | |
|---|---|---|---|---|---|
| < 20 ng/dL (Nadir TST during ADT) | < 0.694 nmol/L (Nadir TST during ADT) | The United States | Androgen deprivation therapy (ADT) | Patients with nadir TST below 20 ng/dL showed significantly improved OS. TST recovery depends on treatment duration and baseline TST levels. More than 50% of patients treated for over 2 years remained castrated for at least 1 year post-ADT | |
| Canada | |||||
| Japan | |||||
| Belgium/Czech Republic/Finland/France/Germany/Hungary/Italy/Russian Federation/Slovakia/Spain | |||||
| < 13 ng/dL | < 0.45 nmol/L | Japan | Chemotherapy (e.g., docetaxel and cabazitaxel) | Patients with lower TST levels responded better to chemotherapy | |
| Netherlands | |||||
| Global meta-data | |||||
| ≥ 345.5 ng/dL | ≥ 12 nmol/L | China/Japan/Spain/Austria | Impact of baseline TST on mHSPC patients | Patients with higher baseline TST had longer TCR (22.4 vs. 19.0 months, p = 0.031) and better cancer-specific and OS. Those with low baseline TST and without combination therapy showed poorer outcomes. Combination therapy is recommended for low TST patients to delay CRPC progression | [96] |
| ≥ 13 ng/dL | ≥ 0.45 nmol/L | Japan | AR-targeted therapy (e.g., enzalutamide and abiraterone) | Higher TST levels are associated with improved efficacy of novel AR inhibitors | |
| Netherlands | |||||
| Global meta-data | |||||
| ≥ 20 ng/dL (post-ADT TST rebound) | ≥ 0.694 nmol/L (post-ADT TST rebound) | Japan | ADT and posttreatment management | Patients experiencing a TST “rebound” (nadir < 20 ng/dL and max ≥ 20 ng/dL) demonstrated enhanced OS and CSS | [110-112] |
| Belgium/Czech Republic/Finland/France/Germany/Hungary/Italy/Russian Federation/Slovakia/Spain | |||||
| The United States | |||||
- Abbreviations: ADT, androgen deprivation therapy; AR, androgen receptor; CRPC, castration-resistant prostate cancer; CSS, cancer-specific survival; mHSPC, metastatic hormone-sensitive prostate cancer; OS, overall survival; PARP, poly (ADP-ribose) polymerase; PD-L1, programmed death-ligand 1; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; TCR, time to castration resistance; TST, testosterone.
7 Serum TST and BAT
The term “bipolar” here refers to the rapid cycling of TST levels from supraphysiologic highs to near-castration lows, repeated over multiple cycles. CRPC cells with high AR levels cannot fully degrade the androgen-stabilized nuclear AR and are vulnerable to cell death when exposed to supraphysiologic TST. High androgen levels also cause lethal double-stranded DNA breaks in PCa cells that have been chronically androgen-deficient. Cells that survive high TST due to low baseline AR levels or adaptive AR downregulation become susceptible to death when re-exposed to low TST in the bipolar treatment cycle [113].
Androgens may also initiate a “hit and run” mechanism through AR, inducing a quiescent state in PCa cells [114]. Thus, by alternating androgen deprivation with supplementation in BAT, cancer cell dormancy is induced or reinforced, potentially inhibiting metastatic progression in early stages.
In PCa, DNA replication is facilitated by AR. In the transition to metastasized Castration-Resistant Prostate Cancer (mCRPC), AR protein expression increases dramatically (by 50–100 times). Nuclear AR in mCRPC cells binds to DNA at origin replication sites (ORS) during the G1 phase as part of the replication origin complex (ORC), enabling DNA replication in the S phase. AR and ORC remain linked from early to late mitosis, and AR degradation is essential for DNA re-licensing in the next cycle. However, with TST supplementation, increased ligand binding stabilizes the ORC-bound AR, preventing full degradation. This ligand-induced stability halts DNA replication re-initiation, leading to cell death in subsequent cycles [115-117]. We have summarized the main mechanisms by which BAT affects PCa cell growth in Figure 1.
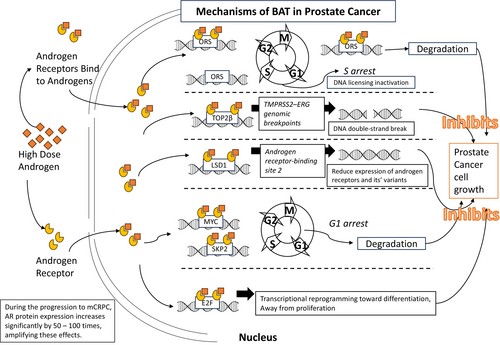
In a Phase 1 clinical trial, high-dose exogenous TST was safely used to treat CRPC patients. Although no patients achieved sustained supraphysiological serum TST levels, the study laid the groundwork for future research directions [118]. Another randomized Phase I study further confirmed the feasibility and tolerability of BAT in early CRPC patients, with 20% of patients experiencing a decline in PSA levels, and no significant impact on QoL or grip strength [119].
In a third open-label, Phase 2, multi-cohort study, 30% of patients achieved a PSA50 response following BAT treatment. Most patients regained sensitivity to enzalutamide upon rechallenge, indicating that BAT could be a safe and effective treatment option [120]. At last, the Phase II BATMAN study showed that after 6 months of ADT induction, 59% of HSPC patients achieved PSA levels below 4 ng/mL after 18 months of BAT treatment. Furthermore, the treatment improved patients' QoL [121].
In Schweizer et al.'s [113] pilot study on BAT, 16 asymptomatic mCRPC patients received BAT for at least 3 months. Results showed that 50% experienced a PSA decrease, with 28.6% seeing reductions over 50%. Imaging also showed controlled soft tissue metastases in 10 patients [113]. BAT gave notable benefits in lipid profiles, QoL, and body composition, offering long-term health advantages for mCRPC patients [122]. The RESTORE study involving 90 patients, reported systemic pain and calf swelling as common side effects, with hot flashes, breast enlargement, and breast pain among the typical sexual side effects [123]. BAT represents a novel therapeutic avenue for CRPC. By leveraging high doses of TST, BAT induces DNA damage and inhibits AR activity, enhancing responses to AR-targeted therapies and chemotherapy. Meta-analyses report PSA response rates of 27%–34% with BAT alone, which increase to 57% when combined with subsequent treatments [124-126].
Notably, the large TRANSFORMER trial (n = 180) compared PFS, safety, and QoL in asymptomatic mCRPC patients treated with BAT versus enzalutamide [125]. Results showed that BAT maintained or improved QoL, particularly in reducing fatigue and enhancing physical and sexual function. Additionally, cross-treatment analysis revealed that patients receiving enzalutamide after BAT responded significantly better than those transitioning from abiraterone to enzalutamide. The PSA-PFS for enzalutamide increased from 3.8 months post-abiraterone to 10.9 months post-BAT, with PSA50 response rates and overall response rates substantially higher (78% vs. 25% for PSA50, 29% vs. 4% for OR), suggesting BAT may partially restore AR sensitivity in resistant PC cells [125].
The BATMAN phase II study further demonstrated BAT's efficacy when alternated with ADT in HSPC. In this study, 76% of patients remained castration-sensitive after two BAT-ADT cycles, and five of seven nonresponders later responded to antiandrogen therapy (bicalutamide or enzalutamide) [121]. Other studies similarly indicate that BAT can reinstate AR sensitivity in previously resistant CRPC patients [120, 123, 125, 127].
Moreover, BAT combined with enzalutamide may enhance the clinical response to PD-1 blockade in metastatic mCRPC, potentially improving outcomes with immune checkpoint inhibitors [128, 129].
Promising directions include combining BAT with radiotherapy (e.g., prostate-specific membrane antigen [PSMA]-targeted therapy) [130], immunotherapy (e.g., PD-L1 inhibitors) [131, 132], or PARP inhibitors like Olaparib [133]. However, challenges such as hypertension and pulmonary embolism remain, necessitating further studies to optimize its application. We have summarized the main studies on BAT conducted to date in Table 3 for ease of reference.
| Patient status | Number | Nationality of subjects | Treatment plan | Result | References |
|---|---|---|---|---|---|
| CRPC | 12 | The United States | 5 mg transdermal TST patch or 1% gel for 1 week or 1 month | 30% of patients showed decreased PSA | [118] |
| Early CRPC with micrometastasis | 15 | The United States | Transdermal TST (25, 50, or 75 mg/day) | Symptom progression in 1 patient; PSA decrease in 3 patients | [119] |
| Asymptomatic CRPC with low to moderate metastasis | 16 | The United States | 400 mg IM TST on Day 1 of a 28-day cycle, plus oral etoposide (100 mg, Days 1–14) | PSA decreased in 50% of patients; imaging showed regression in 50% with soft tissue metastasis | [113] |
| Hormone-sensitive PCa with low metastasis | 33 | The United States | 400 mg IM TST on Days 1, 29, and 57 | PSA < 4 ng/mL in 17 patients at 18 months | [121] |
| mCRPC after enzalutamide | 30 | The United States | BAT cycle (400 mg TST IM on Days 1, 29, 57 for 3 months), followed by 3 months of ADT | 30% achieved PSA decrease; 52% regained enzalutamide sensitivity (PSA decreased) | [120] |
| mCRPC after enzalutamide or abiraterone (RESTORE) | 90 | The United States | 400 mg IM TST on Day 1 of a 28-day cycle plus oral etoposide (100 mg, Days 1–14) | BAT may offer better resensitization to enzalutamide compared to abiraterone | [123, 127] |
| mCRPC (after < or ≥ 6 months of abiraterone) | 180 | The United States | 400 mg IM TST every 28 days or 160 mg enzalutamide daily until clinical/imaging progression | Enzalutamide PSA-PFS tripled from 3.8 months post-abiraterone to 10.9 months post-BAT | [125] |
- Abbreviations: BAT, bipolar androgen therapy; CRPC, castration-resistant prostate cancer; IM, intramuscular; mCRPC, metastasized castration-resistant prostate cancer; PCa, prostate cancer; PFS, progression-free survival; PSA, prostate-specific antigen; TST, testosterone.
8 Conclusion
Recent research has deepened our understanding of the relationship between PCa and androgens, revealing complexities beyond previous assumptions. Yet, questions remain, particularly around optimal TST dosage and BAT cycles.
Although new hormone therapies, including drugs targeting BRCA1/2 mutations and PARP inhibitors, have largely replaced traditional hormone and chemotherapy, serum TST remains a key factor in determining the efficacy of PCa treatments and in predicting patient prognosis. Additionally, TST influences PCa cell proliferation and apoptosis, impacting treatment response and disease progression.
Currently, no clear guidelines exist, leaving clinicians to rely on incomplete but available clinical evidence. Although TST may be necessary in cases, such as successful PCa treatment or active surveillance for low-risk disease, extreme caution is advised for patients with moderate- or high-risk cancer, where benefits must clearly outweigh potential risks. In clinical practice, serum TST is a valuable biomarker for guiding treatment decisions and assessing prognosis, directly influencing outcomes and QoL in advanced PCa patients, as supported by robust clinical evidence.
Author Contributions
Xue Zhao: conceptualization, methodology, software, investigation, writing – original draft, visualization. Shinichi Sakamoto: conceptualization, supervision, validation, methodology, data curation, formal analysis, funding acquisition. Yasutaka Yamada: data curation, writing – review and editing. Kodai Sato: data curation. Takaaki Tamura: data curation. Hiroki Shibata: data curation. Tomokazu Sazuka: data curation. Yusuke Imamura: data curation. Tomohiko Ichikawa: data curation, investigation.
Acknowledgments
The present study was supported by grants from Grant-in-Aid for Scientific Research (C) (20K09555 to Shinichi Sakamoto), Grants-in-Aid for Scientific Research (24K02575 to Shinichi Sakamoto), the Japan China Sasakawa Medical Fellowship (to Xue Zhao), and JST SPRING (Grant Number JPMJSP2109 to Xue Zhao).
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
Tomohiko Ichikawa, Shinichi Sakamoto, and Yusuke Imamura are Editorial Board members of International Journal of Urology and co-authors of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication. The other authors declare no conflicts of interest.




