Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study
Abstract
Objective
To confirm the reproducibility of the effectiveness and safety in photodynamic diagnosis of non-muscle-invasive bladder cancer using 5-aminolevulinic acid in a prospective multicenter non-randomized phase III trial.
Methods
A total of 61 patients with primary or recurrent non-muscle-invasive bladder cancer were prospectively enrolled from five hospitals between May 2015 and March 2016. 5-Aminolevulinic acid (20 mg/kg) was orally administered 3 h before transurethral resection of bladder tumors using white light or fluorescent light. Of 60 evaluable patients, 511 specimens were obtained from tumor-suspicious lesions and normal-looking mucosa. The primary end-point was sensitivity. The secondary end-points were specificity, positive and negative predictive values, and safety.
Results
The sensitivity of the fluorescent light source (79.6%) was significantly higher (P < 0.001) than that of the white light source (54.1%). In total, 25.4% (46/181) of tumor specimens were diagnosed as positive with only the fluorescent light source. In nine (15%) of 60 patients, the risk classification and recommended treatment after transurethral resection of bladder tumors were changed depending on the additional types of tumor diagnosed by the fluorescent light source. The specificity of the fluorescent light versus white light source was 80.6% versus 95.5%. No grade 4–5 adverse event was noted. Hypotension and urticaria were severe adverse events whose relationship to oral 5-aminolevulinic acid could not be excluded.
Conclusions
These findings confirm the diagnostic efficacy and safety of photodynamic diagnosis with 20 mg/kg of oral 5-aminolevulinic acid, and show that transurethral resection of bladder tumors with a fluorescent light source using oral 5-aminolevulinic acid is well tolerated.
Abbreviations & Acronyms
-
- 5-ALA
-
- 5-aminolevulinic acid
-
- AE
-
- adverse event
-
- ALA-PDD
-
- photodynamic diagnosis using 5-aminolevulinic acid
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BCa
-
- bladder cancer
-
- BUN
-
- blood urea nitrogen
-
- CIS
-
- carcinoma in situ
-
- FAS
-
- full analysis set
-
- FL
-
- fluorescent light
-
- HAL
-
- hexaminolevulinate
-
- NMIBC
-
- non-muscle-invasive bladder cancer
-
- PDD
-
- photodynamic diagnosis
-
- PMDA
-
- Pharmaceuticals and Medical Devices Agency
-
- PpIX
-
- protoporphyrin IX
-
- SCr
-
- serum creatinine
-
- T-bil
-
- total bilirubin
-
- TUR
-
- transurethral resection
-
- TURBT
-
- transurethral resection of bladder tumors
-
- UD
-
- urothelial dysplasia
-
- WL
-
- white light
Introduction
BCa is a common cancer of the urogenital system, and approximately 75% of patients treated with TUR present with NMIBC.1 Most patients with NMIBC experience intravesical recurrence (31–78%) within 5 years after the initial treatment, and <1–45% patients with NMIBC experience progression.2 The number of tumors, tumor size, T-category, tumor grade, and the presence of CIS in the NMIBC affect NMIBC recurrence and progression.2-4 In Japan, the BCa treatment algorithm is mainly determined by these factors (Japanese Urology Association guideline for BCa 2015).5 Therefore, accurate diagnosis is essential for NMIBC management to reduce the recurrence and progression risk. However, conventional TURBT under a WL source can fail to detect 4–41% of small papillary tumors, CIS, multifocal growth and microscopic lesions.6, 7 To overcome this problem, TURBT guided by PDD using 5-ALA or HAL has been developed.8-11
5-ALA is a precursor of heme biosynthesis and induces fluorescent endogenous porphyrins, mainly PpIX, which accumulates significantly more in tumor cells than in healthy cells11 because of factors such as decreased ferrochelatase activity.12 Under an excitation wavelength of 405 nm, PpIX emits red fluorescence that peaks at 635 nm, which enables a surgeon to visualize cancer tissues, such as glioma and NMIBC, intraoperatively.10 HAL is an esterified derivative of 5-ALA that improves tissue penetration. Burger et al. found no difference in diagnostic accuracy between 5-ALA and HAL in a retrospective study.13 Meta-analysis showed that photosensitizing agents (5-ALA,14 HAL7 and both13) had similar sensitivity and specificity rates for BCa patients.15 Rink et al. concluded that 5-ALA and HAL are applicable to PDD of BCa.16
The administration route of HAL for NMIBC is limited to intravesical instillation. Conversely, 5-ALA can be administered by both oral and transurethral routes. In Japan, clinical study on ALA-PDD of NMIBC was initiated in 2004.17 Inoue et al. reported that ALA-PDD after intravesical administration is superior to conventional WL cystoscopy in diagnostic accuracy.17 In addition, when oral 5-ALA was compared with intravesical 5-ALA, oral 5-ALA was equally useful to intravesical 5-ALA.17 Oral 5-ALA has already been approved and available as an intraoperative diagnostic drug to visualize glioma in the EU since 2007 and in Japan since 2013, suggesting that safety profiles have been evaluated. Then, we carried out an investigator-initiated phase II/III study of 5-ALA for NMIBC, and found that ALA-PDD using 20 mg/kg of oral 5-ALA could detect more tumors only by PDD compared with ALA-PDD using 10 mg/kg of oral ALA.9 This phase II/III study showed the data supporting effectiveness, the clinically recommended dose (20 mg/kg) and safety of oral 5-ALA for PDD of NMIBC. To confirm the effectiveness and safety, we carried out a phase III study of PDD-assisted TURBT using oral 5-ALA (20 mg/kg) under the guidance of the PMDA.
Methods
This was a prospective, phase III, multicenter, non-randomized, clinical study; the protocol was registered as JapicCTI-152929 and was approved by the institutional review board of each participating institution. Written informed consent was obtained from each enrolled patient before TURBT in accordance with good clinical practice guidelines.18 We abided by the Declaration of Helsinki as a statement of ethical principles for medical research with human subjects, including research on identifiable human material and data (the 64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013). The quality of the trial was controlled by monitoring data management and an independent committee.
Patients
The registration period was from May 2015 to March 2016. In total, five institutions participated in this study. Patients with primary or recurrent NMIBC were enrolled after confirming the inclusion and exclusion criteria, as shown in Table 1.
| Inclusion criteria |
| 1. Indication for TUR of the bladder tumor |
| 2. Ability to give consent, written consent, and compliance with the schedule of hospital visit and the study protocol |
| 3. The age range was 20–85 years at the time of consent |
| 4. Eastern Cooperative Oncology Group performance status of 0–1 |
| 5. The following conditions were required |
| BUN, SCr, AST, ALT and T-bil <1.5-fold the reference value in each institution |
| Neutrophil cell count ≥1500 cells/mm3 |
| Platelet count ≥10 × 104/mm3 |
| Hemoglobin ≥8.5 g/dL |
| Exclusion criteria |
| 1. Cardiac infarction within 3 months before obtaining consent to the present trial, poorly controlled angina pectoris, arrhythmia requiring treatment or congestive heart failure |
| 2. Severe complication (poorly controlled hypertension or diabetes) |
| 3. Interstitial pneumonia or pulmonary fibrosis |
| 4. Poorly controlled infectious diseases |
| 5. Women who are or might be pregnant, or are breast-feeding |
| 6. Active multiple cancers |
| 7. Current or previous hypersensitivity to porphyrins |
| 8. Treatment for BCa within 6 months before obtaining consent to the present trial |
| 9. Participation in another clinical trial within 3 months before obtaining consent to the present trial |
| 10. Ineligibility judged by the investigator due to medical, psychological or other reason |
Investigational agent
The investigational agent administered in this clinical trial was 5-ALA. The agent was approved for sale as an internal diagnostic drug for malignant glioma surgery in 2007 by the European Medicine Agency and in 2013 by PMDA in Japan.19
Apparatus
ALA-PDD was carried out using a D-LIGHT System (Karl Storz GmbH & Co., Tuttlingen, Germany), which consisted of D-Light C (300-W xenon arc lamp) equipped with a band-pass filter to transmit blue light (excitation wavelength 375–445 nm), a HOPKINSII PDD telescope 30° equipped with a long-pass filter to cut off blue light (fluorescence emission wavelength 600–740 nm), and a CCU Tricam SLII3 CCD CH Tricam PDD video camera system that can instantly switch between the blue light mode for fluorescent observation and WL mode for conventional observation.
Procedure
A solution of 5-ALA (20 mg/kg) was credibly dissolved in water immediately before administration and was orally administered 3 h (range 2–4 h) before TURBT. Cystoscopy and tissue collection were carried out essentially the same way as the previous phase II/III study.9 Under general or spinal anesthesia, the bladder was initially examined with a conventional WL source, followed by inspection under an FL source. The findings obtained using the WL and FL sources were recorded, and an electronic data capture system was applied to input at the time of tissue sample collection. A test-positive using the WL source was defined based on the general rule for clinical and pathological studies on renal pelvic cancer, ureteral cancer and BCa.20 A test-positive using the FL source was defined when a tissue emitted a red fluorescence with the FL source. TUR or cold-cup biopsy was carried out to remove tissues that were identified as lesion/test-positive using the WL and/or FL source, followed by cold-cup biopsies of normal-looking lesions. The specimens were classified positive or negative on the basis of judgment by WL and FL sources. Pathological diagnosis of the specimens was made under a blind condition by a pathologist at the central assessment site to determine the T-category (2010 American Joint Committee on Cancer TNM Staging system), tumor grade according to the 2004 World Health Organization classification, the presence of CIS and surface morphology.
Assessment
The primary analysis was carried out on the FAS. The primary end-point was sensitivity. The secondary end-points were specificity, positive predictive value and negative predictive value. Safety was analyzed in the safety analysis set. AEs were evaluated by Common Terminology Criteria of Adverse Events 4.0 within 14 days after the administration of the agent regardless of the presence or absence of a causal relationship with the agent.
Sample size
Population size was determined according to the sensitivities of the FL and WL sources with 20 mg/kg of oral 5-ALA in a phase II/III study.9 Sensitivities for the FL and WL sources with 5-ALA were 75.8% and 47.6%, respectively. Thus, 37 samples were required with a significance level of 5% and a power of 90% using McNemar's test. A total of 12 patients were required for this trial, because a mean of 8.6 samples had been obtained from a patient in the previous report.9 However, the sensitivity for the detection of CIS using the FL source with 5-ALA was much higher than that using the WL source (58.6% vs 17.2%); conversely, the sensitivity for the detection of papillary tumors using the FL source with 5-ALA was slightly higher than that using the WL source (100% vs 94.6%). As flat lesions such as CIS affect total sensitivity, a sufficient number of CIS samples were required. The lower limit of the 95% confidence interval of the rate for CIS (29/124 samples) in the previous study was 15.9%. To obtain 29 CIS samples similarly as the previous study, 56 patients (183 samples) were required.9 Considering the possibility of dropout, the target number of patients was set at 60.
Statistical analysis
Statistical analysis was carried out using sas R9.3 (SAS Institute, Tokyo, Japan). McNemar's test was used to assess the significance of differences in paired data. All statistical tests were two-sided and were considered statistically significant at P < 0.05.
Results
Patients and samples
Written informed consent was obtained from 70 patients. Of these patients, seven patients were excluded due to incompatibility with the inclusion criteria and two patients were excluded due to meeting the exclusion criteria. The remaining 61 patients were enrolled, orally received 20 mg/kg of 5-ALA and were defined as safety analysis set for the analyses of AEs. In total, 60 patients (Table 2) were evaluated as FAS, because one patient who did not undergo ALA-PDD was excluded due to device malfunction.
| Variables | n = 60 |
|---|---|
| Age (years) | |
| Mean (SD) | 70.1 (7.9) |
| Median (range) | 70.5 (53–84) |
| Sex, n (%) | |
| Male | 51 (85) |
| Female | 9 (15) |
| Past history, n (%) | |
| Primary case | 42 (70) |
| Recurrence case | 18 (30) |
| Bodyweight | |
| Mean (SD) | 64.7 (10.7) |
| Median (range) | 64.0 (46–100) |
| Time from 5-ALA administration to TURBT procedure (min) | |
| Mean (SD) | 158 (±29) |
| Median (range) | 160 (112–236) |
| Pathological findings | |
| No malignancy | 4 |
| Dysplasia | 2 |
| Papillary urothelial neoplasm of low malignant potential | 16 |
| Ta (low grade) | 4 |
| Ta (high grade) | 20 |
| T1 (low grade) | 3 |
| T1 (high grade) | 5 |
| CIS (isolated/associated) | 21 (6/15) |
In total, 513 specimens were obtained from the 60 patients. Of the specimens, 181 specimens were diagnosed as tumor-positive and 330 specimens were diagnosed as tumor-negative. Meanwhile, two specimens could not be pathologically diagnosed because of lack of mucosa.
Diagnostic accuracy
Primary end-point
The sensitivity of the FL source was significantly higher than that of the WL source (79.6% [144/181] vs 54.1% [98/181], P < 0.001; Table 3). In total, 25.4% (46/181) of tumor-positive samples were correctly diagnosed as test-positive, with only the FL source as the additional detection (Fig. 1a,b). In contrast, no tumor specimens that were diagnosed as lesion-positive by the WL source, but were test-negative by the FL source, were found in the present study.
| WL | FL | P | |||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Tumor-positive | 98 (0: only WL+) | 83 | 144 (46: only FL+) | 37 | |
| Tumor-negative | 15 | 315 (49: only WL−) | 64 | 266 (0: only FL−) | |
| Sensitivity | 54.1% (95% CI 46.6–61.6) | 79.6% (95% CI 72.9–85.2) | <0.001† | ||
| Specificity | 95.5% (95% CI 92.6–97.4) | 80.6% (95% CI 75.9–84.7) | <0.001† | ||
| Positive predictive value | 86.7% (95% CI 79.1–92.4) | 69.2% (95% CI 62.5–75.4) | <0.001‡ | ||
| Negative predictive value | 79.1% (95% CI 74.8–83.0) | 87.8% (95% CI 83.6–91.3) | 0.004‡ | ||
- †McNemar's test, WL versus FL. ‡The χ2-test, WL versus FL. only FL+, diagnosed as positive by only FL source; only FL−, diagnosed as negative by only FL source; only WL+, diagnosed as positive by only WL source; only WL−, diagnosed as negative by only WL source.
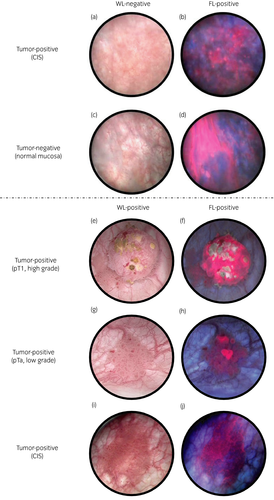
Secondary end-points
The specificity of the FL source was significantly lower than that of the WL source (80.6% [266/330] vs 95.5% [315/330], P < 0.001). The positive predictive value of the FL source was significantly lower than that of the WL source (69.2% [144/208] vs 86.7% [98/113], P < 0.001). Meanwhile, the negative predictive value of the FL source was significantly higher than that of the WL source (87.8% [266/303] vs 79.1% [315/398], P = 0.004).
Sensitivity in each tumor type
The sensitivity of the FL source for flat urothelial tumors was significantly higher than that of the WL source (61.3% [57/93] vs 20.4% [19/93], P < 0.001; Table 4), with 40.9% (38/93) of an additional detection rate of the FL source.
| WL | FL | P | |||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Flat urothelial tumor (n = 93) | 19 | 74 | 57 | 36 | |
| Sensitivity, % (95% CI) | 20.4 (12.8–30.1) | 61.3 (50.6–71.2) | <0.001† | ||
| UD (n = 23) | 2 | 21 | 10 | 13 | |
| Sensitivity, % (95% CI) | 8.7 (1.1–28.0) | 43.5 (23.2–65.5) | 0.005† | ||
| CIS (n = 70) | 17 | 53 | 47 | 23 | |
| Sensitivity, % (95% CI) | 24.3 (14.8–36.0) | 67.1 (54.9–77.9) | <0.001† | ||
| Papillary urothelial tumor (n = 74) | 70 | 4 | 74 | 0 | |
| Sensitivity, % (95% CI) | 94.6 (86.7–98.5) | 100 (95.1–100) | 0.046† | ||
- †McNemar's test, WL versus FL.
The sensitivity of the FL source for papillary urothelial tumors was slightly, but significantly, higher than that of the WL source (100.0% [74/74] vs 94.6% [70/74], P = 0.046; Table 4), with 5.4% (4/74) of an additional detection rate of the FL source.
Change of pathological findings by the FL source
Of 60 patients who underwent TURBT, 24 patients had additional tumor-positive specimens that were detected only by FL diagnosis. Among nine of these patients, the risk classification and recommended treatment after TURBT were changed depending on the additional types of tumor diagnosed by the FL source (Table 5). Of these nine patients, seven patients had additional CIS by the FL source that were not detected by the WL source. One patient was given a diagnosis of CIS by the FL source, even though no BCa was detected by the WL source. One patient was given a new diagnosis of having a higher stage tumor (pT1) by the FL source after a diagnosis of stage pTa was made by the WL source.
| WL | ALA-PDD | No. patients, n (%) | ||
|---|---|---|---|---|
| Types of tumor | Risk classification† | Additional types of tumor | Risk classification† | |
| – | – | pTis | High | 1 (1.7) |
| pTa high grade | Intermediate | pTis | High | 4 (6.7) |
| pTa high grade | Intermediate | pT1 high grade | High | 1 (1.7) |
| pTa high grade | Intermediate | pTis UD | High | 1 (1.7) |
| pT1 high grade | High | pTis | High | 2 (3.4) |
- †Risk classification was determined as described in “Essential content of evidence-based clinical practice guidelines for BCa: the Japanese Urological Association 2015 update” by Kubota et al.5
AEs
Of 61 patients, 58 patients (95.1%) experienced AEs, but none of them died or discontinued the clinical trial as a result of AEs. Grade 4 or higher AEs were not observed. Six patients (eight cases) experienced grade 3 AEs, namely four cases of ALT elevation, one case each of diabetes, bladder perforation, hypotension and urticaria. The bladder perforation and diabetes were not related to 5-ALA. The increased ALT value recovered gradually with (one case) or without (four cases) treatment with anti-inflammatory drugs. No photosensitivity reaction developed. Hypotension and urticaria were severe AEs whose relationship to oral 5-ALA cannot be ruled out. However, the hypotension was improved quickly by a vasopressor under anesthesia, and the urticaria was improved by anti-inflammatory drugs (Table 6).
| n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Investigations | |||||
| ALT increased | 6 (9.8) | 1 (1.6) | 4 (6.6) | 0 (0) | 0 (0) |
| Blood alkaline phosphatase increased | 2 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Aspartate amino transferase increased | 12 (19.7) | 3 (4.9) | 0 (0) | 0 (0) | 0 (0) |
| Blood bilirubin increased | 7 (11.5) | 3 (4.9) | 0 (0) | 0 (0) | 0 (0) |
| γ-Glutamyl transferase increased | 8 (13.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood lactate dehydrogenase increased | 2 (3.3) | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal disorders | |||||
| Abdominal pain | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 1 (1.6) | 2 (3.3) | 0 (0) | 0 (0) | 0 (0) |
| Vascular disorders | |||||
| Hypotension | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) |
| Cardiac disorders | |||||
| Ventricular arrhythmia | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Skin and subcutaneous tissue disorders | |||||
| Urticaria | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) |
- Total n = 61. All AEs related to 20 mg/kg 5-ALA that cannot be ruled out are shown.
Discussion
To our knowledge, this is the first report to show the feasibility of PDD of NMIBC using 20 mg/kg of oral 5-ALA in a phase III trial. Sensitivity of the FL source using 20 mg/kg of oral 5-ALA was significantly higher than that of the WL source, with acceptable AE rates. Furthermore, sensitivities of PDD using 20 mg/kg of oral 5-ALA were higher than those of the WL source for detecting NMIBC, regardless of tumor type, flat urothelial tumors and papillary urothelial tumors. By using the FL source, 40.9% (38/93) of flat urothelial tumors and 5.4% (4/74) of papillary urothelial tumors, which were overlooked as lesion-negative by the WL source, were correctly diagnosed as FL-positive. Meanwhile, no tumor-positive samples that were diagnosed as lesion-positive by the WL source and that were diagnosed as test-negative by the FL source were found. Thus, the FL source was much more useful for detecting flat urothelial tumors than the WL source. The present results were compatible with those of previous studies, and showed that ALA-PDD was able to detect lesions more accurately than the WL source, especially flat lesions.9, 14
In the present trial, the additional tumor types detected by the FL source were very important for nine patients, because they changed the risk classification and the strategy for NMIBC after TURBT (Table 5). In eight patients who received an additional diagnosis of CIS by the FL source, bacillus Calmette–Guérin instillation could be recommended. Furthermore, in one patient who received an additional diagnosis of T1 by the FL source, a second TURBT could be strongly recommended. Thus, ALA-PDD enables more appropriate risk classification and optimizes treatment management of NMIBC.
The major disadvantage of ALA-PDD is its relatively low specificity (Fig. 1c,d). Hungerhuber et al. reported that 1769 (38.2%) of false positive findings were obtained by ALA-PDD from 4630 biopsies.21 False fluorescence (Fig. 1d) is associated with inflammation caused by previous intravesical chemotherapy, recent TURBT,22 female sex23 and oblique illumination of the bladder wall with blue light.24 Therefore, some unnecessary FL-guided resection might be carried out. However, Stenzl et al. reported that more specimens were resected from the 5-ALA arm than from the placebo arm (5-ALA 2.6 vs placebo 2.2 per patient), and the incidence of AEs between the 5-ALA (32.6%) and placebo (33.9%) arms was comparable.25 As a result, an increased number of resected specimens by using ALA-PDD might not affect the incidence of the AEs. Furthermore, Draga et al. reported that the learning curve of the surgeons was proportional to a decrease in the number of false positives up to 12–18 months after the first PDD procedure.26 As compared with the previous phase II/III study and the present phase III study, the specificity was 68.2% and 80.6%, respectively. The present study was carried out in the same institutions as the phase II/III trial. Accordingly, the experiences of TURBT with a FL source might improve specificity and decrease the number of resections for false positives. Another concern about false positive cases in ALA-PDD is whether there are any pathological characteristics in false positive cases that were positive for the FL source, but pathologically negative. In the present trial, all of these samples from false positive cases were normal tissues, and no characteristics of precancerous lesions were noted. However, mucosa that was positive for the FL source, but pathologically negative, was reported to harbor alteration of chromosome 9, which has frequently been reported as a common genetic alteration in BCa, suggesting the possible detection of the precancerous lesion by FL.27
The AEs induced by conventional photosensitive substances, such as porphyrin derivatives, are phototoxic reactions, mainly photosensitivity, and liver disorders.28 In contrast, 5-ALA is a natural amino acid produced by mitochondria in animals and plants, and its toxicity is very low.9 Dunn et al. observed in a randomized study that skin photosensitivity was significantly more common after treatment with Photofrin than after treatment with 5-ALA (43% vs 6%, P < 0.05).29 In the present study, all grade 3 AEs related with 5-ALA were improved soon with or without medication. Conclusively, oral treatment with 20 mg/kg 5-ALA is acceptable with moderate AEs.
A limitation of this trial was the short observation time. Although this trial found that ALA-PDD enabled a more concise diagnosis of NMIBC with acceptable AE rates, this trial did not show whether a more concise diagnosis leads to improved recurrence-free or progression-free survival.
The present clinical trial provided evidence of the effectiveness and safety of PDD with 20 mg/kg of oral 5-ALA, and as a novel diagnostic modality for NMIBC. TURBT with the FL source using oral 5-ALA can be the next standard treatment.
Acknowledgments
The authors express their heartfelt gratitude to Intellim Co. Ltd. for the project management of this clinical trial and to SBI Pharmaceuticals Co., Ltd. for the supply of the investigational product drug for this study.
Conflict of interest
None declared.




