REVASCAT: a randomized trial of revascularization with SOLITAIRE FR® device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset
Abstract
REVASCAT is a prospective, multicenter, randomized trial seeking to establish whether subjects meeting following main inclusion criteria: age 18-80, baseline National Institutes of Health Stroke Scale ≥6, evidence of intracranial internal carotid artery or proximal (M1 segment) middle cerebral artery occlusion, Alberta Stroke Program Early Computed Tomography score of >7 on non-contrast CT or >6 on diffusion-weighted magnetic resonance imaging , ineligible for or with persistent occlusion after intravenous alteplase and procedure start within 8 hours from symptom onset, have higher rates of favorable outcome when treated with the SolitaireTM FR embolectomy device compared to standard medical therapy alone The primary end-point, based on intention-to-treat criteria is the distribution of modified Rankin Scale scores at 90 days. Projected sample size is 690 patients. Estimated common odds ratio is 1●615. Randomization is performed under a minimization process using age, baseline NIHSS, therapeutic window, occlusion location and investigational center. The study follows a sequential analysis (triangular model) with the first approach to test efficacy at 174 patients and subsequent analyses (if necessary) at 346, 518, and 690 subjects. Secondary end-points are infarct volume evaluated on CT at 24 h, dramatic early favorable response, defined as NIHSS of 0–2 or NIHSS improvement ≥8 points at 24 h and successful recanalization in the Solitaire arm according to the thrombolysis in cerebral infarction (TICI) classification defined as TICI 2b or 3. Safety variables are mortality at 90 days, symptomatic intracranial haemorrhage rates at 24 hours and procedure related complications.
Background
Intravenous (i.v.) thrombolysis remains the only approved therapy for acute ischemic stroke. However, it has a short therapeutic window, a strong time dependency, and has only marginal benefit in strokes due to proximal arterial occlusions 1, 2. Endovascular therapy has many theoretic advantages over i.v. thrombolysis. Mechanical thrombectomy reduces and may even preclude the use of chemical thrombolytics, and this may further reduce the risk of intracranial haemorrhage (ICH), allowing faster and sustained recanalization. However, endovascular recanalization techniques have also relevant disadvantages, including delays in initiating treatment, complexity of the procedure, high level of required technical expertise, low availability and risks and expense of an invasive procedure as compared with i.v. tissue plasminogen activator (t-PA). Nevertheless, given the strong relationship between vessel recanalization and good clinical outcomes, the advantages of endovascular stroke therapy as the most efficacious treatment for recanalization of large vessel intracranial occlusions may outweigh its disadvantages and risks, but this needs yet to be proved.
Novel stent retrievers or ‘stentrievers’, intracranial stents that are deployed and retrieved snaring the thrombus showed very promising results 3, 4. Recently, the SOLITAIRE™ With the Intention for Thrombectomy (SWIFT) trial compared two mechanical thrombectomy devices: The MERCI® Retriever and the SOLITAIRE retrievable stent in the arterial recanalization of patients with acute ischemic stroke 5. The SWIFT trial has shown in 113 patients that the SOLITAIRE™ FR device is superior to the MERCI Retriever in achieving successful revascularization (by Corelab, 68% vs. 30%, <0·001), inducing less symptomatic ICH (2% vs. 11%, P = 0·06), reducing mortality (17% vs. 38%, P = 0·02), and increasing good neurologic outcome three-months after stroke (58% vs. 33%). TREVO® Retrieval System has also shown a high rate of revascularization and favorable results 6. Results of the recently reported TREVO 2 trial 7 confirmed the superiority of stentrievers over MERCI embolectomy with respect to recanalization rates [thrombolysis in cerebral infarction (TICI) ≥ 2 in 86·4% vs. 60%, respectively, P < 0·00001], clinical outcomes (modified Rankin score (mRS) ≤ 2 in 40% vs. 21·8%, respectively, P = 0·01) and with no significant difference in the risk of symptomatic intracranial hemorrhage (SICH) (6·8% vs. 8·9%, respectively, P = 0·78). A recently published single-center comparison between MERCI and TREVO/SOLITAIRE showed similar results, with even significant difference in the SICH rate (TICI 2b-3 82% vs. 62%, P = 0·016, mRS ≤ 2 65% vs. 35%, P = 0·002 and SICH 10% vs. 28%, P = 0·01) 8. In none of the aforementioned trials, multimodal imaging showing tissue at risk was used for patient's selection.
The IMS-3 was a phase III, randomized, multicenter, open-label, 900-subject clinical trial conducted to examine whether a combined i.v. and intra-arterial approach to recanalization was superior to standard i.v. t-PA alone when initiated within three-hours of acute ischemic stroke onset 9. The trial was prematurely halted due to futility according to the results of a prespecified interim analysis. The proportion of patients who achieved a mRS score mRS ≤ 2 at 90 days did not differ significantly according to treatment (40·8% with endovascular therapy and 38·7% with i.v. t-PA). Regarding safety results, mortality at 90 days (19·1% and 21·6%, respectively; P = 0·52) and symptomatic intracerebral haemorrhage within 30 hours after initiation of t-PA (6·2% and 5·9%, respectively; P = 0·83) were comparable between the two groups.
Of note, different intra-arterial approaches and devices were allowed during the trial, and patients were randomized after the initiation of i.v. t-PA without knowledge of vessel status. Therefore, there is equipoise on endovascular therapy for acute stroke so far. The ideal thrombectomy trial design should test a single device, randomize patients according to intracranial occlusion, include t-PA nonresponders and use advanced imaging for patients selection beyond 4·5 h. In addition, the trial should be conducted in a few centers with high recruitment capacity and neurointerventional expertise to decrease intercenter variability.
Following this approach, the overall goal of the REVASCAT trial is to establish whether subjects with a baseline National Institutes of Health Stroke Scale (NIHSS) ≥ 6 and large artery occlusion of the anterior territory who can potentially undergo endovascular treatment within eight-hours from the time last seen well have a more favorable outcome at three-months as compared with subjects treated with standard medical therapy alone.
Methods
Study design and participants
REVASCAT is a multicenter, randomized, sequential and blinded–end-point trial. The randomization employs a 1:1 ratio of mechanical embolectomy with approved stentriever SOLITAIRE FR vs. standard medical management alone.
Eligibility criteria
- Patients with acute ischemic stroke ineligible for i.v. thrombolytic treatment (e.g. subject presents beyond recommended time from symptom onset), or treated with i.v. thrombolytic therapy without recanalization after a minimum of 30 mins from start of i.v. t-PA infusion.
- Occlusion (TICI 0–1) of the intracranial ICA (distal internal carotid artery (ICA) or T occlusions), proximal middle cerebral artery (MCA-M1) segment or tandem proximal ICA/MCA-M1 suitable for endovascular treatment, as evidenced by computed tomography angiography (CTA), magnetic resonance angiography (MRA) or angiogram, with or without concomitant cervical carotid occlusion or stenosis. In patients treated with i.v. t-PA, the CTA or MRA confirming occlusion has to be performed at a minimum of 30 mins after start of i.v. t-PA infusion.
- Patient treatable within eight-hours from time last seen well at baseline (i.e. subjects who have stroke symptoms upon awakening will be considered to have their ‘onset’ at beginning of sleep).
- Age ≥ 18 and ≤ 80.
- Baseline NIHSS score must be equal or higher than 6 points.
- No significant pre-stroke functional disability (mRS ≤ 1).
- Informed consent obtained from patient or acceptable patient surrogate.
Exclusion criteria
Clinical
- Known haemorrhagic diathesis, coagulation factor deficiency, or oral anticoagulant therapy with INR > 3·0.
- Baseline platelet count <30·000/μL.
- Baseline blood glucose of <50 mg/dL or >400 mg/dl.
- Severe, sustained hypertension (SBP > 185 mm Hg or DBP > 110 mm Hg).
- Patients in coma (NIHSS item of consciousness >1) (Intubated patients for transfer could be randomized only in case an NIHSS is obtained by a neurologist prior transportation).
- Seizures at stroke onset that would preclude obtaining a baseline NIHSS.
- Serious, advanced, or terminal illness with anticipated life expectancy of less than one-year.
- History of life-threatening allergy (more than rash) to contrast medium.
- Subjects who have received i.v. t-PA treatment beyond 4·5 h from the beginning of the symptoms.
- Renal insufficiency with creatinine ≥3 mg/dl.
- Woman of childbearing potential who is known to be pregnant or lactating or who has a positive pregnancy test on admission.
- Subject participating in a study involving an investigational drug or device that would impact this study.
- Cerebral vasculitis.
- Patients with a preexisting neurological or psychiatric disease that would confound the neurological or functional evaluations; mRS score at baseline must be ≤1.
- Unlikely to be available for 90-day follow-up.
Neuroimaging
-
Hypodensity on CT or restricted diffusion amounting to an Alberta Stroke Program Early CT score (ASPECTS) score of <7 on noncontrast CT, or <6 on DWI magnetic resonance imaging (MRI). ASPECTS may also be evaluated by cerebral blood flow maps of CT Perfusion, or CTA source imaging in patients whose vascular occlusion study (CTA/MRA) confirming qualifying occlusion is performed beyond 4·5 h of last seen well.
-
CT or MR evidence of haemorrhage (the presence of microbleeds is allowed).
-
Significant mass effect with midline shift.
-
Evidence of ipsilateral carotid occlusion, high-grade stenosis or arterial dissection in the extracranial or petrous segment of the internal carotid artery that cannot be treated or will prevent access to the intracranial clot or excessive tortuosity of cervical vessels precluding device delivery/deployment.
-
Subjects with occlusions in multiple vascular territories (e.g. bilateral anterior circulation, or anterior/posterior circulation).
-
Evidence of intracranial tumour (except small meningioma).
Randomization
A ‘Real-Time’ randomization procedure is implemented via the REVASCAT Trial Website. First, the clinical center investigator enters the basic baseline and eligibility information of a subject. If the subject's eligibility status is confirmed, the server allocates the treatment on the basis of a minimization process to balance in a 1:1 ratio the 2 groups both overall as well as within every category of the factors: age (≤70 or >70 years), baseline NIHSS (6–16 or, ≥17-or more), therapeutic window (≤4·5 or >4·5 h), occlusion site (intracranial ICA or M1 segment) and investigational center.
The schedule enrolment, interventions and assessments are shown in Fig. 1.

Template for the schedule of enrolment, interventions, and assessments.
1Only in patients allocated to the Solitaire arm.
2mRS score based on subject's score prior to the stroke symptoms onset.
3mRS to be done by a local blinded evaluator.
4mRS to be done by an independent blinded evaluator.
5CTP or PWI/DWI-MRI should be used at baseline for patient evaluation beyond 4·5 h from symptoms onset.
6Overall stay: should include all hospitalization days since random to discharge (i.e. home, rehab facilities) including stay at referral hospitals.
Treatment
Vascular neurologists and trained interventional neuroradiologists or neurologists in certified comprehensive stroke centers that treat more than 500 acute stroke patients and perform more than 60 acute mechanical thrombectomies every year will treat patients. Neurointerventionalists must have previously performed at least 20 thrombectomies with SOLITAIRE device in acute ischemic stroke patients. Patients in both arms will be admitted at acute stroke units (or ICU if needed) and treated following the European Stroke Organization ESO guidelines (ESO Cerebrovasc Dis 2008).
All interventional therapy must be started earlier than eight-hours relative to the time the subject was last seen well. Treatment initiation is defined as groin puncture. The duration of the interventional procedure should not exceed three-hours. To allow an intention to treat interpretation, no crossover is permitted.
- Only the SOLITAIRE FR device will be allowed in REVASCAT.
- If the SOLITAIRE device fails after a maximum of six passes per vessel, no pharmacological or other mechanical rescue therapies will be allowed.
- Systemic anticoagulation may not be used other than in the heparinized saline infusion as per local interventional procedure standards.
- Balloon angioplasty and/or stenting of extracranial ICA in cases with ICA/M1 tandem occlusions will be allowed as per site specific protocols. For sites that perform stenting in addition to angioplasty for tandem occlusions, it is recommended that aspirin 300 mg and clopidogrel load (600 mg) are administered orally or via nasogastric tube prior to intervention if possible.
- Angioplasty and/or stenting of intracranial vessels beyond the petrous segment of the ICA will not be allowed.
- The use of a balloon guide catheter in the proximal ICA is optional and solely at the discretion of the interventionalist. The rationale for using the balloon guide catheter is to prevent distal embolization including in previously uninvolved territories during device and clot retrieval.
Efficacy end-points
Primary end-point
The primary end-point will be the distribution of the modified Rankin Scale scores at 90 days (shift analysis) as evaluated following a structured interview by two separate certified assessors who will be blinded to treatment.
Clinical secondary end-points
- Early response to treatment as determined by a NIHSS drop of ≥8 or NIHSS 0–2 at 24 (-2/12) hours from randomization or before discharge if patient is discharged prior to the above time limit.
- Barthel Index at 90 days.
- NIHSS at 90 days.
- mRS score (0–2 vs. 3–6) at 12 months.
- Trail Making Test at 90 days.
- Quality of life measured by EuroQol EQ-5D at 90 days and 12 months.
- Cost effectiveness analysis.
- Secondary analyses of the primary end-point: Functional independence defined as mRS ≤ 2 at 90 days and severe dependence defined as mRS > 3 at 90 days.
Neuroimaging secondary end-points
- Final infarct volume measured on 24 (−2/12) h CT (MRI's if available will be used in a separate analysis).
- Median modified ASPECTS score increase defined as CT ASPECTS score at 24 h minus baseline ASPECTS score.
- Vessel recanalization evaluated by CT angiography or MRA at 24 (−2/12) h in both treatment groups.
- Immediate Post-Endovascular Treatment Recanalization (for the mechanical embolectomy group only). Successful recanalization is defined as TICI 2b or 3 in the post-procedure angiography.
Safety end-points
- Clinically significant ICH rates at 24 (−2/+12) hours. All intracerebral haemorrhages will be classified by a central core-lab using the ECASS criteria. Symptomatic ICH will be defined as per the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) definition: deterioration in NIHSS score of ≥4 points within 24 h from treatment and evidence of intraparenchymal haemorrhage type 2 in the 22 to 36 hours follow-up imaging scans. The incidence of any asymptomatic haemorrhage measured at 24 (−2/+12) hours will also be compared.
- Mortality at day 90.
- Procedural related complications: arterial perforation, arterial dissection, and embolization in previously uninvolved vascular territory.
Masking
Each site will designate one or more individual(s) to perform the follow-up evaluation at 24 (± 12) h, 5 (± 2) days or prior to discharge if discharge occurs before three-days and at three-months who can remain blinded to the treatment of each subject. Data entry would not reveal the study arm assignment and patients will be instructed to minimize the chance of disclosing their treatment group to the evaluator.
Regarding the primary end-point, first, a local independent neurologist, not involved in the trial patient management, will record the mRS score in a face to face clinical visit; and second, an experienced and mRS-certified nurse will centrally evaluate mRS score by telephone call, recording the interview in an audio-tape. In case of disagreement between the two assessors, a centralized neurologist will rate mRS by using the audio-tape recording and his mark will be the primary end-point value in this case. All of them will directly introduce mRS score in the database without access to any further information.
All neuroimaging secondary end-points including recanalization at 24 hours, infarct volume and haemorrhage will be determined by the CT/MR core-lab, which will be also blinded to treatment allocation. Successful recanalization in the post-procedure angiography in the mechanical embolectomy group will be classified by a specific core-lab.
Serious adverse events (SAEs) and procedural related complications will be adjudicated by two members of the independent Clinical Events Committee that will be unblinded to treatment arm,
Data safety monitoring board (DSMB)
The purpose of the DSMB is to review, on a regular basis, unmasked accumulated efficacy and safety data. The DSMB will be composed of three stroke neurologists or interventionalists, and a statistician who are not participating in the study and are not affiliated with the sponsor. The role of the DSMB will be to: (1) make recommendations to the Executive Committee regarding stopping or extending the trial based on the pre-planned efficacy interim analysis; and (2) review the occurrence of AEs and make recommendations to the Executive Committee regarding safety of the study.
Imaging core lab
Centralized imaging core labs will be used in this study to provide consistent evaluation of images. Two independent central imaging core labs will be established to independently review CT/MR and angiographic images. One lab will review angiographic images from the procedure to determine clot location and revascularization. Another independent core lab will review CT/MR images obtained at baseline and at 24 h for confirmation of inclusion criteria (occlusion of the intracranial ICA and/or MCA-M1 with or without concomitant cervical carotid occlusion or stenosis; ASPECTS score), and presence/absence of haemorrhage, vessel patency and infarct volume at 24 (−2/+12) hours. Having a CT/MR core lab independent from the angiographic core lab ensures that the CT/MR core lab is blinded to the treatment (Fig. 1). Each core lab will use standardized procedures for neuroimaging evaluation defined in a specific manual. They will provide no information to the investigators with the exception of the deviations from the neuroimaging inclusion criteria.
Clinical events committee (CEC)
A CEC will be in place for the study using 2 physicians knowledgeable in the appropriate disciplines and medical specialties pertinent to the disease state being evaluated in this clinical study. The CEC will be comprised of individuals who are independent of the investigational sites. This committee will be responsible for the review and validation of all complications that occur over the course of the study and the subsequent classification of these complications as related to the device or procedure.
Members of the CEC will review all complications and adjudicate them as defined in the Adverse Event section in the CEC Manual of Operations. The CEC can request additional source documentation and any imaging obtained in support of the adverse event to assist with adjudication.
Statistical design
Effect size measure and primary end-point analysis
We expect that the effect of the revascularization will improve patient status for the first 5 mRS possible cut-points, but not for the change between 5 and 6. In other words, the intervention may ‘shift’ patients to minor values from 5 to 4; from 4 to 3, from 3 to 2; from 2 to 1 and from 1 to 0, but not from 6 to 5. Furthermore, the order preference between those two values, severe disability and death, is not clear neither for patients, clinicians, nor care providers 10. The Ordinal Logistic Analysis (OLR) based on cumulative logits provides a treatment effect in the form of a common estimate of the OR for improvement over considered cut-points. This analysis has been shown to be robust for minor deviations of the assumption of a common OR underlying behind any cut-point 11. This logistic regression will adjust both for the minimization factors and for the interventionist.
Interim analyses rationale
We desired a maximum of 4 looks when approximately 25, 50, 75 and 100% of the sample size finish the follow-up, monitoring and data cleaning processes. A sequential test strategy was designed to have reasonable chances of stopping as soon as possible, either because of better efficacy of the device procedure, because of the futility of the trial or because of safety reasons. We were not interested in proving that the test intervention was inferior to the best medical treatment.
At interim analysis, in case the stopping boundaries are crossed, the DSMB may recommend stopping the study either for efficacy, futility or safety. When addressing safety, DSMB will also consider mortality (mRS = 6) and severe dependency (mRS = 5) at three-months as one single value. In case of early stopping, any over-running patient will be followed until the end of the study and a final analysis will be performed.
Devised effect, power and sample size
The expected proportions of patients having a mRS score 0 or 1 at 90 days are 25% and 35% in the control and treated arms. This results in an expected OR of 1·615. The trial was designed to have 90% chances to conclude efficacy in the case of a clinical advantage of a common OR of 1·615 through any possible mRS cut-points (including 5 to 6) and an overall 2·5% one-sided risk of concluding efficacy in case of no effect.
After a search 12 a triangular test with 3 interim looks plus 1 final analysis was specified with values a = 2·18074 and v1 = 14·008681. The total maximum sample size was 690.
The trials proprieties for the final analysis, pooling mRS values 5 and 6, were validated by simulation including two higher hypothetical OR: 2·00 (15%: 40 over 25% on the 0 or 1 vs. 2 to 6 cut-point) and 2·45 (20%: 45 over 25%). Three hypothetical different scenarios (Figs 2-4) for the treatment effect were analyzed: (A) the targeted studied effects (1615; 2·000 and 2·450) applies exactly to the 6 frontiers or cut-point on the 0 to 6 ranking scale ( Table 1); (B) as before, but the targeted effects are constant for the 5 first frontiers and they are diluted in the last 5 to 6 cut-point resulting in a diluted overall estimate (common along the 6 overall possible frontiers) (Table 2); and (C) as before, but now the diluted overall estimate fits the targeted effects (Table 3).
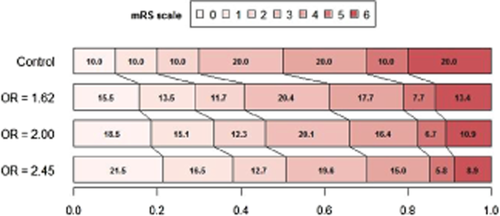
In scenario A, the same OR (either 1·62; 2·00 and 2·45) applies to any possible cut-point.
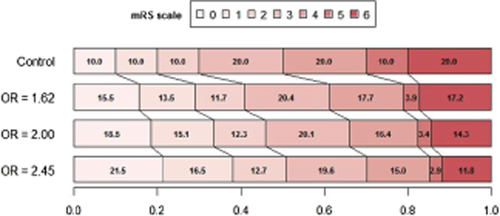
In scenario B, the same OR (1·62; 2·00 and 2·45) applies only to the first 5 cut-points, and it is diluted in the last 5 to 6 frontier. As a consequence, the common (‘averaged’) OR estimated through all cut-points, including the 5 to 6 frontier is smaller.
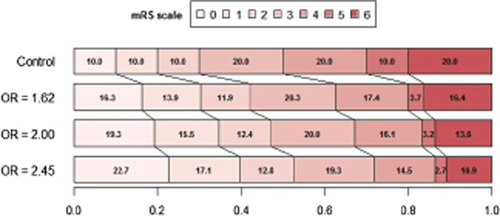
In scenario C, again, the same OR applies to the first 5 cut-points and it is diluted in the last 5 to 6 frontier; but now the common averaged OR results in an estimate of 1·62; 2·00 and 2·45.
| OLR without grouping categories 5 and 6 (1000 simulations) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H0: OR = 1 (Δ = 0) | H1: OR = 1·62 (Δ = 0·10) | H1: OR = 2 (Δ = 0·15) | H1: OR = 2·45 (Δ = 0·2) | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 29·1% | 0·5% | 29·6% | 1·2% | 18·0% | 19·2% | 0·0% | 42·4% | 42·4% | 0·0% | 70·0% | 70·0% |
| 346 | 173 | 49·6% | 0·9% | 50·5% | 3·2% | 41·6% | 44·8% | 0·3% | 47·7% | 48·0% | 0·0% | 29·5% | 29·5% |
| 518 | 259 | 16·0% | 0·7% | 16·7% | 4·7% | 20·4% | 25·1% | 0·1% | 8·6% | 8·7% | 0·0% | 0·5% | 0·5% |
| 690 | 345 | 2·9% | 0·3% | 3·2% | 2·2% | 8·7% | 10·9% | 0·0% | 0·9% | 0·9% | 0·0% | 0·0% | 0·0% |
| 97·6% | 2·4% | 100% | 11·3% | 88·7% | 100% | 0·4% | 99·6% | 100% | 0·0% | 100% | 100% | ||
| Prob(N > 518) | 2·9% | 0·3% | 3·2% | 2·2% | 8·7% | 10·9% | 0·0% | 0·9% | 0·9% | 0·0% | 0·0% | 0·0% | |
| Fixed sample size | – | 564 | 270 | 162 | |||||||||
| Expected Ssize | 335 | 394 | 291 | 226 | |||||||||
| OLR grouping categories 5 and 6 (1000 simulations) | |||||||||||||
| H0: OR = 1 | H1: OR = 1·62 | H1: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 29·7% | 0·3% | 30·0% | 1·2% | 17·6% | 18·8% | 0·0% | 42·4% | 42·4% | 0·0% | 69·8% | 69·8% |
| 346 | 173 | 49·6% | 0·6% | 50·2% | 3·9% | 40·1% | 44·0% | 0·4% | 46·7% | 47·1% | 0·0% | 29·6% | 29·6% |
| 518 | 259 | 15·2% | 1·0% | 16·2% | 5·2% | 22·0% | 27·2% | 0·1% | 9·4% | 9·5% | 0·0% | 0·6% | 0·6% |
| 690 | 345 | 3·1% | 0·5% | 3·6% | 2·0% | 8·0% | 10·0% | 0·0% | 1·0% | 1·0% | 0·0% | 0·0% | 0·0% |
| 97·6% | 2·4% | 100% | 12·3% | 87·7% | 100% | 0·5% | 99·5% | 100% | 0·0% | 100% | 100% | ||
| Prob(N > 518) | 3·1% | 0·5% | 3·6% | 2·0% | 8·0% | 10·0% | 0·0% | 1·0% | 1·0% | 0·0% | 0·0% | 0·0% | |
| Fixed sample size | – | 564 | 270 | 162 | |||||||||
| Expected Ssize | 335 | 395 | 293 | 227 | |||||||||
| Discrepancies | |||||||||||||
| H0: OR = 1 | H1: OR = 1·62 | H0: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 0·6% | −0·2% | 0·4% | 0·0% | −0·4% | −0·4% | 0·0% | 0·0% | 0·0% | 0·0% | −0·2% | −0·2% |
| 346 | 173 | 0·0% | −0·3% | −0·3% | 0·7% | −1·5% | −0·8% | 0·1% | −1·0% | −0·9% | 0·0% | 0·1% | 0·1% |
| 518 | 259 | −0·8% | 0·3% | −0·5% | 0·5% | 1·6% | 2·1% | 0·0% | 0·8% | 0·8% | 0·0% | 0·1% | 0·1% |
| 690 | 345 | 0·2% | 0·2% | 0·4% | −0·2% | −0·7% | −0·9% | 0·0% | 0·1% | 0·1% | 0·0% | 0·0% | 0·0% |
| OLR without grouping categories 5 and 6 (1000 simulations) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H0: OR = 1 (Δ = 0) | |||||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 29·1% | 0·5% | 29·6% | 1·7% | 13·6% | 15·3% | 0·0% | 37·8% | 37·8% | 0·0% | 67·5% | 67·5% |
| 346 | 173 | 49·6% | 0·9% | 50·5% | 4·4% | 37·2% | 41·6% | 0·1% | 49·4% | 49·5% | 0·0% | 30·9% | 30·9% |
| 518 | 259 | 16·0% | 0·7% | 16·7% | 5·0% | 24·2% | 29·2% | 0·3% | 11·2% | 11·5% | 0·0% | 1·6% | 1·6% |
| 690 | 345 | 2·9% | 0·3% | 3·2% | 4·0% | 9·9% | 13·9% | 0·1% | 1·1% | 1·2% | 0·0% | 0·0% | 0·0% |
| 97·6% | 2·4% | 100% | 15·1% | 84·9% | 100% | 0·5% | 99·5% | 100% | 0·0% | 100% | 100% | ||
| Prob(N > 518) | 2·9% | 0·3% | 3·2% | 4·0% | 9·9% | 13·9% | 0·1% | 1·1% | 1·2% | 0·0% | 0·0% | 0·0% | |
| Fixed sample size | – | 564 | 270 | 162 | |||||||||
| Expected Ssize | 335 | 418 | 305 | 233 | |||||||||
| OLR grouping categories 5 and 6 (1000 simulations) | |||||||||||||
| H0: OR = 1 | H1: OR = 1·62 | H1: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 29·7% | 0·3% | 30·0% | 1·3% | 16·6% | 17·9% | 0·0% | 41·2% | 41·2% | 0·0% | 70·6% | 70·6% |
| 346 | 173 | 49·6% | 0·6% | 50·2% | 3·5% | 41·0% | 44·5% | 0·0% | 48·1% | 48·1% | 0·0% | 28·2% | 28·2% |
| 518 | 259 | 15·2% | 1·0% | 16·2% | 3·2% | 24·4% | 27·6% | 0·3% | 9·6% | 9·9% | 0·0% | 1·2% | 1·2% |
| 690 | 345 | 3·1% | 0·5% | 3·6% | 3·2% | 6·8% | 10·0% | 0·1% | 0·7% | 0·8% | 0·0% | 0·0% | 0·0% |
| 97·6% | 2·4% | 100% | 11·2% | 88·8% | 100% | 0·4% | 99·6% | 100% | 0·0% | 100% | 100% | ||
| Prob(N > 518) | 3·1% | 0·5% | 3·6% | 3·2% | 6·8% | 10·0% | 0·1% | 0·7% | 0·8% | 0·0% | 0·0% | 0·0% | |
| Fixed sample size | – | 564 | 270 | 162 | |||||||||
| Expected Ssize | 335 | 397 | 295 | 227 | |||||||||
| Discrepancies | |||||||||||||
| H0: OR = 1 | H1: OR = 1·62 | H0: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 0·6% | −0·2% | 0·4% | −0·4% | 3·0% | 2·6% | 0·0% | 3·4% | 3·4% | 0·0% | 3·1% | 3·1% |
| 346 | 173 | 0·0% | −0·3% | −0·3% | −0·9% | 3·8% | 2·9% | −0·1% | −1·3% | −1·4% | 0·0% | −2·7% | −2·7% |
| 518 | 259 | −0·8% | 0·3% | −0·5% | −1·8% | 0·2% | −1·6% | 0·0% | −1·6% | −1·6% | 0·0% | −0·4% | −0·4% |
| 690 | 345 | 0·2% | 0·2% | 0·4% | −0·8% | −3·1% | −3·9% | 0·0% | −0·4% | −0·4% | 0·0% | 0·0% | 0·0% |
| OLR without grouping categories 5 and 6 (1000 simulations) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H0: OR = 1 | H1: OR = 1·62 | H1: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 29·1% | 0·5% | 29·6% | 0·7% | 19·2% | 19·9% | 0·1% | 46·2% | 46·3% | 0·0% | 75·3% | 75·3% |
| 346 | 173 | 49·6% | 0·9% | 50·5% | 2·9% | 44·6% | 47·5% | 0·2% | 45·5% | 45·7% | 0·0% | 24·3% | 24·3% |
| 518 | 259 | 16·0% | 0·7% | 16·7% | 3·1% | 21·4% | 24·5% | 0·0% | 7·6% | 7·6% | 0·0% | 0·4% | 0·4% |
| 690 | 345 | 2·9% | 0·3% | 3·2% | 2·2% | 5·9% | 8·1% | 0·0% | 0·4% | 0·4% | 0·0% | 0·0% | 0·0% |
| 97·6% | 2·4% | 100% | 8·9% | 91·1% | 100% | 0·3% | 99·7% | 100% | 0·0% | 100% | 100% | ||
| Prob(N > 518) | 2·9% | 0·3% | 3·2% | 2·2% | 5·9% | 8·1% | 0·0% | 0·4% | 0·4% | 0·0% | 0·0% | 0·0% | |
| Fixed sample size | – | 564 | 270 | 162 | |||||||||
| Expected Ssize | 335 | 382 | 281 | 217 | |||||||||
| OLR grouping categories 5 and 6 (1000 simulations) | |||||||||||||
| H0: OR = 1 | H1: OR = 1·62 | H1: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 29·7% | 0·3% | 30·0% | 0·4% | 22·6% | 23·0% | 0·0% | 50·2% | 50·2% | 0·0% | 78·5% | 78·5% |
| 346 | 173 | 49·6% | 0·6% | 50·2% | 2·1% | 46·7% | 48·8% | 0·1% | 43·1% | 43·2% | 0·0% | 21·2% | 21·2% |
| 518 | 259 | 15·2% | 1·0% | 16·2% | 2·3% | 19·6% | 21·9% | 0·0% | 6·3% | 6·3% | 0·0% | 0·3% | 0·3% |
| 690 | 345 | 3·1% | 0·5% | 3·6% | 1·5% | 4·8% | 6·3% | 0·0% | 0·3% | 0·3% | 0·0% | 0·0% | 0·0% |
| 97·6% | 2·4% | 100% | 6·3% | 93·7% | 100% | 0·1% | 99·9% | 100% | 0·0% | 100% | 100% | ||
| Prob(N > 518) | 3·1% | 0·5% | 3·6% | 1·5% | 4·8% | 6·3% | 0·0% | 0·3% | 0·3% | 0·0% | 0·0% | 0·0% | |
| Fixed sample size | – | 564 | 270 | 162 | |||||||||
| Expected Ssize | 335 | 366 | 272 | 211 | |||||||||
| Discrepancies | |||||||||||||
| H0: OR = 1 | H1: OR = 1·62 | H0: OR = 2 | H1: OR = 2·45 | ||||||||||
| N | n | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both | Futility | Positive | Both |
| 174 | 87 | 0·6% | −0·2% | 0·4% | −0·3% | 3·4% | 3·1% | −0·1% | 4·0% | 3·9% | 0·0% | 3·2% | 3·2% |
| 346 | 173 | 0·0% | −0·3% | −0·3% | −0·8% | 2·1% | 1·3% | −0·1% | −2·4% | −2·5% | 0·0% | −3·1% | −3·1% |
| 518 | 259 | −0·8% | 0·3% | −0·5% | −0·8% | −1·8% | −2·6% | 0·0% | −1·3% | −1·3% | 0·0% | −0·1% | −0·1% |
| 690 | 345 | 0·2% | 0·2% | 0·4% | −0·7% | −1·1% | −1·8% | 0·0% | −0·1% | −0·1% | 0·0% | 0·0% | 0·0% |
On those three scenarios, two analysis strategies were compared: considering the full mRS scale with the 6 possible cut-points vs. just 5 (after pooling values 5 and 6). As it was expected, both analyses performed better on the scenarios were their assumptions better hold: the first analysis on scenario A (with almost negligible differences), and the second on B and C (with more relevant advantages). As scenarios B and C are both more realistic and more desired for patients and care providers, the analysis pooling values 5 and 6 was chosen as the final analysis. Its power on the 3 considered scenarios (87·7%, 88·8% and 93·7%) was considered reasonable by the steering committee.
In case of missing scale value and successful contact with patients or relatives, last observation scale value will be carried forward. Worst scale values will be assigned to any documented death or nonsuccessful contact.
Secondary efficacy analyses
To check the consistency of the results under hypothetical unmasking of the local assessor of mRS, the primary analysis will be repeated for the central mRS evaluator. As less reliability is expected for a single ratter, concordance will be based on point and interval estimates and non in significant p values. The common cumulative OR assumption of the modified Rankin scale will be visually evaluated and estimates for any possible cut-point will be also provided. The consistency of the results under alternative missing value assumptions will also be studied 13.
Capture of REVASCAT eligible patients treated outside of the trial
A unique feature in the design of REVASCAT is the existence of a mechanism that enables determination of compliance on the part of the four treating centers with their commitment to enroll all eligible patients. This important requirement ensures minimization of selection bias. The prospective SONIIA registry is an ongoing, government mandated population based database of all reperfusion therapies for acute ischemic stroke in Catalonia that includes endovascular therapies. Because participation in the SONIIA database is government mandated and audited, the capture rate of endovascular acute stroke therapies in this registry is 100%. Entry of endovascular acute stroke cases in the SONIIA registry is blinded to whether the patient is enrolled or not in REVASCAT. However, since the vast majority of acute stroke patients treated with endovascular therapy in the autonomous community of Catalunya are being treated by the four participating hospitals, determination of patients enrolled in REVASCAT relative to the total number of patients meeting REVASCAT eligibility criteria entered in the SONIIA registry will enable continuous monitoring of the true proportion of eligible patients treated outside of the trial. This feature distinguishes REVASCAT from all previous randomized endovascular stroke trials that have been criticized for the fact that the majority of eligible patients were thought to have been treated outside of the trial. Continuous feedback on patients treated outside the trial in REVASCAT will allow the possibility of implementing corrective actions in case of noncompliance. In addition, following completion of REVASCAT, comparison of the primary and secondary outcome end-points between the trial control group and patients treated with endovascular reperfusion therapies outside the REVASCAT trial will provide information of external validity.
Analysis of safety end-points
Mortality at 90 days will be assessed for all enrolled subjects. A Kaplan–Meier analysis will be done and the mortality rate in each arm at 90 days with accompanying 95% confidence intervals will be reported. Every attempt will be made to determine the status of each subject who withdraws from the study so that the withdrawal data can be used as a censoring point.
There is currently no standard for defining clinically significant or symptomatic ICH that is universally accepted. Thus, in addition to the definition above, it is anticipated that in the final analysis, other alternative definitions for symptomatic ICH may also be used and analyzed separately for comparison with other studies and historical literature (e.g. NINDS, ECASS III, MultiMERCI). The CEC will be tasked with defining the criteria to be used in the primary analysis of symptomatic ICH.
A descriptive analysis of study-defined adverse events will be presented in aggregate and by event. The rates and 95% exact Clopper-Pearson confidence intervals will be provided.
Acknowledgements
Trial Steering Committee: A. Dávalos (co-chairman), T. Jovin (co-chaiman), A. Chamorro, E. Cobo (biostatistician), M.A. de Miquel, C. Molina, A. Rovira, L. San Román, J. Serena. Data Safety Monitoring Board: G. Albers (chairman), K. Lees, J. Arenillas, R. Roberts (biostatistician). Crinical Events Committee: J. Martí-Fábregas, B. Jankowitz. Neuroimaging Core Lab: M. Goyal, A. Demchuck. Angiogram Core Lab: R. von Kummer. Principal Investigators (centers): M. Ribó (Hospital Vall d'Hebrón), P. Cardona (Hospital de Bellvitge), A. Chamorro (Hospital Clínic), M. Millán (Hospital Germans Trias I Pujol), Barcelona, Spain.




