Peripheral blood mononuclear cells-expressed miRNA profiles derived from children with metabolic-associated fatty liver disease and insulin resistance
Funding information: Department of Economic Development of Government of Navarra, Grant/Award Numbers: 0011-1365-2019-000000, 0011-1365-2020-00140; Instituto de Salud Carlos III, Grant/Award Number: PI13/01335; Spanish Ministry of Economy and Competitiveness, Grant/Award Number: BES-2017-080770; Spanish Ministry of Industry and Competitiveness, Grant/Award Number: DEP2016-78377-R
Summary
Background
miRNA have been proposed as potential biomarkers of metabolic diseases.
Objectives
To identify potential miRNA biomarkers of early metabolic-associated fatty liver disease (MAFLD) and/or insulin resistance (IR) in preadolescent children.
Methods
A total of 70 preadolescents, aged 8.5–12 years old participated in the study. Hepatic fat was assessed by magnetic resonance imaging. Fasting blood biochemical parameters were measured and HOMA-IR calculated. Peripheral blood mononuclear cells (PBMC)-derived miRNA profiles associated with MAFLD (≥5.5% hepatic fat) and IR (HOMA-IR ≥2.5) were identified using untargeted high-throughput miRNAs sequencing (RNA-seq).
Results
A total of 2123 PBMC-derived miRNAs were identified in children with (21.4%) or without MAFLD. Among them, hsa-miR-143-3p, hsa-miR-142-5p and hsa-miR-660-5p were up-regulated, and p-hsa-miR-247, hsa-let-7a-5p and hsa-miR-6823-3p down-regulated. Importantly, children with MAFLD had consistently higher miR-660-5p expression levels than their peers without it (p < 0.01), regardless of weight status. A total of 2124 PBMC-derived miRNA were identified in children with IR (28.6%) versus children without IR, where thirteen of them were dysregulated (p < 0.05) in children with IR. In addition, children with IR showed higher levels of miR-374a-5p and miR-190a-5p (p < 0.01) and lower levels of miR-4284 and miR-4791 (p < 005), than their peers without IR in both the whole sample and in those with overweight or obesity.
Conclusions
Our study results suggest circulating miR-660-5p as a potential biomarker of the presence of MAFLD in preadolescent children while circulating miR-320a, miR-142-3p, miR-190a-5p, miR-374a-5p and let-7 family miRNAs could serve as potential biomarkers of IR in children.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate transaminase
-
- BMI
-
- body mass index
-
- GGT
-
- gamma-glutamyl-transferase
-
- HbA1c
-
- glycated haemoglobin
-
- HDL
-
- high-density lipoprotein
-
- HOMA-IR
-
- homeostasis model assessment of insulin resistance
-
- IR
-
- insulin resistance
-
- LDL
-
- low-density lipoprotein
-
- MAFLD
-
- metabolic-associated fatty liver disease
-
- miRNA
-
- microRNA
-
- MRI
-
- magnetic resonance imaging
-
- NW
-
- normal weight
-
- OB
-
- obesity
-
- OW
-
- overweight
-
- SPSS
-
- statistical package for social sciences
-
- T2D
-
- type 2 diabetes mellitus
-
- TG
-
- triglycerides
1 INTRODUCTION
Metabolic-associated fatty liver disease (MAFLD) is the most common liver disorder and the second most common cause of liver transplantation.1 MAFLD has been considered the hepatic manifestation of metabolic syndrome and of systemic insulin resistance (IR).2 The interaction between IR and MAFLD cause a vicious circle, where IR has been determined as one of the inductors of MAFLD, increasing hepatic de novo lipogenesis and impairing insulin-mediated suppression of adipose tissue lipolysis by inducing free fatty acids flux into the liver.3, 4 In turn, MAFLD has been also directly associated with the aggravation of IR and, in consequence, with an increased risk of developing type 2 diabetes (T2D), already in childhood.3, 5
The MAFLD term has been recently agreed among different expert groups in order to reflect more accurately the current knowledge of fatty liver disease associated with metabolic dysfunction.6, 7 The definition of paediatric MAFLD is based on the evidence of intrahepatic fat accumulation in addition to one of the following three criteria: excess overall adiposity, presence of prediabetes or T2D, or evidence of metabolic dysregulation defined as the presence of at least two cardiometabolic risks according to sex and age percentiles.7 It is estimated that MAFLD is present in nearly 10% of general paediatric population8 and in 30% of children with overweight or obesity.9
The development and progression of paediatric MAFLD is complex and multifactorial, and the underlying mechanisms have not been entirely elucidated.10 However, there is evidence that dietary habits, environmental and genetic factors can lead to the development of metabolic alterations directly associated with hepatic fat accumulation and inflammation.11, 12 Although this disease is reversible and easily treatable in the early stages, its asymptomatic evolution, together with its high prevalence and costly (magnetic resonance imaging, MRI) and/or invasive (liver biopsy) diagnosis methods make early identification and treatment difficult.11 For that reason, the search for potential biomarkers has become a priority line in MAFLD research. Nowadays, there is evidence that excess adiposity and lifestyle factors such as sugar-rich diets and sedentary behaviours are strong risk factors for the development and progression of hepatic steatosis through epigenetic mechanisms.10, 13, 14
MicroRNAs (miRNAs), one of the major forms of epigenetic modulation, are short, noncoding RNA molecules (21–23 nucleotides) that have been proposed as potential biomarkers and therapeutic targets for MAFLD14, 15 and type 2 diabetes in adults.16 In children, there are still few studies examining the miRNAs expression levels in relationship with obesity-related comorbidities, IR or MAFLD.17-24 These studies, however, were performed through targeted analysis of several candidate miRNAs previously identified in adult studies.19-24 To date, as far as we are aware, there are no previous studies developed through a high-throughput untargeted search of miRNAs in paediatric population with MAFLD and/or IR. Therefore, the main objective of the present work was to identify potential miRNA biomarkers of early MAFLD and/or IR in preadolescent children, and, secondly, to analyse the associations of miRNA expression levels with cardiometabolic risk factors.
2 METHODS
2.1 Study design and participants
This cross-sectional formed part of the PREDIKID project (ClinicalTrials.gov ID: NCT03027726) whose overall aims were: (1) to evaluate the effect of a 22-week family-based multidisciplinary intervention program including exercise on insulin resistance syndrome (IRS) risk in children with a high risk of developing T2D, and (2) to identify the profile of microRNA in peripheral blood mononuclear cells in children with a high risk of developing type 2 diabetes, and its response to a multidisciplinary intervention program including exercise. Details of sample calculation, randomization, the characteristics of the study participants, methodological procedures and measurements taken are available elsewhere.25
For the current proposal, baseline data of 70 preadolescent children aged 8.5–12 years old and with complete and valid data on MRI-diagnosed hepatic steatosis (5.5% hepatic fat), IR and miRNA levels were analysed. Having other hepatic pathologies such as viral hepatitis, toxic hepatitis or autoimmune diseases were considered as exclusion criteria.
The study protocol, which complies with the ethical guidelines of the Declaration of Helsinki (2013 revision), was approved by The Euskadi Clinical Research Ethics Committee. Participants were recruited at the Paediatric Endocrinology Unit of the University Hospital of Araba, and at primary care clinics. The parents or legal guardians of each childprovided written, informed consent.
2.2 Measurements
2.2.1 Hepatic fat and insulin resistance
Hepatic fat percentage was assessed by MRI using a Magnetom Avanto system (Siemens Healthcare, Erlangen, Germany).25 The presence of MAFLD was determined as a hepatic fat percent ≥5.5%26 in addition to one of the three following criteria: overweight or obesity, presence of prediabetes or T2D, or as evidence of metabolic dysregulation defined as the presence of at least two cardiometabolic risks according to sex and age percentiles.7 The homeostasis model assessment of insulin resistance [HOMA-IR = insulin (mU/L) × glucose (mmol/L)/22.5] was calculated by fasting serum concentrations of glucose and insulin.27 HOMA-IR ≥2.5 determined the presence of IR.
2.2.2 Anthropometric and biochemical parameters
Body mass (SECA 760), height (SECA 220), and waist circumference (SECA 201) were measured in duplicate following standard protocols. Thereafter, the body mass index (BMI) (kg/m2) and the waist-to-height ratio (WHtR) were calculated.28 Weight status was defined according to the body mass index (BMI) age and sex-specific cut-off values provided by Word Obesity Federation.29
The plasma concentrations of cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), glycated haemoglobin (HbA1c), glucose, insulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl-transferase (GGT) were measured in fasting blood samples serum using standard protocols.25
2.2.3 RNA purification and miRNA analysis
Total RNA from peripheral blood mononuclear cells was isolated using RNAeasy Kit (Quiagen). miRNAs profiles were analysed using specific RNA-seq methodology. Briefly, gene libraries were prepared using TruSep Small RNA Sample preparation kit (Illumina, Inc) following manufacturer's instructions. Libraries with 145–160 bp size were selected to undergo deep sequencing on Illumina's MiSeq Next Generation Sequencing system. Sequencing reactions were performed on Illumina's MiSeq Reagent Kit V3. Analysis of results was pre-processed and analysed using MiSeq Reporter, Bowtie, SAMtools and miRDeep software tools; as well as R/Bioconductor packages.
2.3 Bioinformatic analysis
Assignation of mapped sequencing reads to miRNA expression data using miRbase version 21 database was performed with featureCounts R function.30 Differential expression of miRNAs was tested using DESeq2 R package.31
2.4 Statistical analysis
Differences in anthropometric and clinical characteristics between children with or without MRI-diagnosed MAFLD and between children with or without HOMA-IR determined IR were analysed using the independent t-test or x2 test. T-test was performed to analyse differences in miRNAs expression between: (i) children with or without MAFLD, and (ii) children with or without IR. The miRNA expression levels were log2- transformed for analysis. Partial correlations were performed to examine the association between miRNAs expression levels and biochemical parameter concentrations adjusting for sex, age and BMI. Statistical analyses were carried out with statistical software SPSS v.23.0 (IBM, Armonk, New York). Significance was set at α = 0.05.
3 RESULTS
Clinical and anthropometric characteristics of participants according to the presence (21.4%) or absence of MAFLD, and to the presence (28.6%) or absence of IR are shown in Table 1. Children with MAFLD had significantly higher waist-to-height ratio, diastolic blood pressure and lower HDL, than their peers without MAFLD (Table 1). TG and ALT levels tended to be higher in children with MAFLD (p < 0.07) when compared to those without MAFLD. Children with IR had significantly higher weight, BMI, TG, glucose and insulin levels and lower HDL levels than their peers without IR (Table 1).
| Non-MAFLD | MAFLD | p | Non-insulin resistance | Insulin resistance | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||
| Characteristics | ||||||||||
| Age (years) | 55 | 11.3 (1.2) | 15 | 10.6 (1.0) | 0.025 | 50 | 11.0 (1.2) | 20 | 11.5 (1.1) | 0.100 |
| Girls (N%) | 55 | 31. 56 | 15 | 7. 47 | 0.352 | 50 | 24. 48 | 20 | 14. 70 | 0.116 |
| Body composition | ||||||||||
| Height (cm) | 55 | 149.4 (7.3) | 15 | 146.8 (9.0) | 0.299 | 50 | 148.1 (7.5) | 20 | 150.88 (7.9) | 0.182 |
| Weight (kg) | 55 | 54.1 (9.4) | 15 | 55.7 (15.4) | 0.623 | 50 | 52.2 (8.4) | 20 | 60.05 (14.1) | 0.006 |
| Body mass index (kg/m2) | 55 | 24.2 (3.2) | 15 | 25.4 (4.5) | 0.335 | 50 | 23.8 (2.9) | 20 | 26.07 (4.5) | 0.013 |
| NW/OW/OB (N/%) | 55 | 8,27,20.15,49,36 | 15 | 2,4,9.13,27,60 | 50 | 8, 23,19/16,46,38 | 20 | 2,8,10/10,40,50 | ||
| Waist to height ratio (×100) | 55 | 50.18 (4.30) | 15 | 53.60 (5.89) | 0.010 | 50 | 50.0 (0.49) | 20 | 52.0 (0.48) | 0.246 |
| Hepatic fat (%) | 55 | 3.7 (0.9) | 15 | 9.3 (3.7) | <0.001 | 50 | 4.7 (2.8) | 20 | 5.3 (3.3) | 0.474 |
| Blood pressure | ||||||||||
| Systolic (mmHg) | 55 | 95 (10) | 15 | 95 (8) | 0.863 | 50 | 95 (9) | 20 | 94 (11) | 0.727 |
| Diastolic (mmHg) | 55 | 61 (7) | 15 | 65 (6) | 0.028 | 50 | 61 (7) | 20 | 64 (6) | 0.052 |
| MAP (mmHg) | 55 | 84 (8) | 15 | 85 (6) | 0.578 | 50 | 84 (8) | 20 | 84 (7) | 0.820 |
| Biochemical parameters | ||||||||||
| Cholesterol (mg/dL) | 55 | 162.6 (25.5) | 15 | 155.7 (34.6) | 0.485 | 50 | 164.4 (29.3) | 20 | 152.7 (21.6) | 0.071 |
| High-density lipoprotein (mg/dL) | 55 | 51.1 (11.8) | 15 | 43.7 (7.1) | 0.004 | 50 | 52.1 (11.7) | 20 | 43.2 (7.0) | <0.001 |
| Low-density lipoprotein (mg/dL) | 55 | 96.8 (21.1) | 15 | 94.5 (30.8) | 0.783 | 50 | 98.6 (24.9) | 20 | 90.6 (17.9) | 0.139 |
| Triglycerides (mg/dL) | 55 | 72.9 (31.6) | 15 | 87.6 (24.6) | 0.065 | 50 | 68.7 (26.2) | 20 | 94.4 (34.1) | 0.005 |
| HbA1c_IFCC (mmol/mol) | 37 | 35.6 (3.1) | 12 | 36.0 (3.3) | 0.696 | 34 | 35.3 (3.3) | 15 | 36.5 (2.6) | 0.206 |
| Glucose (mg/dL) | 55 | 84.7 (5.3) | 15 | 84.7 (5.8) | 0.989 | 50 | 83.1 (5.2) | 20 | 88.6 (3.7) | <0.001 |
| Insulin (μl/ml) | 55 | 10.4 (4.9) | 15 | 13.0 (5.5) | 0.121 | 50 | 8.5 (2.3) | 20 | 17.0 (5.1) | <0.001 |
| HOMA-IR | 55 | 2.20 (1.12) | 15 | 2.74 (1.23) | 0.114 | 50 | 1.76 (0.48) | 20 | 3.72 (1.17) | <0.001 |
| Aspartate aminotransferase (U/L) | 55 | 23.1 (4.3) | 15 | 24.5 (4.3) | 0.287 | 50 | 23.9 (4.2) | 20 | 22.1 (4.6) | 0.131 |
| Alanine aminotransferase (U/L) | 55 | 18.6 (5.0) | 15 | 25.2 (12.4) | 0.060 | 50 | 20.4 (8.0) | 20 | 19.0 (6.7) | 0.452 |
| Gamma-glutamyl-transferase (U/L) | 55 | 14.3 (3.6) | 15 | 16.7 (5.1) | 0.100 | 50 | 14.6 (4.5) | 20 | 15.5 (2.8) | 0.295 |
- Abbreviations: HbA1c, glycated haemoglobin; HOMA-IR, homeostatic model assessment; IR, insulin resistance; MAFLD, metabolic-associated fatty liver disease; MAP, mean arterial pressure; NW, normal weight, OB, obesity; OW, overweight. Bold values indicate p value < 0.05.
A total of 2123 circulating miRNAs were identified in our sample of children with or without MAFLD (Table S1), where six of them were significantly dysregulated (p < 0.05) in children with MAFLD – hsa-miR-143-3p, hsa-miR-142-5p and hsa-miR-660-5p were up-regulated, and p-hsa-miR-247, hsa-let-7a-5p and hsa-miR-6823-3p were down-regulated (Table 2). We observed that miR-660-5p expression levels were consistently higher in children with MAFLD than in their peers without it (Table 3). Thus, we observed similar results in the whole sample (p < 0.01), and when we analysed separately those children with overweight or obesity (p < 0.05) and children with normal weight (p < 0.02). In addition, MAFLD was significantly related to higher let-7a-5p, miR-142-5p and miR-142-5p expression levels only in children with normal weight.
| miRNAs | Fold change (log2) | p |
|---|---|---|
| Children with MAFLD versus children without MAFLD (N = 70) | ||
| p-hsa-miR-247 | −1.00 | 0.010 |
| hsa-let-7a-5p | −0.56 | 0.019 |
| hsa-miR-143-3p | 0.70 | 0.027 |
| hsa-miR-142-5p | 0.50 | 0.046 |
| hsa-miR-6823-3p | −0.88 | 0.047 |
| hsa-miR-660-5p | 0.51 | 0.049 |
| Children with IR versus children without IR (N = 70) | ||
| hsa-miR-320a | 1.02 | 0.002 |
| hsa-let-7d-5p | 0.87 | 0.002 |
| hsa-miR-4284 | −1.03 | 0.002 |
| hsa-let-7a-5p | 0.61 | 0.007 |
| hsa-miR-374a-5p | 0.69 | 0.009 |
| hsa-let-7 g-5p | 0.58 | 0.012 |
| hsa-miR-185-5p | 0.65 | 0.014 |
| hsa-miR-142-3p | 0.50 | 0.021 |
| hsa-let-7b-5p | 0.60 | 0.029 |
| hsa-miR-15b-5p | 0.61 | 0.029 |
| hsa-miR-4791 | −0.71 | 0.033 |
| hsa-let-7f-5p | 0.34 | 0.037 |
| hsa-miR-190a-5p | 0.54 | 0.038 |
- Abbreviations: IR, insulin resistance; MAFLD, metabolic-associated fatty liver disease. Bold values indicate p value < 0.05.
| Children without MAFLD | Children with MAFLD | p | Non-MAFLD and normal weight | MAFLD and normal weight | p | Non-MAFLD and overweight/obesity | MAFLD and overweight/obesity | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | ||||
| miR-247 | 55 | 3.12 (1.96) | 15 | 2.22 (1.55) | 0.072 | 8 | 2.24 (1.89) | 2 | 2.60 (0.64) | 0.676 | 47 | 3.27 (1.95) | 13 | 2.17 (1.65) | 0.053 |
| let_7a_5p | 55 | 8.75 (0.94) | 15 | 8.49 (0.93) | 0.358 | 8 | 8.54 (0.35) | 2 | 8.11 (0.06) | 0.011 | 47 | 8.78 (1.01) | 13 | 8.55 (1.00) | 0.465 |
| miR-143-3p | 55 | 3.00 (1.71) | 15 | 3.79 (1.73) | 0.131 | 8 | 2.75 (1.30) | 2 | 5.35 (1.42) | 0.188 | 47 | 3.05 (1.78) | 13 | 3.55 (1.68) | 0.357 |
| miR-142-5p | 55 | 6.52 (1.35) | 15 | 7.13 (1.16) | 0.091 | 8 | 6.67 (0.73) | 2 | 8.21 (0.08) | <0.001 | 47 | 6.49 (1.43) | 13 | 6.96 (1.15) | 0.228 |
| miR-6823-3p | 55 | 1.38 (3.29) | 15 | 0.13 (0.51) | 0.149 | 8 | 2.10 (2.80) | 2 | 0.00 (0.00) | 0.340 | 47 | 1.26 (3.37) | 13 | 0.15 (0.54) | 0.247 |
| miR-660-5p | 55 | 1.69 (1.28) | 15 | 2.69 (1.00) | 0.006 | 8 | 1.30 (1.15) | 2 | 3.12 (0.43) | 0.015 | 47 | 1.75 (1.30) | 13 | 2.62 (1.05) | 0.020 |
| Children without IR | Children with IR | p | Non-IR and normal weight | IR and normal weight | p | Non-IR and overweight/Obesity | IR and overweight/Obesity | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | ||||
| miR-320a | 50 | 3.20 (1.78) | 20 | 4.10 (1.64) | 0.054 | 8 | 3.18 (1.59) | 2 | 3.96 (0.45) | 0.268 | 42 | 3.21 (1.83) | 18 | 4.10 (1.73) | 0.079 |
| let-7d-5p | 50 | 4.34 (1.54) | 20 | 5.01 (1.55) | 0.110 | 8 | 4.19 (0.87) | 2 | 3.73 (0.85) | 0.585 | 42 | 4.37 (1.64) | 18 | 5.16 (1.56) | 0.088 |
| miR-4284 | 50 | 1.96 (2.93) | 20 | 0.27 (2.56) | 0.003 | 8 | 1.54 (2.66) | 2 | 0.80 (4.50) | 0.856 | 42 | 2.04 (3.00) | 18 | 0.39 (2.44) | 0.002 |
| let-7a-5p | 50 | 8.62 (0.91) | 20 | 8.91 (1.12) | 0.318 | 8 | 8.52 (0.37) | 2 | 8.23 (0.19) | 0.210 | 42 | 8.64 (0.98) | 18 | 8.98 (1.16) | 0.282 |
| miR-374a-5p | 50 | 2.04 (1.47) | 20 | 3.25 (1.29) | 0.002 | 8 | 2.36 (1.54) | 2 | 3.21 (0.11) | 0.165 | 42 | 1.98 (1.47) | 18 | 3.26 (1.37) | 0.003 |
| let-7 g-5p | 50 | 7.15 (0.98) | 20 | 7.46 (1.17) | 0.308 | 8 | 7.17 (0.65) | 2 | 7.12 (0.09) | 0.872 | 42 | 7.15 (1.04) | 18 | 7.50 (1.23) | 0.305 |
| miR-185-5p | 50 | 2.04 (1.73) | 20 | 2.76 (1.82) | 0.138 | 8 | 1.86 (1.10) | 2 | 2.36 (0.05) | 0.235 | 42 | 2.07 (1.84) | 18 | 2.81 (1.92) | 0.181 |
| miR-142-3p | 50 | 3.29 (1.47) | 20 | 3.93 (1.37) | 0.094 | 8 | 3.47(0.63) | 2 | 4.87 (0.51) | 0.088 | 42 | 3.26 (1.58) | 18 | 3.82 (1.40) | 0.178 |
| let-7b-5p | 50 | 6.43 (1.46) | 20 | 6.94 (1.41) | 0.188 | 8 | 6.25 (0.64) | 2 | 5.74 (0.04) | 0.062 | 42 | 6.47 (1.58) | 18 | 7.07 (1.43) | 0.155 |
| miR-15b-5p | 50 | 4.90 (1.63) | 20 | 5.25 (2.08) | 0.504 | 8 | 5.39 (0.93) | 2 | 4.49 (1.10) | 0.440 | 42 | 4.81 (1.73) | 18 | 5.34 (2.17) | 0.367 |
| miR-4791 | 50 | 3.37 (2.82) | 20 | 1.37 (3.51) | 0.030 | 8 | 2.74 (2.69) | 2 | 2.21 (5.50) | 0.915 | 42 | 3.50 (2.86) | 18 | 1.28 (3.45) | 0.012 |
| let-7f-5p | 50 | 8.68 (0.63) | 20 | 8.77 (0.81) | 0.644 | 8 | 8.76 (0.29) | 2 | 8.34 (0.02) | 0.005 | 42 | 8.66 (0.67) | 18 | 8.82 (0.85) | 0.491 |
| miR-190a-5p | 50 | 0.53 (0.90) | 20 | 1.37 (1.11) | 0.005 | 8 | 0.18 (0.58) | 2 | 2.05 (0.49) | 0.052 | 42 | 0.60 (0.93) | 18 | 1.29 (1.14) | 0.031 |
- Abbreviations: IR, insulin resistance; MAFLD, metabolic-associated fatty liver disease; SD, standard deviation. Bold values indicate p value < 0.05.
When comparing children with IR versus children without IR, a total of 2124 circulating miRNAs were identified (Table S2), where thirteen of them were significantly (p < 0.05) dysregulated in children with IR – hsa-miR-320a, hsa-let-7d-5p, hsa-let-7a-5p, hsa-miR-374a-5p, hsa-let-7 g-5p, hsa-miR-185-5p, hsa-miR-142-3p, hsa-let-7b-5p, hsa-miR-15b-5p, hsa-let-7f-5p and hsa-miR-190a-5p were up-regulated, whereas hsa-miR-4284 and hsa-miR-4791were down-regulated (Table 2). Children with IR showed significantly higher levels of miR-374a-5p and miR-190a-5p (p < 0.01) and lower levels of miR-4284 and miR-4791 (p < 005), than their peers without IR in both the whole sample and in those with overweight or obesity (Table 3). In addition, miR-let-7f levels were negatively associated with IR only in children with normal weight (p < 0.01).
3.1 Association of miRNA expression levels with biochemical parameters
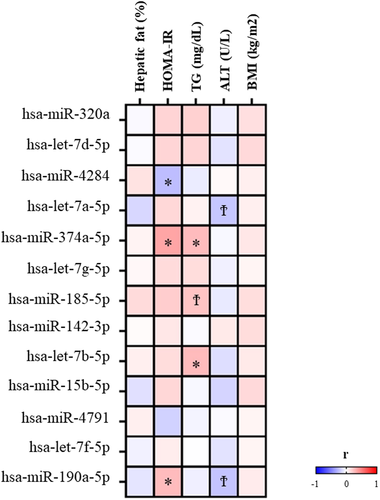
Figures 1 and 2 show the associations of MAFLD and IR, respectively, previously identified miRNA expression levels with cardiometabolic risk factors. Among MAFLD-associated miRNAs, it was observed that lower miR-247 (p = 0.017) and higher miR-660-5p (p = 0.067) expression levels were associated with higher percentage hepatic fat and that higher expression levels of miR-142-5p were correlated with ALT plasma concentrations (p = 0.031). Among IR-associated miRNAs, miR-374a-5p and miR-190a-5p were positively correlated (p = 0.004 and p = 0.035, respectively) and miR-4284 inversely (p = 0.034) associated with HOMA-IR. In addition, miR-374a-5p and let-7b-5p miRNA expression showed significant correlations with TG plasma concentrations (p = 0.035 and p = 0.031, respectively).

4 DISCUSSION
In the present study, we conducted an untargeted high-throughput miRNAs sequencing and specific circulating miRNA profiles associated with MAFLD and IR in preadolescent children were detected.
To date, there is very limited data on the associations of circulating miRNAs with MAFLD. In adults, miR-122 is the most studied miRNA associated with the presence and severity of MAFLD.32 Other miRNAs such as miRNA-99a and miRNA-34a, have also been associated with MAFLD.33, 34 In children, as far as we are aware, there are only three previous studies examining differences in miRNA expression levels between children with and without MAFLD. In contrast to our findings, these studies reported that the miRNA-122 was dysregulated in children with suspected MAFLD. Thus, two previous studies conducted in children and adolescents aged 8–18 years old,19, 24 showed that miR-122 and miR-34a-5p expression levels were significantly elevated in those with obesity and ultrasound-based19 or MRI based24 diagnosed-MAFLD compared with children with overweight or obesity without MAFLD. The association of the miR-122 levels with hepatic enzyme levels was also reported in three European cohorts of pre-pubertal children.20 However, previous studies were conducted following candidate miRNAs analysis of biomarkers of fatty liver in adults, and the untargeted approach for identifying novel biomarkers in children is lacking.
In our study approach of untargeted RNA sequencing, we did not detect significant differences in miR-122 or miR-34a levels between children with and without MRI-diagnosed MAFLD. Our results, however, show consistent associations of the miR-660 with MAFLD in preadolescent children. Indeed, we observed that (i) miR-660 was upregulated in children with MAFLD, (ii) children with MAFLD had higher mean expression levels than children without MAFLD, (iii) the results were consistent in children with overweight/obesity and in children with normal-weight, and (iv) mean expression levels of miR-660 were correlated with hepatic fat percent. Further studies conducted in vitro and in vivo animal models have associated miR-66035 with the proliferation and activation of hepatic stellate cells and liver fibrosis which may explain our findings. These findings suggest that the miR-660-5p could be a potential specific biomarker of MAFLD, independently of the presence of overweight or obesity.
We also observed that the miR-142-5p was upregulated in children with MAFLD, and normal weight than their control peers. These results are in line with studies in vitro and in vivo with animal models showing that the miR-142-5p was related to the accumulation of lipids in the hepatocytes and with increased hepatic steatosis.36 Nevertheless, these findings should be taken with caution. Indeed, we did not find any consistent and significant differences in miR-142-5p in the whole sample of children and mean expression levels of miR-142-5p were not significantly correlated with the percentage of hepatic fat.
Nowadays, there are very few studies analysing circulating levels of miRNAs in children with IR21-24 and the results are controversial. Mohany et al. examined three circulating miRNAs (miR-486, miR-146b and miR-15b) in a sample of 120 children aged 6–14 years.23 The authors reported that the circulating levels of the three miRNAs were significantly higher in children with obesity and with type 2 diabetes compared to either healthy controls or children with obesity but without type 2 diabetes. Lischka et al. analysed the expression of 16 circulating miRNAs in children with severe obesity and observed that circulating levels of two of them, miR-34a and miR-122, were significantly higher in those children with prediabetes.24 In adults and animal models, many other miRNAs have been identified as potential biomarkers of IR or type 2 diabetes. Likewise, according to a meta-analysis of 39 case–control studies, miR-148b, miR-223, miR-130a, miR-19a, miR-26b and miR-27b could be proposed as biomarkers of diabetes.37
In the current study, children with IR had elevated levels of miR-320a. This finding is in concordance with a previous study in children with obesity aged 2.0–5.8 years in which a specific search of 179 mRNAs was conducted.21 In adults, circulating miR-320a has been previously associated with IR and with the progression of prediabetes to diabetes.38, 39 In addition, this miRNA has been proposed as a predictor of the response to several pharmacological therapies for diabetes.38, 39 In mice, it was observed that this miRNA could damage pancreatic b-cells, increase ROS levels and induce β-cell apoptosis.38, 40
We also found that the circulating miR-190a-5p levels were consistently higher in children with IR independently of their weight status and that it was significantly correlated with HOMA-IR. In patients with type 2 diabetes, the miR-190a-5p was associated with the risk of developing diabetic retinopathy41 In animal models, miR-190a-5p expression levels were higher in liver tissues of mice with liver fibrosis than in their respective controls.42
We observed significant differences in mean expression levels of miR-142-3p between children with obesity and with and without IR, in agreement with previous findings in adults43, 44 and children.21 In a sample of 250 school children, Al-rawaf et al. studied the association of specific miRNAs with different parameters associated with metabolic syndrome and reported higher levels of circulating miR-142 in those with higher HOMA-IR.22 The circulating miR-142-3p was also found up-regulated in adults with morbid obesity45 and T2D46 and was proposed as a potential biomarker for acute and chronic inflammation.47
Likewise, we observed that miR-4791 and miR-4284 were down-regulated, and miR-374a-5p was up-regulated in preadolescent with IR and that there were significant differences in mean expression levels between in children with and without IR, either in the whole sample or in children with overweight or obesity, but not in normal weight children. These results suggest that the excess of overall adiposity might be influencing these miRNAs expression levels. There are very few studies examining these miRNAs and most of them have been explored in cancer disease.48-50 Interestingly, in concordance with our results, one previous case–control study in Asian patients with or without prediabetes or T2D, observed that the miR-347a-5p was correlated with HOMA-IR.51
The use of the high-throughput untargeted analysis of circulating miRNAs methodology and the MRI-based diagnosis of MAFLD should be considered as important strengths of the current study. More studies on bigger number of preadolescent girls and boys of multi-ethnic origin and with varied weight status categories are needed before the use of the proposed miRNAs as biomarkers at population and clinical level. Indeed, both pathological conditions, MAFLD and IR, have a strong hereditability that recommends to confirm or refute our findings according to ethnic origin, as well as by sex and weight status categories.
In conclusion, our study findings provide additional knowledge of the possible epigenetic regulation in MAFLD and IR. Disease-specific miRNAs were detected among paediatric population, where miR-660-5p, miR-320a, miR-142-3p, miR-190a-5p, miR-374a-5p and let-7 family miRNAs of special interest. Our study results suggest circulating miR-660-5p as a potential biomarker of the presence of MAFLD in preadolescent children while circulating miR-320a, miR-142-3p, miR-190a-5p, miR-374a-5p and let-7 family miRNAs could serve as potential biomarkers of IR in children.
AUTHOR CONTRIBUTIONS
Maddi Oses collected and analysed the data, drafted the manuscript and takes full responsibility for the integrity of the data analyses, generated the figures, participated in the interpretation of the results, and critically revised the manuscript for important intellectual content. María Medrano collected the data and critically revised the manuscript. Idoia Labayen designed the study, coordinated and supervised data collection, drafted the manuscript, participated in the interpretation of the results, and critically revised the manuscript for important intellectual content. Maddi Oses, María Medrano, Javier Margareto Sánchez, María Puy Portillo, Concepción María Aguilera, Signe Altmäe, and Idoia Labayen critically revised the manuscript for its intellectual content and approved the final version.
FUNDING INFORMATION
This project was funded by the Spanish Ministry of Health “Fondos de Investigación Sanitaria del Instituto de Salud Carlos III” (PI13/01335), the Spanish Ministry of Industry and Competitiveness (DEP2016-78377-R), by EU Fondos Estructurales de la Unión Europea (FEDER) funds (“Una manera de hacer Europa”) and Department of Economic Development of Government of Navarra (0011–1365–2019-000000, 0011–1365–2020-00140). Signe Altmae is supported by Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and European Regional Development Fund (FEDER) grant RYC 2016 21199. Maddi Oses is supported by a grant from the Spanish Ministry of Economy and Competitiveness, grant number; BES-2017-080770.
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.




