A New Diagnostic Approach for Myelodysplastic Neoplasms Using a Combination of Scores Based on Flow Cytometry and Automated Hematology Sysmex XN Analyzers
Funding: The authors received no specific funding for this work.
ABSTRACT
Introduction
The first-step in diagnosis of myelodysplastic neoplasms (MDS) is essentially based on bone marrow cytomorphology. However, cytomorphology of MDS is often a difficult exercise, subject to inter-operator variability. Our study aims to evaluate whether the combination of two dysplasia scores, the extended Ogata score and the MDS-CBC score, could improve the screening of MDS patients among patients with chronic cytopenia.
Methods
Extended Ogata score and MDS-CBC score have been measured on a retrospective cohort of 63 patients with a clinical suspicion of MDS based on the presence of cytopenia. Among these patients, 33 patients were diagnosed as MDS (MDS group) and 30 patients were diagnosed with another cause of cytopenia (non-MDS cytopenic control group).
Results
Our results show excellent performance of the combined scores in predicting MDS when the two scores are concordant: positive predictive value (PPV) = 96% and negative predictive value (NPV) = 92%. In comparison, in the same cohort, extended Ogata score alone showed a PPV = 90% and NPV = 79%, MDS-CBC score alone showed a PPV = 85% and NPV = 86%.
Conclusion
For the first time, our results show that the combination of these two dysplasia scores constitutes a useful and rapid tool for the assessment of dysplasia associated with MDS. In the MDS diagnostic process, the use of combined scores could constitute a valuable tool to enable early strong prediction of MDS in cytopenic patients and to target patients who initially require additional genetic assays.
1 Introduction
Myelodysplastic neoplasms (MDS) are a group of complex and heterogeneous clonal hematopoietic disorders affecting multipotent stem cells. These neoplasms are defined by cytopenia and morphologic dysplasia of hematopoietic myeloid lineage [1]. MDS mainly affects the elderly, with a median age at diagnosis ranging between 70 and 75 years [2]. One of the main complications of MDS is progression to acute myeloid leukemia.
The diagnosis of MDS should be considered in any patient with at least one chronic cytopenia [1]. Currently, bone marrow cytomorphology, remains the reference assay for dysplasia evaluation in the first-step diagnosis approach of MDS. The recommended threshold for dysplasia is set at 10% of cells for all lineages [3]. Genetic abnormalities are frequently associated with MDS but are not specific. However, cytogenetic and molecular assays complete cytomorphology assays for the diagnosis of MDS [1]. The WHO 2022 classification takes into account both cytomorphology and genetics to group MDS entities as those having defining genetic abnormalities and those that are morphologically defined [3]:
“MDS with defining genetic abnormalities” grouping MDS with low blasts and isolated 5q deletion (MDS-5q), MDS with low blasts and SF3B1 mutation (MDS-SF3B1) and MDS with biallelic TP53 inactivation (MDS-biTP53).
“MDS morphologically defined” grouping MDS with low blasts (MDS-LB), MDS, hypoplastic (MDS-h) and MDS with increased blasts (MDS-IB).
Currently, the most widely used prognostic scores, IPSS-R and IPSS-M [4, 5], also take into account the results of cytomorphology, cytogenetic and molecular assays.
The morphological assessment of dysplasia is subjective and operator-dependent [6, 7]. This evaluation is particularly difficult in cases where the signs of dysplasia are minimal, in the absence of blasts or ring sideroblasts excess. However, the assessment of dysplasia is an important issue in first-step diagnosis of MDS, as it provides guidance for further genetic analyses to help clinicians in their diagnostic decisions. Considering the importance and complexity of dysplasia evaluation, more precise and reproductive parameters are needed to optimize the indication of complex, time-consuming and costly genetic complementary assays. Among these parameters, two dysplasia scores have recently stood out for their performance, rapid and relative ease of use: the extended Ogata score using flow cytometry and the MDS-CBC score using Sysmex XN automated hematology systems.
Cell analysis by flow cytometry has been studied for its potential in MDS since the 1990s. Over 20 diagnostic scores composed of immunophenotypic abnormalities observable in various hematopoietic compartments have been published [8]. Due to its relative technical and analytical simplicity, the Ogata score is currently the most widely used in laboratories. The Ogata score takes into account variations of four immunophenotypic parameters: increase in the percentage of CD34+ myeloid progenitors among leukocytes, decrease in the percentage of B-cell progenitors among CD34+ cells, increase or decrease in the ratio between the mean fluorescence intensity (MFI) of CD45 on lymphocytes and myeloid progenitors, and decrease in the ratio between the mode values of SSC of granulocytes and lymphocytes [9]. Nevertheless, the performance of the Ogata score is debatable because it present an average sensitivity ranging between 39% and 76% [9-13]. In order to improves Ogata score sensitivity, Bardet et al. propose the extended Ogata score with two additional parameters: aberrant expression of CD7 on myeloid progenitors and aberrant expression of CD56 on monocytes [10]. The extended Ogata score sensitivity was 75% for MDS without blast excess [10].
More recently, authors have explored the potential of automated cell hematology analyzers parameters to detect dysplasia associated with MDS [14-16]. Thus, dysplasia scores using several parameters of hematology analyzers have been published. Among these scores, the myelodysplastic neoplasms-complete blood count (MDS-CBC) score is calculated with three parameters measured on Sysmex XN analyzers: structural dispersion of neutrophil granulocytes (Ne-WX) a research use only (RUO) parameter, mean corpuscular volume (MCV) and absolute neutrophil count (ANC). Ne-WX and MCV are increased and ANC is decreased in MDS patients [17]. The MDS-CBC score is promising, demonstrating good performances for detection of dysplasia associated with MDS (specificity and sensitivity above 80%) [17-19]. Whereas monocytic score derived from hematology analyzers is widely investigated in CMML [17, 19-22], there are fewer publications available on the MDS-CBC score [17-19]. Additionally, various applications of this score have been proposed: the MDS-CBC score has been studied in order to screen blood smears examination [19], as a diagnostic parameter of MDS in the presence of cytopenias [17, 18].
Our study aims to evaluate for the first time whether the combination of the MDS-CBC and extended Ogata score would improve the prediction of MDS in cytopenic patients and consequently optimize the selection of patients requiring additional genetic exploration (cytogenetic and molecular assays).
2 Materials and Methods
2.1 Patients
Patients were retrospectively included at the Saint-Philibert University Hospital, Lomme (France) from December 2022 to June 2024. Patients with clinical suspicion of MDS, based on the presence of persistent and unexplained cytopenia, were enrolled. The inclusion criteria reflect a real-world diagnostic experience of MDS because these patients underwent explorations for unexplained cytopenia. Complete blood count for calculation of MDS-CBC score, bone marrow cytomorphology and flow cytometry with extended Ogata score were performed for inclusion. According to the WHO 2022 classification [3], patients were further diagnosed as MDS (MDS group) or non-MDS cytopenic control group (non-MDS group) using data from bone marrow cytology, cytogenetic, and molecular assays. Cytogenetic data were available for 43 patients, and molecular data were available for 36 patients. The patients with presence of bone marrow infiltration by a lymphocytic clone or diagnosed with acute leukemia were excluded.
The study was conducted according to the Declaration of Helsinki and was approved by the internal hospital ethics committee.
2.2 Bone Marrow Cytology
Bone marrow smears were stained with May-Grünwald-Giemsa (MGG) stain using the Sysmex SP10 module. The bone marrow differential count was performed by two experimented cytologists on a total of 500 cells. Microscopic examination of bone marrow smears was initially performed at low magnification to assess marrow cellularity and megakaryocyte morphology, followed by examination at high magnification to perform differential cell counts and evaluate the granulocytic and erythroid lineages dysplasia. The characterization of dysplasia for each lineage was performed according to WHO recommendations, with a threshold of 10% dysplastic cells to qualify a lineage as dysplastic [1].
2.3 Flow Cytometry
Flow cytometry was performed on bone marrow samples collected in EDTA tubes. An optimal processing time of 24 h from sample collection was desired, but a maximum delay of 72 h was acceptable [23].
For analysis, 100 μL of bone marrow sample was incubated for 15 min at room temperature and protected from light with the following panel of antibodies: CD7-PC7 (clone: 8H8), CD10-PC5.5 (clone: ALB1), CD14-FITC (clone: J3-119), CD19-ECD (clone: RMO52), CD34-AA700 (clone: N901), CD45-KO (clone: 581), CD56-PE (clone: J33) (Beckman Coulter). Subsequently, erythrocyte lysis using Versalyse solution (Beckman Coulter) was performed according to the manufacturer's technical instructions. After lysis, samples were analyzed on the Navios flow cytometer (Beckman Coulter) with a minimum acquisition of 100.000 CD45+ events [23]. The extended Ogata score was calculated as described in literature, taking into account the following parameters [9, 10, 12]: percentage of CD34+ myeloid progenitors among CD45+ cells (normal threshold < 2%), percentage of B-cell progenitors among CD34+ cells (normal threshold > 5%), ratio: MFI CD45 lymphocytes/MFI CD45 myeloid progenitors (normal range 4–8), ratio: mode SSC granulocytes/mode SSC lymphocytes (normal threshold > 6), percentage of myeloid progenitors with aberrant CD7 expression (normal threshold < 30%), percentage of monocytes with aberrant CD56 expression (normal threshold < 30%).
Each parameter outside the normal range counts as 1 point, and as determined in literature, an extended Ogata score ≥ 2 supports the diagnosis of MDS [9, 10, 12].
Flow cytometry gating was performed using Kaluza software (Beckman Coulter). The gating strategy is available in Figures S1 and S2.
2.4 MDS-CBC Score
The MDS-CBC score was conducted using blood samples collected in EDTA tubes with a mean delay of 3 h and 30 min between sample collection and processing. Measurements of parameters necessary for calculating the MDS-CBC score (ANC, MCV, and NE-WX) were performed by the Sysmex XN module. The MDS-CBC score has been collected after verifying the absence of measurement bias by the analyzer (no contamination of erythrocyte measurements by macroplatelets or small lymphocytes, no incorrect leukocyte classifications).
According to Boutault et al., a MDS-CBC score ≥ 0.2 leads to MDS diagnosis [17]. Zhu et al. propose a positivity threshold of 0.23 [18]. To define the best threshold of the MDS-CBC score to predict MDS related dysplasia in our cohort, we calculated the best Youden index from the ROC curve plot. The optimal threshold was 0.23, which corresponds to the threshold proposed by Zhu et al. [18]. Thus, the score of 0.23 was chosen as the positivity threshold in our study.
2.5 Statistical Analyses
Statistical tests were conducted using R software (Version: 4.4.0). The area under the curve (AUC) of ROC curves and determination of Youden index were performed using the ROCit package [24]. Mean comparisons were conducted using the parametric Student's t-test for large sample sizes (n ≥ 30) or the non-parametric Wilcoxon–Mann–Whitney test for small sample sizes (n < 30). Chi-square test was used to determine the association between categorical variables. For all statistical tests, p < 0.05 was considered statistically significant.
3 Results
3.1 Characteristics of the Study Population
Sixty-three patients were included (36 males and 27 females (sex ratio M/F = 1.33)). The mean age was 71.8 years. All patients had at least one cytopenia (Hb < 13 g/dL for male or Hb < 12 g/dL for female, platelets < 150G/L and ANC < 1.8G/L). Among these patients, 33 were diagnosed with MDS (MDS group) and 30 were diagnosed with other causes of cytopenia (non-MDS group). According to WHO 2022 classification, among the MDS patients, 18 (54%) were classified as MDS-LB, 7 (21%) as MDS-IB1, 1 (3%) as MDS-IB2, 1 (3%) as MDS-5q, 3 (9%) as MDS-SF3B1, and 3 (9%) patients classified as CMML. CMML were included because they are myelodysplastic/myeloproliferative neoplasms and the literature shows that the extended Ogata score is able to detect dysplasia associated with CMML [10]. Furthermore, we have recently shown that the MDS-CBC score can also be positive in oligomonocytic CMML [25]. Among the non-MDS causes of cytopenia, we find eight cytopenia due to other hemopathy (27%), four cytopenia due to hepatic or renal causes (13%), four drug-induced cytopenia (13%), three immune thrombocytopenic purpura (10%), two clonal cytopenia of undetermined significance (CCUS) (7%), one cytopenia due to vitamin B9 deficiency (3%), one thrombotic microangiopathy (3%) and one multifactorial cytopenia (3%). Cytopenia without identified causes were classified as idiopathic cytopenia (n = 6%, 20%) (Table 1). Among the eight patients diagnosed with “other hemopathies,” we identified: two monoclonal gammopathy of undetermined significance (MGUS), one marginal zone lymphoma (MZL), one monoclonal B lymphocytosis (MBL), one diffuse large B-cell lymphoma (DLBCL), one multiple myeloma (MM), one aplastic anemia (AA), and one primary myelofibrosis (PMF).
| MDS (n = 33) | Non-MDS (n = 30) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n (%) | Mean (min–max) | Variable | n (%) | Mean (min–max) | ||
| Age | 75, 2 (51–93) | Age | 67, 5 (23–86) | ||||
| Gender | Male | 19 (58) | Gender | Male | 16 (53) | ||
| Female | 14 (42) | Female | 14 (47) | ||||
| Complete blood count | Hb (g/dL) | 10, 8 (6, 5–14, 8) | Complete blood count | Hb (g/dL) | 12, 4 (8, 2–7, 4) | ||
| MCV (fL) | 100, 2 (74, 6–113, 4) | MCV (fL) | 93, 4 (82, 1–118) | ||||
| Platelets (G/L) | 171 (26–472) | Platelets (G/L) | 187 (3–961) | ||||
| ANC (G/L) | 2, 66 (0, 4–10) | ANC (G/L) | 4, 07 (0,9–9, 4) | ||||
| WHO 2022 classification | 33 | Causes | Drug-induced cytopenia | 4 (13) | |||
| “MDS with defining genetic abnormalities” | MDS-SF3B1 | 3 (9) | |||||
| MDS-5q | 1 (3) | Hepatic or renal insufficiency | 4 (13) | ||||
| “MDS morphologically defined” | MDS-LB | 18 (55) | CCUS | 2 (7) | |||
| MDS-IB1 | 7 (21) | Other hemopathy | 8 (27) | ||||
| MDS-IB2 | 1 (3) | ITP | 3 (10) | ||||
| CMML | 3 (9) | Vitamin B9 deficiency | 1 (3) | ||||
| IPSS-R score | 30 | Thombotic microangiopathy | 1 (3) | ||||
| IPSS-R 1 | Very low | 4 (13) | Multifactor | 1 (3) | |||
| Low | 12 (40) | Idiopathic | 6 (20) | ||||
| Intermediate | 9 (30) | ||||||
| IPSS-R 2 | High | 2 (7) | |||||
| Very high | 3 (10) | ||||||
| IPSS-M score | 23 | ||||||
| IPSS-M 1 | Very low | 4 (17) | |||||
| Low | 9 (39) | ||||||
| Moderate low | 3 (13) | ||||||
| IPSS-M 2 | Moderate high | 1 (4) | |||||
| High | 5 (22) | ||||||
| Very high | 1 (4) | ||||||
- Abbreviations: ANC = absolute neutrophil count; CMML = chronic myelomonocytic leukemia; IB = increased blast; ITP = immune thrombocytopenic purpura; LB = low blast; MCV = mean corpuscular volume.
The mean age was significantly higher in the MDS group than in the non-MDS group (p < 0.01). There was no significant difference observed in the sex ratio (M/F) between the two groups (p = 0.94). MDS group had lower mean levels of hemoglobin (p < 0.05) and absolute neutrophils counts (ANC) (p < 0.05) than non-MDS group. Platelets count was not significantly different in the two groups (p = 0.28) (Table 1).
The IPSS-R prognostic score was calculated for each patient with MDS and the IPSS-M score was also calculated for MDS patients with available molecular data. To obtain larger samples of patients for statistical analyses, we have grouped together sub-categories of low or high-risk patients for each of the two prognosis scores: low-risk group IPSS-R1 (grouping very low, low and intermediate IPSS-R category), high-risk group IPSS-R2 (grouping high and very high IPSS-R category), low-risk group IPSS-M1 (grouping very low, low and moderate low IPSS-M category) and high-risk group IPSS-M2 (grouping moderate high, high and very high IPSS-M category) (Table 1).
3.2 Parameters Composing Extended Ogata Score and MDS-CBC Score
Each parameter composing the extended Ogata score was significantly different between MDS group and non-MDS group. Among these parameters, the decrease in the percentage of B-cell progenitors and the SSC ratio were the most significantly different between MDS group and non-MDS group and the most sensitive parameters to detect MDS (p < 0.001; Se = 0.70 and p < 0.001; Se = 0.55, respectively). The percentage of CD7+ myeloid progenitors and CD56+ monocytes had low sensitivity but were the most specific parameters to detect MDS (Se = 0.15; Sp = 1 and Se = 0.21; Sp = 0.97, respectively) (Figure S3).
Our results also show that each parameter composing the MDS-CBC score was significantly different between MDS and non-MDS groups: Ne-WX and MCV was significantly higher in MDS group than in non-MDS group (p < 0.001 and p < 0.01, respectively) and ANC was significantly lower in MDS group than in non-MDS group (p < 0.01) (Figure S4).
3.3 Results and Performances of Isolated Extended Ogata and MDS-CBC Scores
Among the 33 patients in the MDS group, extended Ogata score was positive in 26 patients (79%) and negative in 7 patients (21%) and MDS-CBC score was positive in 29 patients (88%) and negative in 4 patients (12%) (Table 2).
| Scores | Results | MDS (n = 33) | Non-MDS (n = 30) | Performances |
|---|---|---|---|---|
| Extended Ogata score | Positive (≥ 2) | 26 | 3 | Se = 79%, Sp = 90%, PPV = 90%, NPV = 79% |
| Negative (< 2) | 7 | 27 | ||
| MDS-CBC score | Positive (≥ 0.23) | 29 | 5 | Se = 88%, Sp = 83%, PPV = 85%, NPV = 86% |
| Negative (< 0.23) | 4 | 25 | ||
| Combined scores | ||||
| Combined score only DP and DN patients | DP | 24 | 1 | Se = 92%, Sp = 96%, PPV = 96%, NPV = 92% |
| DN | 2 | 23 | ||
| Combined score considering patients with at least one positive score | DP + SP | 31 | 7 | Se = 94%, Sp = 77%, PPV = 82%, NPV = 92% |
| DN | 2 | 23 | ||
| Combined score considering patients with at least one negative score | DP | 24 | 1 | Se = 73%, Sp = 97%, PPV = 96%, NPV = 76% |
| DN + SP | 9 | 29 | ||
- Abbreviations: DN = double negative, DP = double positive, NPV = negative predictive value, PPV = positive predictive value, Se = sensitivity, SP = simple positive, Sp = specificity.
Among the 30 patients in the non-MDS group, extended Ogata score was positive in 3 patients (10%) and negative in 27 patients (90%) and MDS-CBC score was positive in 5 patients (17%) and negative in 25 patients (83%) (Table 2).
Overall, in our cohort, the performance of the extended Ogata score was as follows: sensitivity (Se) = 79%, specificity (Sp) = 90%, positive predictive value (PPV) = 90%, negative predictive value (NPV) = 79%, and AUC = 0.89 (95%CI = [0.81–0.97]) (Table 2). In the category of MDS without blast excess, sensitivity decreased to 73%, while sensitivity reached 100% in the category of MDS with blast excess. In the CMML group, two out of three cases were positive for the extended Ogata score.
The performance of the MDS-CBC score for the diagnosis of MDS was as follows: Se = 88%, Sp = 83%, PPV = 85%, NPV = 86%, and AUC = 0.87 (95%CI = [0.78–0.96]) (Table 2). In the category of MDS without blast excess sensitivity decreased to 86%, while sensitivity reached 100% in the category of MDS with blast excess. In the CMML group, two out of three cases were positive for the MDS-CBC score.
In the whole cohort, the concordance between the extended Ogata score and bone marrow cytomorphology was 83%, and the concordance between the MDS-CBC score and bone marrow cytomorphology was 78%.
3.4 Results and Performances of the Combined Extended Ogata and MDS-CBC Scores
To combine the two scores, we have chosen to classify patients into three categories as follows: Double Positive (DP) when both scores were positive, Double Negative (DN) when both scores were negative and Single Positive (SP) when only one of the two scores was positive.
In the whole cohort, the concordance of the MDS-CBC score and extended Ogata score, represented by the DN and DP cases, was 79%.
In the MDS group (33 patients), 2 patients (6%) were classified as DN, 7 patients (21%) were classified as SP (2 Ogata+/CBC− and 5 Ogata−/CBC+) and 24 patients (73%) were classified as DP. A total of 31 patients (94%) presented at least one positive score (SP + DP) (Table 2).
In the non-MDS group (30 patients), 23 patients (77%) were classified as DN, 6 patients (20%) were classified as SP (2 Ogata+/CBC− and 4 Ogata−/CBC+) and 1 patient (3%) was classified as DP. A total of 7 patients (23%) presented at least one positive score (SP + DP) (Table 2).
DP results predicted the diagnosis of MDS with a PPV = 96%. Conversely, DN results excluded the diagnosis of MDS with a NPV = 92%. SP results didn't improve the performance of individual scores (Table 2). In the category of MDS without blast excess, DP results predicted the diagnosis of MDS with a PPV = 93% and DN results excluded the diagnosis of MDS with a NPV = 96%. In the MDS with excess blasts category, all patients had DP results. In the CMML group, two out of three cases had a DP result and the other had a DN result.
3.5 Prognostic Consideration
Regarding prognosis, the mean of the extended Ogata score was higher in IPSS-R2 group than in IPSS-R1 group (p < 0.05). Similarly, the mean of the extended Ogata score was higher in IPSS-M2 group than in IPSS-M1 group (p < 0.01) (Figure 1).
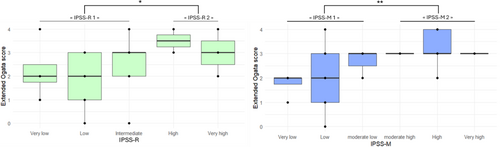
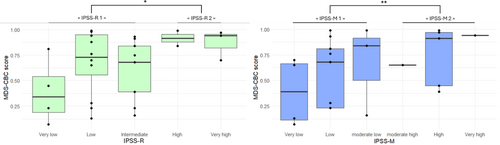
The mean of the MDS-CBC score was higher in IPSS-R2 group than in IPSS-R1 group (p < 0.05). Similarly, the mean of the MDS-CBC score was higher in IPSS-M2 group than in IPSS-M1 group (p < 0.01) (Figure 2).
Interestingly, when combining the two scores, we observe that patients with the higher-risk categories of prognostic score (IPSS-R2 and IPSS-M2) were all DP.
4 Discussion
In the presence of cytopenia, the diagnosis of MDS is based in first instance on the evidence of bone marrow dysplasia. However, cytomorphological diagnosis of MDS is often challenging, particularly in cases where the signs of dysplasia are minimal, in the absence of blasts or ring sideroblasts excess. This challenge has led to explore alternative diagnostic parameters such as extended Ogata and MDS-CBC scores in order to evaluate dysplasia associated with MDS.
In our study, we evaluated the value of the extended Ogata score and the MDS-CBC score both independently and in combination for the diagnosis of MDS in a retrospective cohort of 63 patients. To our knowledge, this is first time that these two scores have been studied in combination. Inclusion criteria, which were clinical suspicion of MDS in the presence of persistent and unexplained cytopenia, allows to simulate real-world MDS diagnosis conditions.
First of all, our study confirms the performances published in literature of the extended Ogata and the MDS-CBC scores for detecting dysplasia associated with MDS [10, 17, 18]. Then, we demonstrate that each parameter of these two scores is significantly different in MDS group rather than in non-MDS group.
Regarding the extended Ogata score alone, we demonstrated robust diagnostic performance for MDS in cases of positive results (Sp = 90%, PPV = 90%). However, this score still lacks sensitivity to rule out a diagnosis of MDS when it is negative (Se = 79%, NPV = 79%). The sensitivity of a positive extended Ogata score was 73% for MDS without blast excess diagnosis and 100% for MDS with blast excess diagnosis. In their study proposing the extended Ogata score [10], Bardet et al. founded a sensitivity of 75% in MDS without blast excess and a sensitivity ranging from 97% to 100% in MDS with blast excess, as well as a specificity of 87%. Our data were close to those founded in this study.
Concerning the MDS-CBC score alone, using a positive threshold of 0.23, we founded good performance for the diagnosis of MDS (Se = 88%, Sp = 83%, PPV = 85% and NPV = 86%). The sensitivity of a positive MDS-CBC score was 86% for MDS without blast excess and 100% for MDS with blast excess. The performances we found for MDS-CBC score confirm the initial published results: indeed, using a same positive threshold of 0.23, Zhu et al. founded a sensitivity of 92% and a specificity of 83% [18].
The major contribution of our study is the demonstration, for the first time, that the combination of the MDS-CBC score with the extended Ogata score improves the prediction of MDS diagnosis when both scores are concordant, compared with the use of scores alone. Indeed, in our cohort, the combined score has demonstrated impressive performance for predicting MDS when patients have DP scores (PPV = 96%). In our study, the only DP case not diagnosed as MDS is debatable because the patient was diagnosed with primary myelofibrosis with the presence of JAK2, ASXL1, TET2, and U2AF1 mutations. Considering the molecular data, the presence of an underlying MDS in this patient cannot be entirely ruled out. Furthermore, in our study, a DN result excludes the diagnosis of MDS with a high NPV of 92%. Regarding the two discordant patients with MDS and DN results, one of them was categorized as MDS-SF3B1 and the other as CMML. Concordant results from combined score provides better performance compared to each score used separately. The advantage of combining these two scores stems from their assessment of distinct but complementary parameters on different matrices. In contrast, SP results didn't improve the performance of individual scores and were insufficient to confirm or exclude the diagnosis of MDS: seven MDS patients and six non-MDS patients had SP results.
Overall, in our cohort, eight patients with negative or non-contributing cytomorphology results had positive results for combined score (four DP results and four SP results). Interestingly, dysplasia was certainly underestimated in half of these patients because the bone marrow was poor or hemodiluted (three patients) or the MDS was masked by a vitamin B12 deficiency (one patient).
Furthermore, our results also demonstrated that the extended Ogata score and the MDS-CBC score were higher in the high-risk than in the low-risk prognostic categories of the IPSS-R and IPSS-M scores. The association between Ogata score results and prognostic classification has been demonstrated previously [26], but this is the first time that the association between MDS-CBC score and prognostic classification has been shown. Interestingly, all high-risk patients classified in the IPSS-R2 and IPSS-M2 groups had DP results for the combined score.
Based on our results, we propose a fast and robust algorithm for the first-step diagnosis of MDS to optimize the technical laboratory execution of complementary cytogenetic and molecular assays (Figure 3). As extended Ogata score and MDS-CBC score assays are cheaper and faster than genetic assays, this algorithm could lead to cost saving by limiting genetic tests performing at the initial evaluation of DN patients. In our cohort, this would have prevented performing three karyotypes and six NGS panels for non-MDS patients. However, we would have missed one patient with MDS-SF3B1. Therefore, in case of DN result, the ongoing presence of unexplained cytopenia during patient follow-up should lead to genetic testing. This algorithm could also encourage genetic testing in patients with at least one positive score (SP + DP) to avoid missing a MDS diagnosis and to accelerate patient management. However, this algorithm could sometimes lead to genetic testing for non-MDS patients in case of SP result. In our cohort, genetic testing would have been performed for six non-MDS patients with a SP result. Cytogenetic was performed for two of these patients and founded no abnormalities. Molecular assays were performed for only one of these patients and founded an IDH2 mutation, classifying the patient as CCUS. Furthermore, we observed that the PPV of DP was 96%, strongly supporting the diagnosis of MDS. Therefore, DP result in combined score could lead to close patient monitoring despite negative cytology. This is especially evident when the scores are elevated, as our findings indicate an association with a worse prognosis.
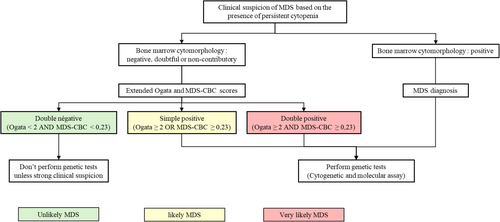
The main limitation of this study is the small sample size in each of the populations, which is particularly evident in the comparison between the different IPSS-R and IPSS-M categories. Therefore, it would be interesting to test the combination of the extended Ogata score and the MDS-CBC score on larger sample sizes. Ideally, future studies should be conducted prospectively in a multicenter setting to evaluate the inter-center reproducibility of this approach. It would also be interesting to observe whether non-MDS patients with a SP result evolve more frequently into MDS during follow-up.
Another limitation of this study is that genetic tests (cytogenetics and molecular biology) were not performed for all patients. Consequently, for 20 non-SMD patients without genetic results, an underlying MDS cannot be completely ruled out. Among these patients, 10 patients corrected their cytopenias, thus reasonably ruling out an underlying MDS. Seven patients had identified causes of irreversible cytopenias, and for three patients no hematological follow-up was performed. The presence of an underlying MDS in these patients is therefore unlikely.
It should also be noted that this combined score can only be performed on the Sysmex XN hematology analyzers. It would also be interesting to test a similar approach using scores developed on other cellular hematology analyzers.
5 Conclusion
Although the extended Ogata score is becoming widespread in hematology laboratories, the MDS-CBC score is still rarely used. Thus, it seems pertinent to include the MDS-CBC score in the analysis panel for chronic cytopenia evaluation, particularly given that MDS-CBC is easy to consider as it is automatically computed by Sysmex XN hematology analyzers. It is therefore an objective, fast, and cost-effective quantitative test. Our study demonstrated the potential of the combination of these scores to confirm or exclude the diagnosis of MDS when the results of the two scores are concordant. We also showed that considering the results of this combination could have limited initial genetic tests in the case of double-negative results, and conversely, should encourage their realization in the case of at least one positive result.
In conclusion, we report for the first time that combining MDS-CBC score with extended Ogata score strongly improves the prediction of MDS diagnosis compared with the use of scores alone. In the context of clinical suspicion of MDS in the presence of unexplained cytopenia, this combination of scores will be helpful in guiding genetic testing and predicting MDS diagnosis.
Author Contributions
L.F. and A.C. draft the paper. L.F., A.C., J.H. and M.M. performed flow cytometry analyses. A.C., J.H. and M.M. performed bone marrow cytomorphology analysis. B.P. performed genetic analysis. A.W. and V.T. provided clinical data. L.F., A.C. and B.P. wrote the manuscript. The manuscript was approved by all authors.
Ethics Statement
The study was conducted according to the Declaration of Helsinki and was approved by the internal hospital ethics committee.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.