Immune reconstitution in children after haploidentical haematopoietic stem cell transplantation
Abstract
Introduction
Immune reconstitution (IR) kinetics of paediatric patients underwent haploidentical haematopoietic stem cell transplantation (HSCT) with post-transplant cyclophosphamide (PTCy) have not been extensively studied. We compared IR patterns of children receiving HSCT from haploidentical (n = 92) and HLA-matched donors (n = 36), and analysed risk factors for viral infection in these patients.
Methods
We prospectively measured lymphocyte subset numbers before HSCT and at 1, 3, 6 and 12 months after HSCT. Blood cytomegalovirus (CMV), Epstein–Barr virus, adenovirus, BK virus (BKV) and urine adenovirus and BKV viral loads were measured at designated time points.
Results
The median numbers of total T and T helper cells at 1 month were significantly lower in the haploidentical group compared with the HLA-matched group. Haploidentical HSCT recipients had significantly lower median numbers of several T cell subsets and B cells for 1 year after HSCT. The median NK cell count of the haploidentical group was lower at 1 month. BKV haemorrhagic cystitis, blood CMV and urine adenovirus reactivation were more frequently found in the haploidentical group. Post-haploidentical HSCT patients receiving anti-T lymphocyte globulin (ATG) had significantly lower median numbers of total T cells (at 1 month) and T helper cells (at 6 and 12 months) and higher rate of blood BKV reactivation compared with those without ATG.
Conclusion
Paediatric patients who undergo haploidentical HSCT with PTCy are likely to have delayed IR and an increased risk of viral reactivation/infection compared with HLA-matched HSCT. The addition of ATG to PTCy delayed T cell recovery and increased risk of BKV reactivation.
1 INTRODUCTION
Haploidentical donors have been widely used as an alternative donor for allogeneic haematopoietic stem cell transplantation (HSCT). Outcomes of patients with haematologic malignancies, who receive HSCT from haploidentical donors, have been comparable with matched related donors (MRD).1, 2 However, haploidentical HSCT increases the risk of infection, especially viral reactivation and infection, which is a significant cause of transplant-related mortality.3-5 Thus, identification of the risk factors for infection in patients after haploidentical HSCT may help transplanters to monitor and prevent infection and provide early treatment to these patients.
Immune reconstitution (IR) is the process of immune recovery after HSCT. The type of stem cell donor has been shown to affect the IR rate that is generally slower in haploidentical HSCT compared with MRD HSCT. After haploidentical HSCT, patients have lower numbers of total T cells, T helper cells, cytotoxic T cells and NK cells during the first 2 months than MRD HSCT patients.6-8 B cells recover late after HSCT, but B cell numbers are comparable between these two groups.7, 8 The speed of immune recovery is associated with the risk of infection and overall survival (OS) of patients who undergo allogeneic HSCT. Rapid IR, particularly T cell subsets, is associated with a low risk of infection and better clinical outcomes.7, 9 Conversely, delayed T cell subset recovery increases the risk of viral reactivation and infection as well as treatment-related mortality in patients after allogeneic HSCT.10, 11 For example, high cytomegalovirus (CMV) peak titres are frequently observed in allogeneic HSCT recipients with delayed early T cell reconstitution at 1 and 3 months and increased risk of non-relapse mortality.12 Risk factors for the development of Epstein–Barr virus (EBV) post-transplantation lymphoproliferative disorder in patients who undergo haploidentical HSCT include a low cytotoxic T cell count and immunoglobulin (Ig) M level at day 30.13 Therefore, IR is a significant factor associated with viral infection and the prognosis of post-allogeneic HSCT recipients.
IR has been studied in several settings of haploidentical HSCT, because of the various treatment platforms. Additionally, most data have been analysed from adult patients with haematologic malignancies. Information on IR and its association with viral reactivation and infection in paediatric patients with malignant and non-malignant diseases has been inadequate. Therefore, this study analysed the pattern of IR and its effects on viral reactivation and infection in children and adolescents who received haploidentical HSCT with post-transplant cyclophosphamide (PTCy).
2 MATERIALS AND METHODS
We retrospectively reviewed data from paediatric patients who received allogeneic HSCT at Ramathibodi Hospital, Mahidol University between January 2015 and December 2019. We excluded patients who had died within 1 month after transplantation, received a second transplant, did not receive a conditioning regimen, or had inadequate medical record data. We collected patient and donor characteristics, data of the transplantation procedure, transplant-related complications, IR and viral reactivation and infection during the first year after HSCT. This study was approved by our Institutional Committee on Human Rights Related to Research Involving Human Subjects (protocol number COA. MURA2020/305).
2.1 Transplant procedure
2.1.1 Preparative regimens
All patients had completed pre-transplantation evaluation of health, organ functions, persistent infection and viral serology. Each patients underwent double-lumens Hickman catheter placement before starting preparative regimens. The preparative regimens were designed in accordance with the patients' underlying diseases and donor types (Tables S1, S2 and S3).14, 15 Most patients with non-malignant haematologic diseases received intravenous (i.v.) busulfan and cyclophosphamide (BUCY) for MRD HSCT, BUCY and Anti-T lymphocyte globulin (ATG) for unrelated donor (URD) HSCT, and i.v. ATG, fludarabine and busulfan for haploidentical HSCT. Patients with malignant haematologic diseases received i.v. cytosine arabinoside, cyclophosphamide, and total body irradiation (TBI) or BUCY for MRD HSCT, i.v. cytosine arabinoside, cyclophosphamide, TBI, and ATG or BUCY and ATG for URD HSCT, and i.v. fludarabine, thiotepa and busulfan for haploidentical HSCT.
2.2 Graft-versus-host disease prophylaxis
Patients who underwent MRD or URD HSCT received a calcineurin inhibitor (cyclosporine A or tacrolimus) starting on day −2 and methotrexate on days +1, +3, +6 and +11 or mycophenolate mofetil starting on day +1. For haploidentical HSCT, the GVHD prophylaxis included cyclophosphamide on days +3 and +4, followed by tacrolimus and mycophenolate mofetil starting on day +5.
2.3 Anti-microbial prophylaxis
Patients received antibiotic prophylaxis consisting of oral ciprofloxacin and penicillin starting from the beginning of preparative regimens. Oral antibiotics were continued until neutrophil engraftment unless the patients developed fever. In these cases, broad spectrum antibiotics were administered after a septic work-up. Oral acyclovir and itraconazole were prescribed from day 0 to prevent herpes simplex and fungal infections, respectively. Both acyclovir and itraconazole were discontinued when neutrophil engraftment was observed. If the patients developed herpes simplex or fungal infections, infectious disease specialists were consulted and then appropriate anti-microbial agents were administered to the patients.
2.4 Engraftment and post-HSCT complications
We collected data of neutrophil and platelet engraftments that were defined as the first day when absolute neutrophil counts exceeded 500 cells/cu mm and platelet counts exceeded 20 000 cells/cu mm for 3 consecutive days without transfusion.16 Post-transplantation complications were recorded, such as mucositis, hepatic veno-occlusive disease and engraftment syndrome, and GVHD classified into acute, late acute and chronic GVHD.17-20
2.5 Immune reconstitution analysis
We prospectively collected patient blood samples to measure lymphocyte subset numbers before HSCT and at 1, 3, 6 and 12 months after HSCT. Flow cytometry performed by the dual platform lyse-wash method was used to analyse the type and number of common lymphocyte subsets (total T cells [CD3+], T helper cells [CD3 + CD4 + CD8-], cytotoxic T cells [CD3 + CD4-CD8+], double-negative T cells [CD3 + CD4-CD8-], B cells [CD19 + CD20+] and NK cells [CD3-CD16/56+]), T cell subsets (naïve T helper cells [CD3 + CD4 + CD45RA+], memory T helper cells [CD3 + CD4 + CD45RO+], naïve cytotoxic T cells [CD3 + CD8 + CD45RA+], memory cytotoxic T cells [CD3 + CD8 + CD45RO+] and regulatory T cells [CD4++CD25hiCD127lo]) and dendritic cells (DCs) (total DCs [HLA-DR++Lin-], plasmacytoid DCs [HLA-DR++Lin-CD123 + CD11c-] and myeloid-derived DCs [HLA-DR++Lin-CD123-CD11c+]).21
2.6 Viral reactivation and disease monitoring
We prospectively monitored viral reactivation by regularly measuring viral loads of CMV, ADV, Epstein–Barr virus (EBV) and BKV using viral load tests of Cobas® for CMV, ELITe MGB® and R-GENE® for ADV, and Sentosa® for EBV and BKV. We measured blood viral load of CMV every week and that of ADV, EBV and BKV every 2 weeks starting at day 0 until day +100 and then every month thereafter for 1 year post-HSCT. We measured urine ADV and BKV viral loads every 2 weeks starting at day 0 until day +100. Viral reactivation was defined as a detectable viral copy number above the standard level of each method. CMV, ADV or EBV disease was diagnosed when patients had viremia and proven evidence of organ infection.22, 23 BKV and ADV haemorrhagic cystitis were diagnosed by detectable BKV and ADV viral loads, respectively, in urine (with or without viremia) with lower urinary tract symptoms such as dysuria or haematuria.24 We performed a nasal swab to detect common respiratory tract pathogens in patients who presented with signs or symptoms of upper or lower respiratory tract infection by multiplex PCR using the NxTAG® Respiratory Pathogen Panel.
2.7 Statistical analysis
We used STATA/IC 15.0 for Windows (StataCorp, TX, USA) to analyse statistical data. The Chi-squared test was used to compare categorical data, whereas the Mann–Whitney U test was used to compare continuous data. p < 0.05 was considered statistically significant. We used the Kaplan–Meier method to calculate the probabilities of event free survival (EFS), OS and GVHD free survival rates. Logistic regression analysis was performed to identify risk and protective factors for CMV reactivation and BK haemorrhagic cystitis. To evaluate the cut-off point of T helper and cytotoxic T cell counts for CMV reactivation and BK haemorrhagic cystitis, we conducted ROC curve analysis.
3 RESULTS
A total of 128 of 140 paediatric patients who underwent allogeneic HSCT were enrolled in this study. For comparison and analysis, we divided the patients into two groups of 92 patients (72%) who received haploidentical HSCT (haplo group) and 36 patients (28%) who received MRD (n = 29) and URD (n = 7) HSCT (HLA-matched group).
Patient characteristics and transplant-related data are shown in Table 1. Patients in the haplo group were more frequently positive for CMV IgG and received more CD34+ cells from peripheral blood stem cell collection. Neutrophil engraftment was faster in the HLA-matched group compared with the haplo group (11.5 vs. 14 days, p < 0.001). The median days for platelet engraftment were comparable between these groups (14 vs. 15.5 days, p = 0.908). Engraftment syndrome was more frequently found in the haplo group than the HLA-matched group (29% vs. 11%, p = 0.038). The frequencies of mucositis and hepatic veno-occlusive disease were not different between haplo and HLA-matched groups. Acute GVHD grade I–IV more commonly occurred in the haplo group than the HLA-matched group (58% vs. 33%, p = 0.014). A higher proportion of patients in the haplo group received corticosteroids during the first year after HSCT (73% vs. 42%, p = 0.001). The median follow-up times of haplo and HLA-matched groups were 903 days (range, 511–1369 days) and 1089 days (range, 612–1433 days), respectively.
| HLA matched (N = 36) | Haploidentical (N = 92) | p-value | |
|---|---|---|---|
| Recipient | |||
| Age (years) (median, IQR) | 8.3 (6.1–11.2) | 8.4 (3.8–11.2) | 0.567 |
| Sex: male/female | 22/14 (61%/39%) | 52/40 (57%/43%) | 0.636 |
| Donor | |||
| Age (years) (median, IQR) | 11 (6.2–16.0) | 37.9 (33.9–42.5) | <0.001 |
| Sex: male/female | 19/17 (53%/47%) | 35/57 (38%/62%) | 0.129 |
| Diagnosis | |||
| Malignant diseases | 17 (47%) | 41 (45%) | 0.318 |
| Acute lymphoblastic leukaemia | 12 (29%) | 9 (53%) | |
| Acute myeloid leukaemia | 15 (37%) | 5 (29%) | |
| Chronic myeloid leukaemia | 0 | 1 (6%) | |
| Langerhan cell histiocytosis | 2 (5%) | 0 | |
| Myelodysplastic syndrome | 1 (2%) | 0 | |
| Neuroblastoma | 10 (24%) | 2 (11%) | |
| Non-Hodgkin's lymphoma | 1 (2%) | 0 | |
| Non-malignant diseases | 19 (53%) | 51 (55%) | 0.395 |
| Adrenoleukodystrophy | 0 | 1 (2%) | |
| Chronic granulomatous disease | 0 | 2 (4%) | |
| Dyskeratosis congenita | 1 (5%) | 0 | |
| Gaucher disease | 0 | 2 (4%) | |
| Hb E/β-thalassemia | 11 (58%) | 30 (59%) | |
| HbH disease | 2 (11%) | 0 | |
| Haemophagocytic lymphohistiocytosis | 1 (5%) | 1 (2%) | |
| Hurler syndrome | 0 | 3 (6%) | |
| Krupple-like factor-1 disease | 0 | 1 (2%) | |
| Severe aplastic anaemia | 2 (11%) | 4 (8%) | |
| Severe combined immunodeficiency | 0 | 2 (4%) | |
| Wiskott-Aldrich syndrome | 1 (5%) | 1 (2%) | |
| β-Thalassemia major | 1 (5%) | 3 (6%) | |
| Pyruvate kinase deficiency | 0 | 1 (2%) | |
| CMV IgG | |||
| D+/R+ | 19 (58%) | 81 (91%) | <0.001 |
| D+/R- | 3 (9%) | 8 (9%) | |
| D−/R+ | 7 (21%) | 0 | |
| D−/R- | 4 (12%) | 0 | |
| Anti-T lymphocyte globulin containing conditioning regimen | 18 (50%) | 60 (65%) | 0.113 |
| Type of stem cell | |||
| Peripheral blood | 31 (86%) | 92 (100%) | 0.001 |
| Bone marrow | 4 (11%) | 0 | |
| Peripheral blood and bone marrow | 1 (3%) | 0 | |
| CD34+ cell count (million cells/kg) (median, IQR) | 6.4 (4.5–8.5) | 8.7 (7.4–10.3) | <0.001 |
| Acute GVHD | 12 (33%) | 53 (58%) | 0.014 |
| Acute GVHD grade 2–4 | 8 (22%) | 38 (41%) | 0.043 |
| Acute GVHD grade 3–4 | 5 (14%) | 16 (17%) | 0.63 |
| Late acute GVHD | 0 | 16 (17%) | 0.006 |
| Chronic GVHD | 6 (17%) | 27 (29%) | 0.14 |
| Outcome | |||
| Rejection/graft failure | 0 | 4 (4%) | 0.576 |
| Alive | 31 (86%) | 74 (80%) | 0.452 |
| Dead | 5 (14%) | 18 (20%) | 0.452 |
| Relapsed malignant disease | 3 (8%) | 11 (12%) | 0.756 |
| Alive | 1 (3%) | 1 (9%) | |
| Dead | 2 (7%) | 10 (91%) | |
- Abbreviations: CMV, cytomegalovirus; D, donor; GVHD, graft-versus-host disease; Ig G, immunoglobulin G; IQR, interquartile range; R, recipient.
3.1 Transplant outcomes
The 5-year OS rates of the patients underwent HLA-matched and haploidentical HSCT were 86.1% (95% CI, 69.8–93.9%) and 77.1% (95% CI, 65.3–85.3%), respectively (p = 0.494). The 5-year aGVHD and cGVHD free survival rates were 77.3% (95% CI, 62.1–86.9%) and 79.2% (95% CI, 68.5–86.6%), respectively. For the patients with malignant diseases, the 5-year OS and EFS rates were 68.2% (95% CI, 52.5–79.7%) and 65.8% (95% CI, 51.8–71.7%), respectively. The 2-year cumulative incidence of relapse was 25.2% (95% CI, 15.5–39.6%). Non-relapse mortality rate was 3.9% (95% CI, 1.8–8.8%) for this group. The 5-year OS of relapse patients was only 11.7% (95% CI, 0.7–39.2%).
3.2 Immune reconstitution
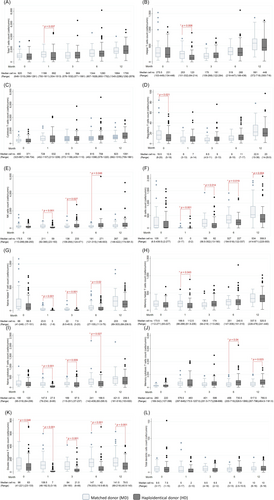
The median number of lymphocyte subsets before HSCT and at 1, 3, 6 and 12 months after HSCT in both groups are shown in Figure 1. The median number of total T cells and T helper cells in the first month was significantly lower in the haplo group compared with the HLA-matched group. The median NK cell count of the haplo group was lower in the first month (59 vs. 210.5, p < 0.001), but rapidly increased to higher than that of the HLA-matched group at 3 and 6 months after HSCT. The haplo group had significantly lower median numbers of B cells at 1, 3, 6 and 12-months after HSCT. The median IgM level of the haplo group was significantly lower than that of the HLA-matched group at 1 month (0.47 mg/mL [range, 0.26–0.81 mg/mL] vs. 0.354 mg/mL [range, 0.21–0.48 mg/mL], p = 0.026) and 3 months (0.52 mg/mL [range, 0.34–0.74 mg/mL] vs. 0.3 mg/mL [range, 0.18–0.52 mg/mL], p < 0.001), whereas the median IgA level of the haplo group was significantly lower than that of the HLA-matched group at all four time points during the first year after HSCT (Figure S1).
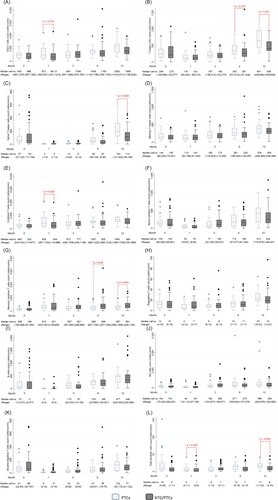
In the haplo group, there was a subgroup of the patients (65%) receiving ATG within the preparative regimens (ATG/PTCy group). The number of lymphocyte subsets before HSCT and at 1, 3, 6 and 12 months after HSCT in non-ATG (PTCy) and ATG/PTCy groups are shown in Figure 2. The median total T cell count at 1 month was significantly lower in the ATG/PTCy group (652 cells/cu mm [range, 20–4635 cells/cu mm]) compared with the non-ATG group (972 cells/cu mm [range, 242–4479 cells/ cu mm]) (p = 0.018). The median T helper cell counts in the ATG/PTCy group at 6 and 12 months were 261 cells/cu mm (range, 28–1606 cells/ cu mm) and 408 cells/cu mm (range, 25–962 cells/cu mm). Both T helper cell counts were significantly lower than those in the non-ATG group, which were 330 cells/cu mm (range, 111–861 cells/cu mm) at 6 month (p = 0.019) and 641 cells/cu mm (range, 274–1291 cells/cu mm) at 12 month (p = 0.007), respectively. There was no difference in number of NK cell or B cell counts between these two groups during the first year after HSCT.
3.3 Factors affecting IR
We analysed the association between clinical characteristics and lymphocyte subsets number after HSCT using multivariate analysis. Patients receiving higher dose of CD34 cells (≥5 million cells/kg) had significantly higher median T helper cells at 6 (p = 0.036) and 12 months (p = 0.019). Patients with malignant diseases had higher median cytotoxic T cells at 12 month (p = 0.02). Patients underwent haploidentical HSCT had significantly lower B cells at 6 (p = 0.008) and 12 months (p = 0.014). Interestingly, younger age patients (<5 years) had faster recovery of T helper cells (p < 0.001), cytotoxic T cells (p = 0.007) and B cells (p = 0.02) at 12 month compared to older age patients (≥5 years).
We evaluated the association between IR and the patients' outcomes in this study. We found that patients who death after HSCT had lower median T helper cells (p = 0.021) and NK cells at 1 month (p = 0.007).
3.4 Viral reactivation and infection
The frequencies of viral reactivation and infection in patients after HSCT are shown in Table 2. We found more frequent blood CMV and urine adenovirus reactivation in the haplo group than that in the HLA-matched group by regular viral monitoring. Patients who had CMV viremia at >1000 copies/mL received pre-emptive therapy with intravenous ganciclovir or foscarnet.
| Infection | HLA-matched (N = 36) | Haploidentical (N = 92) | p-value |
|---|---|---|---|
| Common viral respiratory tract infection | |||
| N (%) | 7 (19.44) | 35 (38.04) | 0.044 |
| Episode | 10 | 48 | 0.753 |
| CMV disease | |||
| N (%) | 2 (5.56) | 8 (8.70) | 0.724 |
| ADV disease | |||
| N (%) | 3 (8.33) | 11 (11.96) | 0.756 |
| EBV disease | |||
| N (%) | 1 (2.78) | 1 (1.09) | 0.485 |
| BKV disease (BKV haemorrhagic cystitis) | |||
| N (%) | 4 (11.11) | 25 (27.17) | 0.051 |
| Episode | 4 | 26 | |
| Viral reactivation (blood) | |||
| CMV reactivation | 11 (30.56) | 77 (83.70) | <0.001 |
| ADV reactivation | 6 (16.67) | 28 (30.43) | 0.113 |
| EBV reactivation | 2 (5.56) | 4 (4.35) | 0.674 |
| BKV reactivation | 10 (27.78) | 29 (31.52) | 0.679 |
| Viral reactivation (urine) | |||
| ADV reactivation | 4 (11.11) | 26 (28.26) | 0.039 |
| BKV reactivation | 22 (61.11) | 49 (53.26) | 0.422 |
- Abbreviations: ADV, adenovirus; BKV, BK virus; CMV, cytomegalovirus; EBV, Epstein–Barr virus.
Common respiratory viral infections were more frequently found in the haplo group than the HLA-matched group (p = 0.044). Occurrences of CMV disease (CMV retinitis, CMV pneumonitis, CMV gastrointestinal disease and CMV-associated haemophagocytosis), ADV disease (disseminated adenovirus infection, adenovirus pneumonia and adenovirus haemorrhagic cystitis) and EBV disease (EBV-associated haemophagocytosis and EBV esophagitis) were not different between both groups. Ten patients experienced ADV haemorrhagic cystitis, seven patients in the haplo group and three patients in the HLA-matched group. BKV haemorrhagic cystitis tended to occur in the haplo group more commonly than the HLA-matched group. CMV reactivation also occurred more in the haplo group than the HLA-matched group (p < 0.001). The median day of detection of CMV reactivation was day +21 (range, day +14 to +32) in the haplo group and day +28 (range, day +20 to +45) in the HLA-matched group (p = 0.194). Among patients with CMV reactivation, 40 patients (52%) in the haplo group and 7 patients (63%) in the HLA-matched group received pre-emptive treatment for CMV infection by i.v. ganciclovir or foscarnet. Eventually, CMV disease developed in six (15%) and two (28%) patients in haplo and HLA-matched groups, respectively. Two patients developed CMV disease without receiving pre-emptive treatment.
In the haplo group, the patients received ATG had more frequent blood BKV reactivation than those did not receive ATG (40% vs. 15.6%, p = 0.019). Twenty-five patients developed BKV haemorrhagic cystitis, which occurred in 20 patients (33.3%) in ATG group and 5 patients (15.6%) in non-ATG group (p = 0.087). There were no differences of the frequencies of CMV, EBV or ADV reactivations between ATG and non-ATG group.
3.5 Risk factors for CMV reactivation and BKV haemorrhagic cystitis
Because of the higher frequencies of CMV reactivation and BKV haemorrhagic cystitis in the haplo group, we further analysed the risk factors for these complications (Table S4). We analysed the association between the lymphocyte subset number and CMV reactivation and BKV haemorrhagic cystitis. A T helper cell count of <150 cells/cu mm at 1 month post-HSCT was associated with CMV reactivation (72.22% sensitivity and 63.29% specificity, AUROC = 0.706). Using multivariate analysis, the risk factors for development of CMV reactivation were haploidentical HSCT and positive for recipient CMV IgG, whereas a T helper cell count of ≥150 cells/cu mm in the first month was a protective factor (Table 3).
| Variable | OR (95% CI) | p-value |
|---|---|---|
| CMV reactivation | ||
| Haploidentical HSCT | 10.38 (2.91–37.06) | <0.001 |
| Steroids used in 100 days | 3.03 (0.75–12.25) | 0.119 |
| aGVHD | 1.89 (0.46–7.71) | 0.375 |
| Donor CMV IgG+ | 0.67 (0.09–4.76) | 0.686 |
| Recipient CMV IgG+ | 4.91 (1.10–21.82) | 0.037 |
| T helper cells at 1 month ≥150 cells/cu mm | 0.33 (0.11–0.99) | 0.049 |
| BKV haemorrhagic cystitis | ||
| aGVHD grade 2–4 | 5.13 (1.60–16.46) | 0.006 |
| Blood BKV reactivation within 100 days | 9.02 (2.54–32.01) | 0.001 |
| Urine BKV reactivation within 100 days | 14.07 (1.53–129.07) | 0.019 |
| Cytotoxic T cells at 1 month ≥340 cells/cu mm | 0.28 (0.08–0.96) | 0.044 |
- Abbreviations: aGVHD, acute graft-versus-host disease; BKV, BK virus; CI, confidence interval; CMV, cytomegalovirus; HSCT, haematopoietic stem cell transplantation; Ig, immunoglobulin; OR, odds ratio.
A cytotoxic T cell count of <340 cells/cu mm in the first month after HSCT was associated with BKV haemorrhagic cystitis (75.82% sensitivity and 54.17% specificity, AUROC = 0.686). For BKV haemorrhagic cystitis, acute GVHD grade 2–4, blood BKV reactivation and urine BKV reactivation were independent risk factors, whereas a cytotoxic T cell count of ≥340 cells/cu mm in the first month decreased the risk of BKV haemorrhagic cystitis (Table 3).
4 DISCUSSION
Our study revealed the IR kinetics of post-allogeneic HSCT paediatric patients and the associations between IR and viral infection during the early post-HSCT period. We found discrepancies in the pattern of lymphocyte subset recovery between patients who received haploidentical and HLA-matched HSCT. We studied specific groups of patients who had not been extensively examined previously. We performed HSCT in paediatric patients who were diagnosed with various malignant and non-malignant disorders. Additionally, we used the PTCy platform and unmanipulated peripheral blood stem cells for all patients who underwent haploidentical HSCT.
In this study, paediatric patients had delayed recovery of most T cell subsets in the early phase after haploidentical HSCT. During the first few months after haploidentical HSCT with PTCy, T cell recovery depends on the donor naïve T cell compartment.25 Our haploidentical HSCT recipients generated significantly lower naïve T cells that persisted for at least 6 months after HSCT. Therefore, these patients produced lower numbers of total T cells, T helper cells and memory T cells compared with HLA-matched recipients during the first 30 days. Compared with another haploidentical HSCT platform, paediatric and adult patients who received the granulocyte colony-stimulating factor primed grafts, intensive immunosuppression, anti-thymocyte globulin, and combined peripheral blood stem cell and bone marrow (GIAC) protocol using extensive immunosuppression with T cell-replete graft consisting of G-CSF-primed bone marrow and peripheral blood stem cells had a similar pattern of T cell recovery to our patients.7 The patients had significantly lower number of CD3+, CD4+ and CD4+ memory T cells on day +30 and CD4+ naïve T cells on days +30, +90 and +180, post-GIAC haploidentical HSCT compared with HLA-matched HSCT.7
We found that post-haploidentical HSCT patient B cells disappeared in the first month and then slowly increased during the first year. Conversely, the B cell number in adult patients who received haploidentical HSCT with PTCy, MMF and tacrolimus reached the normal donor range by day +60.9 Our findings were consistent with B cell recovery in children and adults, who receive the GIAC protocol for haploidentical/mismatched HSCT.26 These patients had low B cell numbers at day +90, +180 and + 360 and about 45% of the patients had B cell levels within the normal range. There are several factors that influence B cell reconstitution, including the stem cell source, serotherapy, intensity of the preparative regimen, TBI and acute and chronic GVHD.27 Our haploidentical recipients more frequently developed acute GVHD and received glucocorticoids that may be factors affecting delayed B cell reconstitution.26, 27
Recently, there have been several studies on IR after haploidentical HSCT in adults with hematologic malignancies.28-30 In T cell subset recovery, we found that the haplo-group (PTCy) had significantly lower T helper cell count at the first month after HSCT compared with non-PTCy group. However, adult patients receiving PTCy showed persistent lower total CD 4+ cell count throughout the first year after HSCT compared with receiving conventional GVHD prophylaxis.28 There were several factors might lead to the incompatible results such as the differences of recipients' age, underlying diseases and prior therapy and preparative regimens between the studies. Interestingly, a previous study compared IR in adult patients between receiving ATG and PTCy.29 The study showed that the ATG group had a faster reconstitution of CD8+ T, NK, NKT and γδT cells but slower reconstitution of CD4+ T cells and B cells. Moreover, using higher dose (60 mg/kg) of ATG was associated with delayed IR compared to the lower dose (30 mg/kg).30 We also analysed IR in haplo group who received ATG and not received ATG prior stem cell infusion. The patients underwent haploidentical HSCT with ATG showed significantly delayed T helper cells compared with those without ATG. Our finding supports the effect of ATG on IR post-HSCT in the previous study.29
CMV and BKV infections are common infectious complications in patients after haploidentical HSCT. CMV infection rates are higher in recipients after haploidentical HSCT compared with post-HLA-matched HSCT.31 The incidence of CMV infection in adult patients who received haploidentical HSCT with PTCy ranged from 44% to 69%.3, 32 We found CMV reactivation in 84%, but CMV disease had developed in only 9% of our paediatric haplo group. The high incidence of CMV reactivation might be related to the high frequency of CMV IgG D+/R+ in our cohort. Moreover, CMV-negative blood products were not available for recipients with negative CMV IgG. We identified significant risk factors for CMV reactivation, including haploidentical HSCT, recipient CMV IgG+ and a T cell number at 1 month of ≥150 cells. Haploidentical HSCT and CMV-seropositive recipients have been reported as risk factors for development of CMV infection in adult patients.31, 32 We found a significant association between T cell recovery and CMV reactivation in the haploidentical setting. Delayed T cell reconstitution increases the risk of a high CMV viral load and non-relapsed mortality.12 Additionally, the numbers of T cell subsets predict a risk of CMV reactivation. Post-allogeneic HSCT with low absolute CD4+ T cells (<55 cells/cu mm) and/or CD16+ NK cells (<84 cells/cu mm) are significant risk factors for CMV reactivation.33 Our study also demonstrated that patients with a T helper cell count of ≥150 cells/cu mm had a low risk of CMV reactivation.
BKV haemorrhagic cystitis is a common viral disease after haploidentical HSCT with PTCy with an incidence ranging from 55% to 62%.34, 35 Our findings were similar to a previous study showing that patients in the haplo group had a higher frequency of BKV haemorrhagic cystitis compared with the HLA-matched group.35 High grade haemorrhagic cystitis causes patient suffering and renal impairment as well as increases the risk of mortality.36 However, effective treatment for BKV haemorrhagic cystitis has been not successfully established. Viral monitoring and risk factor identification may improve patient outcomes by receiving early treatment. Several risk factors for this complication after allogeneic HSCT have been reported, such as a young age, haploidentical donor graft, high plasma and urine BKV viral loads, corticosteroid therapy and acute GVHD.35, 37-39 In this study, we analysed risk factors for developing BKV haemorrhagic cystitis in the haploidentical HSCT with PTCy setting, including the presence of acute GVHD as well as blood and urine BKV viral loads. Additionally, cytotoxic T cells of ≥340 at 1 month were a significant protective factor against this disease. Although delayed IR is associated with BKV haemorrhagic cystitis, our data revealed a correlation between a specific T cell subset and the occurrence of this condition in the haploidentical HSCT with PTCy platform.40
We have acknowledged several limitations of this study including retrospective nature and the heterogeneity of our patients specially underlying diseases, preparative regimens and ATG administration. To reduce bias, we analysed the factors associated with IR using multivariate analysis. We further analysed the effect of ATG in haploidentical setting by subgroup analysis of the haplo group. Therefore, the results might be more specific and explicit for applying to the future research and practices.
In conclusion, paediatric patients who underwent haploidentical HSCT had delayed IR of total T cells, B cells, T helper cells, naive T helper cells, naive cytotoxic T cells and double-negative T cells, especially the first 3 months after HSCT compared with HLA-matched HSCT. CMV and BKV infection were frequently detected in post-haploidentical HSCT recipients. Delayed IR was associated with the risk of CMV and BKV infection in this setting.
AUTHOR CONTRIBUTIONS
Saranthorn Apasuthirat, Nopporn Apiwattanakul and Samart Pakakasama initiated and designed the study; Usanarat Anurathapan, Karan Paisooksantivatana, Ekawat Pasomsub and Suradej Hongeng registered data; Saranthorn Apasuthirat collected data; Karan Paisooksantivatana and Ekawat Pasomsub performed laboratory tests; Nintita Sripaiboonkij Thokanit, Saranthorn Apasuthirat, Nopporn Apiwattanakul and Samart Pakakasama analysed data; Saranthorn Apasuthirat, Nintita Sripaiboonkij Thokanit and Samart Pakakasama wrote manuscript; and all authors contributed to manuscript review and provided comments on the manuscript.
CONFLICT OF INTEREST STATEMENT
All authors declare they have no conflicts of interests.
Open Research
DATA AVAILABILITY STATEMENT
The anonymized data obtained and analyzed in this study are available from the corresponding author on reasonable request.