Role of flow cytometry in evaluation of the cellular therapy products used in haematopoietic stem cell transplantation
Abstract
Cellular therapy nowadays includes various products from haematopoietic stem cells (HSC) collected from bone marrow, peripheral blood, and umbilical cord blood to more complex adoptive immune therapy for the treatment of malignant diseases, and gene therapy for inherited immune deficiencies. Broader utilization of cellular therapy requires extensive quality testing of these products that should fulfil the same requirements regarding composition, purity, and potency nevertheless they are manufactured in various centres. Technical improvements of the flow cytometers accompanied by the increased number of available reagents and fluorochromes used to conjugate monoclonal antibodies, enable detailed and precise insight into the function of the immune system and other areas of cell biology, and allows cell evaluation based on size, shape, and morphology or assessment of cell surface markers, as well as cell purity and viability, which greatly contributes to the development and progress of the cell therapy. The aim of this paper is to give an overview of the current use and challenges of flow cytometry analysis in quality assessment of cellular therapy products, with regard to basic principles of determining HSC and leukocyte subpopulation, assessment of cells viability and quality of thawed cryopreserved HSC as well as the importance of validation and quality control of flow cytometry methods according to good laboratory practice.
1 INTRODUCTION
The field of cellular therapy has been constantly evolving since the first bone marrow (BM) transplantation in the 1950s, and currently has been successfully implemented as a treatment for patients with malignant, congenital, or acquired diseases of the haematopoietic system.1 Cellular therapies that initiated with haematopoietic stem cell (HSC) transplantation, now are becoming more complex including adoptive immune therapy for malignant diseases and gene therapy for the treatment of inherited immune deficiencies.2 The novel promising cellular therapies are immune effector cells (IECs), such as gene-modified T cells and natural killer (NK) cells.3 Broader utilization of cellular therapy requires extensive quality testing of these products that should fulfil the same requirements regarding composition, purity, and potency nevertheless they are manufactured in various centres.4 In order for cellular therapy products to be exported from one centre to another for further clinical use, it is critical to have mechanisms in place to ensure that the cell collection and processing procedures yield safe, effective, and comparable products at all centres.
Initially, the evaluation of the quality of BM graft was limited to enumeration of total nucleated cells and colony forming unit (CFU) testing, while nowadays various assays have been used for assessing the quality of cellular therapy products. The assessment of the cell type may involve evaluations based on cell size, shape, and morphology, or the evaluation of cell surface markers by flow cytometry. The purity of the cells is also often evaluated by flow cytometry, as well as cell viability measured by dye exclusion assays. Flow cytometry has applications in various fields such as immunology, cellular biology, bacteriology, virology, cancer biology and infectious disease monitoring. It has seen dramatic advances over the last 30 years, allowing detailed and precise insight into the function of the immune system and other areas of cell biology.5 This paper aims to give an overview of the current use and challenges of flow cytometry analysis in the quality assessment of cellular therapy products used in HSC transplantation setting.
2 FLOW CYTOMETRY: PRINCIPLES, INSTRUMENTS, REAGENTS
Flow cytometry is a technique utilized in many different settings, both in the routine laboratory and in research facilities. With the advancement of technology, the field of flow cytometry is also evolving, so today special types of instruments have been designed for specific purposes, for example, system that combines microscopy and flow cytometry or flow cytometry with mass spectrometry. This technique allows simultaneous analysis of cell characteristics of mixed cell population from peripheral blood (PB) and BM as well as solid tissues that can be dissociated into single cells, with cell sorting for further analysis, which is one of the main application of flow cytometry.5
Technical improvements of the flow cytometer are accompanied by an increase in the number of available reagents and fluorochromes used to conjugate monoclonal antibodies which results in the complexity of the analysis and requires the use of newer cluster data analysis algorithms. All that improves methods of data mining allow useful information to be extracted from the high-dimensional data now available from flow cytometry.5
In addition to immunophenotyping, which is the most used application in flow cytometry, apoptosis analysis, cell cycle analysis and cell sorting are also used in the quality assessment of cellular therapy products.5-8
3 ENUMERATION OF HAEMATOPOIETIC STEM CELL
HSC sources currently used for transplantation are BM, mobilized PB and umbilical cord blood (UCB).9 Each type of HSC graft requires a different method of collection and processing, and has its advantages and disadvantages. BM has been almost completely replaced as a source of HSC with peripheral blood stem cells (PBSC), due to easier collection with leukapheresis procedure without the need for general anaesthesia and more rapid haematopoietic reconstitution after transplantation.10, 11 HSCs from UCB have a high clonogenic potential and because they are immunologically naive and immature, can be transplanted with only partial histocompatibility. But due to their limited volume, UCB is mainly applicable in transplantation of paediatric patients.9
Regardless of the source of HSCs, haematopoietic stem and progenitor cells appear morphologically as either small lymphocytes in the case of the earliest stem cells or in the case of maturing progenitors, as blast forms. Therefore, they can be identified only by functional assays or by immunophenotypic surface marker analysis. Phenotype of HSC is CD34+/CD45dim/SSClow/FSClow and intermediate.12
Later progenitors may be functionally assayed in soft agar culture systems. When supported by the proper growth factors, they form colonies of their progeny that can be enumerated and expressed as a particular number of CFUs per total number of cells plated. Functional assays for enumerating different species of HSCs require several weeks of sterile culture incubation. Therefore, these assays have some disadvantages, and the most significant being poor intra- and inter-laboratory reproducibility, non-standardization, and long turnaround time.13, 14 Hence, functional haematopoietic cell assays in transplantation clinical practice are generally limited to quality-control procedures of cryopreserved cells and are rarely used in routine laboratory testing.
The measurement of CD34+ cells by flow cytometry has, however, become the universal assay for measuring the potency of HSC products. The evaluation of absolute CD34+ cell count in patient PB is also important for the decision of optimal timing to start the leukapheresis procedure. According to the Joint Accreditation Committee of International Society of Cellular Therapy (JACIE) standards, enumeration of viable CD34+ cells must be performed in fresh PBSC products to access the graft quality.15 Minimal CD34+ cell count for one transplantation is ≥2 × 106 CD34+ cells/kg of recipient's body weight, while the optimal is 5 × 106 CD34+ cells/kg of body weight, which is associated with faster recovery of neutrophil and platelet count after transplantation.16, 17
Although laboratories for quality control in transplant centres use different protocols for determining CD34+ cells, the protocol of International Society of Hemotherapy and Graft Engineering (ISHAGE) is the most commonly used (Figure 1).18 This protocol has been continuously updated, by introducing counting beads and including viability dyes.12 However, there are still differences between laboratories using the ISHAGE protocol: some use single platform method, while others dual platform.19 The benefit of the single platform is the simultaneous determination of the percentage and absolute count of CD34+ cells using fluorescent microspheres (beads), which can be in liquid phase or in lyophilized form. The main advantage of lyophilized beads is that the tube contains an exact number of beads which allows the calculation of the absolute cell count. Furthermore, there is a difference in the cell labelling protocol which depends on whether the single or dual platform method is used. Some laboratories use lyse-wash method (dual platform), while others use lyse-no wash method (single platform) which lasts shorter and potential cell loss is prevented. Although the ISHAGE protocol for CD34+ cell determination is used in many laboratories, the mentioned differences indicate that standardization is necessary.
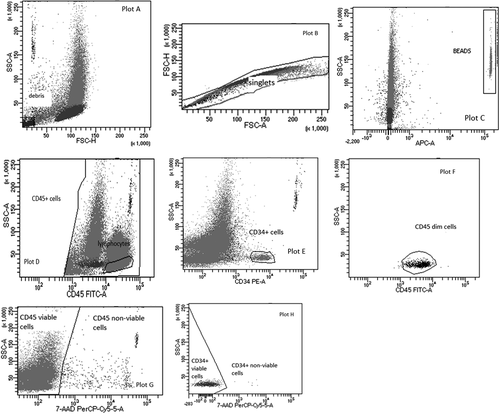
Although according to EBMT guidelines, CD34+ evaluation is not mandatory for the assessment of BM many centres include it in quality testing.20 If the laboratory performs determination of viable CD34+ cells in BM samples, it is necessary to carefully set up protocols and templates for cells analysis on flow cytometer. Compared to CD34+ cells enumeration in PB or in PBSC graft, the analysis of BM samples is challenging because they contain high red blood cell count and may contain fat, cell clumps which all complicate the analysis. Therefore, the settings on the flow cytometer must be adjusted to reduce debris and allow a more accurate determination of the population of CD34+ cells.
4 ASSESSMENT OF CELLS VIABILITY
The viability of cells was firstly evaluated using the dye exclusion tests, based on the principle that live cells possess intact cell membranes that exclude certain dyes, whereas dead cells do not. The cell suspension is mixed with dye and then visually examined to determine whether cells take up or exclude dye. The most common dyes used for performing dye exclusion tests are trypan blue, eosin Y, acridine orange, or propidium iodide. Dye exclusion is a simple and rapid technique, but the limitation of the method is that viability is determined indirectly from the assessment of cell membrane integrity. It is important that the test be performed accurately because a small amount of dye uptake indicative of cell injury may go unnoticed.21, 22
As already mentioned, in addition to the number of CD34+ cells, according to the JACIE standards it is necessary to determine the viability of collected and processed/cryopreserved cells. In the assessment of CD34+ cells viability, the most commonly used dye is 7-aminoactinomicin D (7-AAD), which binds to GC region of the cell DNA, and allows the determination of necrotic cells and those in late apoptosis (7-AAD positive) using flow cytometer.22 Besides 7-AAD, some laboratories still used dye excluding tests in routine work, but because 7-AAD has become a standard part of the ISHAGE protocol, most laboratories use it to determine the viability of CD34+ cells.21 The main disadvantage of the method with 7-AAD, as well as the previously mentioned dye exclusion tests, is a failure to detect cells in early apoptosis, which may lead to overestimation of graft cell viability.23 Several studies have shown that the method using Annexin V (Ann V) is suitable for detection of cells in early apoptosis.14, 24 Annexin V is a protein that has a high affinity for negatively charged phospholipids, such as phosphatidylserine, which are characteristic of the cytoplasmic side of the viable cell membrane. But in early apoptosis phosphatidylserine becomes exposed on the cell surface due to cell membrane asymmetry and can be detected using a reagents containing Ann V protein and Ann V binding buffer with calcium, which allows binding Ann V to negatively charged phospholipids. It may be useful to evaluate the apoptotic status of progenitor cells before beginning ex vivo manipulation procedures such as stem cell expansion or gene therapy.25 Other assays for the determination of apoptotic cells that are used in research settings are method with DNA binding dye Syto 16, method for detecting activation of caspases, TUNEL (TdT dUTP Nick End Labeling) assay for detection of endonuclease digestion of DNA, and method for detection of mitochondrial apoptosis using dyes that determine mitochondrial membrane potential and chromation condensation in the nucleus using method with specific dye.5, 26
5 ASSESSMENT OF QUALITY OF THAWED CRYOPRESERVED HSC
Fresh HSCs, once harvested, are only viable for several hours to a few days, limiting their use and geographical reach. Currently, HSCs and other cell therapies are cryopreserved using the same techniques with cryoprotectant dimethyl sulfoxide (DMSO) at a slow cooling rate.27 Cryopreservation of HSCs allows their transportation from the site of processing to the site of clinical use, creates a larger window of time in which cells can be administered to patients, and enables sufficient time for quality control and regulatory testing. Despite these benefits, during processing, cells are exposed to some factors (e.g., centrifugation, condition of storage, and thawing process) that can lead to the reduction of the cell count in graft, but also to a decline in cell viability after thawing.27
The gold standard for cryopreservation for HSCs is still DMSO. The timing of cell exposure to DMSO is very important because DMSO can directly impact cellular function by affecting metabolism, enzyme activity, apoptosis, and cell cycle. In addition, the effect of DMSO depends on the type of cells, the stage of cell differentiation, the duration of exposure, and DMSO concentration.28 Since DMSO has the toxic effect on the cells in thawed graft and could also cause adverse reactions during the infusion, in some transplant centres it is removed from thawed HSCs before transplantation.27 However, the question is how many cells are lost and damaged by this process, because it is known that the number of viable CD34+ cells in a thawed product is actually a real number transplanted to the patient.11, 29 In addition to the DMSO, some other factors affect the recovery of the cells after cryopreservation, such as cell concentration, pre-freeze storage conditions, freezing rate, and storage temperature, which also affect cell viability.27
It is well known that ‘single platform’ method in combination with 7-AAD represents the reference method for determining the count of viable CD34+ cells in fresh samples. However, for analysing thawed cryopreserved samples modification of the method is necessary. It is recommended to adjust the gates Side scatter versus Forward scatter and CD45 versus SSC in order to allow the acquisition of a higher number of dead and live cells. Therefore, analysis of thawed samples requires the adaption of gatting strategy and acquisition settings for the purpose of more precise and accurate analysis of HSC.11, 30
According to NetCord – Foundation for the Accreditation of Cellular therapy (FACT) standards for Cord Blood Banks the post-thaw CD34+ cell viability should be ≥70%.31 There are no such recommendations for minimal CD34+ cell post-thaw viability of PBSCs, and it is only required that the viability of nucleated cells should be >50% after freezing and thawing of apheresis product.32 It is therefore questionable how to perform the quality control of the PBSC graft after thawing and which assays and methods should be used. Although some studies evaluated the methods for post-thaw viability of PBSC and CB, as well as attempted to standardize process from sample preparation to acquisition on flow cytometer, still there are no guidelines defining each step in the process after thawing of cryopreserved samples, for example, exact conditions of sample thawing, is it necessary to wash cells, dilute the samples and remove cryoprotectant before labelling, and acquisition on flow cytometer.33, 34
In quality specifications for UCB, post-thaw CFU is still one of the requirements, but due to the before mentioned disadvantages of CFU assay, in routine work it would be desirable to use assay with higher reproducibility and a shorter turnaround time for faster assessment of graft quality.35, 36 Although 7-AAD is the most commonly used dye for the determination of cell viability on flow cytometer in HSC transplants, the lack of a test is that it cannot determine cells in early apoptosis. Since it is known that in early apoptosis the functioning of the cell is impaired, the question is whether such damaged cells have the possibility of proliferation. Thus, it would be useful to determine the viability of cells, in addition to the method with 7-AAD use the method for determining cells in early apoptosis, such as the method with annexin V. Few studies showed that for UCB samples, assay using 7-AAD and Ann V was a feasible method for prediction of CFU results.14, 24 Duggleby and coworkers24 showed that significant numbers of CD34+ AnnV+ events were found within the 7AAD-gated population on their custom protocol for determining viable CD34+ cells. In their study, the measured results indicated a good correlation between nonapoptotic cells (CD34+ AnnV−) and CFU results, so they confirmed the fact that the current standard enumeration of CD34+ viable cells does not fully reflect potency after -thawing because standard enumeration with 7-AAD does not measure the early apoptotic cell. Radke and coworkers14 in their study presented that in comparison to the standard ISHAGE protocol, the method with AnnV resulted in similar good correlations between CFU and CD34+ cells seeded, which leads to an improved conformability with the theoretically expected colony formation. The results of these studies were especially important because the Ann V method can be used in the case the number of viable cells needs to be determined as soon as possible before transplantation.
6 ASSESSMENT OF LEUKOCYTE SUBPOPULATION
In addition to CD34+ cell determination, the quality assessment of allogenic HSC graf must include enumeration of other leukocyte subpopulation, especially CD3+ T cells. Besides T cells, for different types of cell therapy it is necessary to determine leukocytes subpopulation in the graft. Nowadays, flow cytometers used in laboratory work enable the detection of several cell markers at once, depending on the laser and detector configuration of flow cytometer (e.g., 10-colour flow cytometer with three lasers [violet 405 nm, blue 488 nm, and red 638 nm]). Therefore, it is possible to determine leukocytes subpopulations using monoclonal antibodies (mAb) in one test tube, reducing the price and time to test results. Leukocyte subpopulations are usually detected using anti-CD 19 mAb for B-cells, anti-CD 16 and anti-CD 56 for NK cells, anti-CD3, anti-CD 4 and anti-CD8 mAb for subpopulation of T-cells and anti-CD 14 and anti-CD16 for monocytes (Table 1).
When the laboratory defines the protocol for detecting cells of interest, it is necessary to choose the antibodies (Abs) and fluorochromes which will allow accurate cell identification. This is especially important when performing multiple analysis in one step (e.g., enumeration of lymphocytes subpopulation). In addition to individual Ab, there are commercial kits that contain predefined Abs labelled with fluorochromes and reagents for determining cells of interest, for example, for determining HSC or lymphocyte subpopulations.16, 37, 38 The advantage of these kits is that they contain pre-assembled Ab panels, which facilitates the creation and implementation of protocols in laboratories that have no experience with setting up in-house methods. Furthermore, some manufacturers offer software for acquisition and analysis which have an auto-gating algorithm for isolation of the cells of interest, which also contributes to more user-friendly cell analysis, for example, BD FACSCanto Clinical software (BD Bioscience, San Jose, CA).39
Transplantation of allogeneic HSC is sometimes followed with additional administration of donor lymphocytes, for example, donor lymphocyte infusion (DLI). Lymphocytes for DLI could be collected during allo-HSC collection procedure or from unstimulated PB, and cryopreserved as a simple and effective therapeutic option for patients in case of disease relapse.40, 41 The purpose of DLI is to enhance donor T-cells potency against leukaemic cells (graft-vs.-tumour effect), that is, to treat disease relapse and improve immune recovery.42
In the fresh apheresis products which will be cryopreserved for DLI, CD3+ cells are determined on flow cytometer. Since there are only a few reports on how this cell population tolerates the cryopreservation and thaw process, and because of difference among laboratories in cryopreservation protocols, each centre should determine the viability of CD3+ cells in the thawed sample before infusion.38
In the case of partial HLA matching, the HSC graft could be manipulated before administration in order to remove unwanted cells. The transplantation of HSC graft obtained from haploidentical donors carries an increased risk of developing graft-versus host disease (GvHD). In order to avoid that potential complication after transplantation, immunomagnetic techniques can be used prior to the infusion of the graft: indirect T cell depletion by the enrichment of CD34+ cells or the depletion of unwanted CD3+ T cells and CD19+ B cells.43-45 The method of enrichment of target CD34+ cells is based on magnetic isolation technique using anti-CD 34 mAb conjugated to superparamagnetic iron dextran particles and magnetic cell separator.45 After selection, the number of viable CD34+ and CD3+ cells is determined using flow cytometer. The challenge in the analysis is to determine the count of CD3+ cells in the positive fraction after selection. The residual number of CD3+ cells is usually less than 1% and for analysis is needed special protocol for the rare number of T cells. In addition, multigating strategy protocol should be implemented.46 Nowadays, two different T-cell depletions are used in haploidentical HSC transplantation: in vitro T-cell depletion of PBSC and posttransplantation cyclophosphamide for in vivo T-cell depletion.47 Immunological techniques for depletion of T-cell receptor alpha/beta and CD19+ cells is even more demanding than enrichment techniques. After the depletion process, very few residual TCRαβ+ and CD19+ cells must be determined using flow cytometry.48 For the analysis of the products obtained using immunological techniques, it is very important to devise gating strategies, set up a protocol on flow cytometer and then accurately analyse the cells of interest, because regardless of their very small number in the product, the effect of the procedure is assessed according to result from flow cytometer.
7 IMMUNE EFFECTOR CELLS
Adoptive cellular therapy with IECs is an exciting and rapidly developing field that is evolving from a clinical manufacturing model, generally occurring in academic institutions, to an industry model with centralized manufacturing. Immune effector cells currently comprise cells that express broad cytotoxicity against tumours or targeted cytotoxicity against tumour-associated antigens, as well as the cells that induce tolerance by suppressing inflammatory responses or enhancing immune recognition. Their identity, enumeration, and viability are critical information for initiating manipulation and releasing the products for infusion.49, 50
Despite rapid development, IECs therapy still faces several challenges such as manufacturing processes, logistic and coordination aspects and toxicity profiles. Therefore, the Immune Effector Cell Task Force was created the standards and accreditation program for IEC.51 The standards clearly state that relevant and validated assay should be employed to evaluate cellular therapy products undergoing manipulation that alters the function of the target cell population, where multi-colour flow cytometry is the technology of choice for cell surface marker detection, viability and enumeration. Accurate quantification of viable absolute numbers of cells is a prerequisite for several activities: standardization of input cellular material for CAR-transduced T or NK cell manufacturing, cell selection, in-process quality controls and dosing of IEC.50, 51
CAR-T cell therapy is increasingly applied in clinical practice, in which genetic modification optimizes the T cells to actively proliferate and recognize cancer cells.49 As with CD34+ cells collection for HSC transplantation, obtaining a sufficient concentration of T-cells is a critical part of the collection process. It is therefore important to perform quality control of leukapheresis products using anti-CD3 mAb, because quality of the final product depends on the quality of the starting material used in CAR-T cells manufacturing.42, 52 After the manufacturing process, a sample of the CAR-T cell product is taken for quality control, which includes, among other things, phenotyping, viability and purity of effector cell population.42
In addition to the determination of the quality of the leukapheresis product, flow cytometry is used in the monitoring of expansion and persistence of the therapeutic cells after the infusion and in the evaluation of treatment response in patients who received graft with CAR-T cells.53
8 QUALITY CONTROL AND VALIDATION OF FLOW CYTOMETRY METHODS
Before the introduction of new methods in routine work, it is necessary to perform validation that usually includes assessment of precision and accuracy, linearity, limit of quantification, method comparison between two or more flow cytometers and carryover, and sometimes a sample stability study.37, 39, 50
8.1 Stability of the cellular therapy product samples
Sample stability study is an important part of validation of the protocol used for quality assessment, especially when implementing a new type of cell therapy product. Several studies have examined the stability of fresh leukapheresis samples and thawed cryopreserved UCB samples.37, 39, 54, 55 The results showed that fresh leukapheresis samples were stable up to 24 h stored at 4°C.37, 39 The results of stability studies of thawed UCB samples were conflicting. Lee et al.54 in their study showed that thawed UCB samples were stable up to 6 h after thawing, regardless of whether samples were stored at room temperature or at 4°C. On the other hand, Huang and colleagues reported that CD34+ cell viability decreased significantly after only 20 min when thawed UCB samples were stored at RT.55 Krasna et al.56 evaluated the stability of the HSC products after immunoselection and reported that CD34+ cells after selection were less stable than CD34+ cells in leukapheresis product during refrigerated storage up to 6 days. Since the results of the studies conducted so far have varied depending on the type of cellular therapy products and storage conditions, each laboratory should perform a sample stability study as part of the validation protocol.
Before performing validation, it is necessary to optimize the settings of clones combined with the best possible fluorochromes and also antibody concentration to minimize nonspecific background fluorescence.
In routine work, after initialization and start up procedure, lasers on flow cytometer need to be adjusted and commercial reagents (fluorescent beads) are most commonly used for this purpose. If necessary, spectar overlap compensation can be performed.57
8.2 Internal and external quality control
According to good laboratory practice, it is necessary to provide quality control for the methods used in routine laboratory work. Internal quality control should be performed every day before routine work, for which commercial controls are used, usually from the same manufacturer as the reagents. Beside internal control, it is also important to participate in external quality assessment (EQA). Samples for EQA are usually analysed several times per year, and laboratories decide in which scheme will participate (e.g., for CD34 count, or for lymphocyte immunofentoyping). Laboratories that are part of HSC transplantation program usually participate in the UK NEQAS quality scheme and/or in national schemes.58, 59 It is very important that samples from EQA are processed in the same way as routine samples, because the external control allows periodic verification of the method, and at the same time checks the technical performance of the test. Validation and verification of the methods, internal and EQA are part of the accreditation procedure.60 If the laboratory wants to meet the requirements for accreditation, it is necessary to implement the mentioned procedures.
9 CONCLUSION
Flow cytometry methods nowadays open new views and insights into the quality of various cellular products used in the field of HSC transplantation which greatly contributes to the development and progress of cell therapy. They are now in routine use in clinical as well as in research laboratory work, and application of good laboratory practice, constant education and training are needed for the implementation and maintenance of the methods in this fast-growing field of laboratory testing.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data from this study are available from the corresponding author upon reasonable request.