Comparison of RNA- and DNA-based methods for measurable residual disease analysis in NPM1-mutated acute myeloid leukemia
Louise Pettersson and Sofie Johansson Alm these authors contributed equally to this work.
Linda Fogelstrand and Mats Ehinger these authors share senior authorship.
Abstract
Introduction
Reverse transcriptase quantitative PCR (RT-qPCR) is considered the method of choice for measurable residual disease (MRD) assessment in NPM1-mutated acute myeloid leukemia (AML). MRD can also be determined with DNA-based methods offering certain advantages. We here compared the DNA-based methods quantitative PCR (qPCR), droplet digital PCR (ddPCR), and targeted deep sequencing (deep seq) with RT-qPCR.
Methods
Of 110 follow-up samples from 30 patients with NPM1-mutated AML were analyzed by qPCR, ddPCR, deep seq, and RT-qPCR. To select DNA MRD cutoffs for bone marrow, we performed receiver operating characteristic analyses for each DNA method using prognostically relevant RT-qPCR cutoffs.
Results
The DNA-based methods showed strong intermethod correlation, but were less sensitive than RT-qPCR. A bone marrow cutoff at 0.1% leukemic DNA for qPCR or 0.05% variant allele frequency for ddPCR and deep seq offered optimal sensitivity and specificity with respect to 3 log10 reduction of NPM1 transcripts and/or 2% mutant NPM1/ABL. With these cutoffs, MRD results agreed in 95% (191/201) of the analyses. Although more sensitive, RT-qPCR failed to detect leukemic signals in 10% of samples with detectable leukemic DNA.
Conclusion
DNA-based MRD techniques may complement RT-qPCR for assessment of residual leukemia. DNA-based methods offer high positive and negative predictive values with respect to residual leukemic NPM1 transcripts at levels of importance for response to treatment. However, moving to DNA-based MRD methods will miss a proportion of patients with residual leukemic RNA, but on the other hand some MRD samples with detectable leukemic DNA can be devoid of measurable leukemic RNA.
Abbreviations
-
- AML
-
- acute myeloid leukemia
-
- AUC
-
- area under the ROC curve
-
- BM
-
- bone marrow
-
- CI
-
- confidence interval
-
- det
-
- detectable
-
- ddPCR
-
- droplet digital PCR
-
- ELN
-
- European LeukemiaNet
-
- κ
-
- kappa value
-
- LOD
-
- limit of detection
-
- MRD
-
- measurable residual disease
-
- MFC
-
- multiparameter flow cytometry
-
- NPA
-
- negative percent agreement
-
- NPV
-
- negative predictive value
-
- NGS
-
- next generation sequencing
-
- PB
-
- peripheral blood
-
- PPA
-
- positive percent agreement
-
- PPV
-
- positive predictive value
-
- qPCR
-
- quantitative PCR
-
- ROC
-
- receiver operating characteristic
-
- RT-qPCR
-
- reverse transcriptase quantitative PCR
-
- R s
-
- Spearman's rank correlation
-
- deep seq
-
- targeted deep sequencing
-
- undet
-
- undetectable
-
- VAF
-
- variant allele frequency
1 INTRODUCTION
Acute myeloid leukemia (AML) with mutated NPM1 is recognized as a distinct entity in the 2016 revision to the WHO classification of myeloid neoplasms.1 Mutations in the NPM1 gene are common in AML, occurring in approximately 30% of all adult cases and in 50%-60% of those with a normal karyotype.1, 2 More than 55 different NPM1 mutations have been described, of which type A is the most common, constituting 80% of the variants in adult AML.3, 4
Assessment of measurable residual disease (MRD) has two major clinical purposes: to evaluate treatment response after induction and consolidation therapy and to detect pending relapse allowing for preemptive treatment.5-7 NPM1 mutations are particularly suitable as leukemia-specific targets for MRD detection since they are common, usually occur de novo, are present in the whole leukemic cell population, and are stable over the course of disease in most cases.2, 3 Numerous studies have shown the importance of NPM1 MRD for prognosis.8-13 In bone marrow (BM), a less than 3 log10 reduction of NPM1 transcripts as compared to the diagnostic level after two cycles of chemotherapy or a failure to reduce NPM1-mutated transcript levels to <200/104 ABL copies post treatment entail a higher risk of relapse and death.9, 11, 14 In peripheral blood (PB), the persistence of NPM1-mutated transcripts after two courses of cytoreductive therapy is an independent prognostic factor for death.15 Yet, there is no single threshold, time point or material (BM vs PB), that consistently is the most predictive among clinical trials.9, 12-16 Consequently, there is a lack of consensus regarding optimal timing and relevant cutoffs for clinical assessment of MRD. In 2018, the European LeukemiaNet (ELN) published the first consensus guidelines for MRD in AML.7 In these, molecular MRD is recommended over the use of multiparameter flow cytometry (MFC) for NPM1-mutated AML. Among the molecular MRD techniques, reverse transcriptase quantitative PCR (RT-qPCR), performed on RNA, is currently preferred because of its high sensitivity, reflecting numerous copies of mutated mRNA for each copy of DNA. It is also the most commonly used method in large clinical studies.6, 9, 11, 12, 14, 15
With the evolution of high-throughput sequencing techniques (next generation sequencing; NGS), new molecular targets have been identified, resulting in the possibility and need for DNA-based MRD analysis.17-20 We and others have demonstrated the power of the DNA-based methods targeted deep sequencing (deep seq) by NGS, quantitative PCR (qPCR), and droplet digital PCR (ddPCR) for MRD detection.8, 21-30 Compared with RT-qPCR, DNA-based methods offer certain advantages. They allow for assessment of other mutations than RT-qPCR, which can only be used for highly expressed genes/gene fusions, and are not affected by variations in gene expression, thus more accurately measuring the fraction of leukemic cells. qPCR is accurate and highly sensitive 8, 29, 31 and neither ddPCR nor deep seq require standard curves or reference genes.25, 28 In addition, deep seq can readily identify all types of NPM1 mutations without the need for mutation-specific primers or probes.21 From a practical point of view, DNA preparation allows for more flexible protocols than those for RNA preparation, and the stability of DNA simplifies the logistics of sample transport.
There is a shortage of comparative studies between RNA and genomic DNA for MRD detection and consequently a lack of knowledge if DNA-based methods can add relevant MRD information or even replace RT-qPCR. Therefore, the aim of this study was to compare MRD results from NPM1 mutations measured by three different genomic DNA MRD methods; qPCR, ddPCR, and deep seq, with the gold standard RT-qPCR performed on RNA. The results show strong correlation between the three different DNA methods with RT-qPCR standing out as the most sensitive. As compared to a defined RT-qPCR BM cutoff of prognostic relevance for assessment of treatment response, the DNA-based MRD methods offer high negative and positive predictive values.
2 MATERIALS AND METHODS
2.1 Patient samples and cell line
The study included samples from 32 patients (15 males and 17 females) diagnosed with AML with NPM1 mutation between 2013 and 2019 and analyzed for NPM1 MRD status by RT-qPCR or qPCR on DNA at Clinical Chemistry/Center for Medical Genomics, Sahlgrenska University Hospital or Clinical Pathology, Lund University Hospital. Mean age at diagnosis was 56 years (range 23-76). In 30 patients, the type A mutation was confirmed at diagnosis and in two patients, the type B and DD5 mutations, respectively. Eleven patients had a concomitant FLT3-ITD; 19 were wild type and in two cases FLT3 status was not determined. To select samples for inclusion in the study, we considered all routine clinical follow-up samples in morphological remission analyzed with RT-qPCR from adult AML patients with mutated NPM1 between 2013 and 2018, thus excluding samples taken at morphological relapse. Due to a high number of samples with undetectable mutated NPM1 transcript, we selected samples with detectable transcripts and the first undetectable sample after any positive time point (including the diagnostic time point), excluding other negative samples. This strategy generated a large MRDlow/undetectable (low/undet) group (for MRD classification, see below) despite exclusion of many repeatedly RT-qPCR negative samples, since only a minority of all clinically analyzed samples were MRDhigh. To balance the ratio of MRDhigh and MRDlow/undet samples, an additional eleven MRDhigh samples from 2019 were included. In total, 132 MRD samples were analyzed; 110 type A (67 BM, 43 PB) from 30 patients analyzed with all three DNA-based methods and 2 type B (1 BM, 1 PB) and 20 type DD5 (7 BM, 13 PB) from 2 patients analyzed with ddPCR and deep seq. DNA was isolated from white blood cells or in a minority of cases from mononuclear cells (14 MRD and 8 diagnostic samples) using QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer's protocol. RNA was isolated from the same material using the QIAsymphony RNA Kit (Qiagen). cDNA synthesis was performed from 900 ng RNA with Superscript (Invitrogen). The leukemia cell line OCI-AML3 (Leibniz Institute DSMZ, no.: ACC 582) was used for dilution experiments with wild-type DNA from healthy blood donors.
2.2 Reverse transcription quantitative polymerase chain reaction (RT-qPCR) and definition of measurable residual disease
Assessment of MRD with RT-qPCR for NPM1 was performed as part of clinical routine according to Kronke et al with ABL1 as reference gene.14 All assays were run in triplicate (with 2.5 µL of cDNA for each 25 µL reaction) on Rotor-Gene Q (Qiagen). The limit of blank and thus the definition of positivity, was based on analysis of 25 samples with wild-type NPM1. The mean number of mutant transcripts was 3.3 for each sample, defining 15 transcripts (3.3 + 3SD) in at least two of three replicates as positivity. Using dilution of RNA from a diagnostic NPM1-mutated AML sample with RNA from a wild-type control sample with the same level of ABL1 expression, the method was shown to be linear down to the above defined level of positivity (Supplementary Figure 1A). In this study, the following definitions were used for MRD classification, based on previous clinical studies showing the prognostic impact of NPM1 MRD. In the first analysis comparing the assay performances of RNA vs DNA-based methods, positivity in the RT-qPCR as defined above was used as cutoff to define MRD positivity (MRDdetectable) in BM and PB. In the second analysis, where the DNA-based methods were compared with RT-qPCR for their ability to capture samples with a high MRD level, BM follow-up samples were dichotomized into MRDhigh and MRDlow/undet. MRDhigh was defined as a less than 3 log10 reduction of NPM1 transcripts as compared to the diagnostic level 9 and/or by demonstrating >200 mutant NPM1 copies/104 ABL copies (>2%),14 and MRDlow/undet as below both of these cutoffs (either detectable at low level or undetectable NPM1 mutation; negative).14, 32 For PB samples, the MRD cutoff was presence (ie, detectable) or absence (ie, undetectable) of leukemic transcripts.15
2.3 Quantitative polymerase chain reaction (qPCR)
qPCR for the NPM1 type A mutation was performed as previously described.8, 29 Briefly, samples were run in duplicates with each PCR reaction containing 125-500 ng DNA. A standard curve was generated for each experiment from a dilution series of the heterozygote NPM1 type A positive cell line OCI-AML3.6, 8, 29 For quantification and determination of limit of detection (LOD), we adhered to the criteria previously defined for acute lymphoblastic leukemia (ALL) 33 and NPM1-mutated AML.6 LOD based on serial dilutions of positive samples was at least 0.001% using 500 ng of input DNA. No false positives were recorded when 30 NPM1 wild-type patient DNA samples or DNA from the AML cell line NB4 were amplified.29
2.4 Digital droplet polymerase chain reaction (ddPCR)
ddPCR was performed with the IBSAFE technology (SAGA Diagnostics AB, Lund, Sweden).28 All assays, using 100 ng of DNA per reaction, were run in duplicates using the Bio-Rad QX Droplet Digital PCR System. The specificity of the ddPCR assays for NPM1 mutations type A, B and DD5 was verified by negative signal for samples with other NPM1 mutations and NPM1 wild-type samples (data not shown). At least 38 human control samples were amplified with mutation-specific PCR primers with no false positives (data not shown and28). The control material was either purchased purified human genomic DNA from Promega or in house preparations of genomic DNA from 10 healthy blood donors. As previously reported, the LOD for the type A mutation by IBSAFE using 180 ng of DNA input, has been shown to be at least 0.0017% variant allele frequency (VAF).28
2.5 Deep sequencing with next generation sequencing (deep seq)
As previously described, the mutation-rich area of NPM1 was amplified with PCR from 100 ng DNA.21 Sequencing was performed on a MiSeq (Illumina, San Diego, CA) using paired-end reads and unique dual indexing with zero allowed mismatches.25 Mutated and wild-type reads were called with an in house-developed tool including variants encountered in the literature and/or the clinical laboratory. The tool “NPM1 Deep Seq” and a list of included variants are available at https://github.com/ClinicalGenomicsGBG/NPM1_DeepSeq. Briefly, VAF of NPM1 mutation is calculated as the number of reads with mutated NPM1 divided with the number of reads with mutated NPM1 plus the number of reads with the wild-type sequence. In all samples, the coverage was >7 × 105 reads. The limit of blank was defined as the mean +3 SDs of 21 normal samples; 8 mutated reads, corresponding to approximately 0.001% VAF. As previously shown, at a sequencing depth of 5 × 105 the method is linear down to 0.02% VAF.22
2.6 Statistical analyses
Data were analyzed using IBM SPSS Statistics version 25 and 26 and GraphPad QuickCalcs. Plots were made in GraphPad Prism version 8. Correlation and agreement between methods were assessed using Spearman's rank correlation (Rs), Bland-Altman plots, and Cohen's kappa coefficient statistic including 95% confidence interval (CI). For assessment of diagnostic accuracy of the DNA-based methods, receiver operating characteristic (ROC) curves were generated with RT-qPCR data as the reference (true) value. Area under the ROC curve (AUC) >0.8 was defined as good and >0.9 as outstanding diagnostic accuracy.34 After having chosen MRD cutoffs for the different DNA methods; sensitivity / positive percent agreement (PPA), specificity / negative percent agreement (NPA), positive predictive value (PPV) and negative predictive value (NPV) including 95% CI were calculated for each MRD DNA method for PB and BM samples separately, for the respective cutoffs. Kappa values (κ) 0.61-0.74 were considered substantial agreement and κ > 0.75 excellent agreement.35, 36 P < .05 was considered statistically significant.
3 RESULTS
3.1 Comparison between DNA-based methods and RT-qPCR
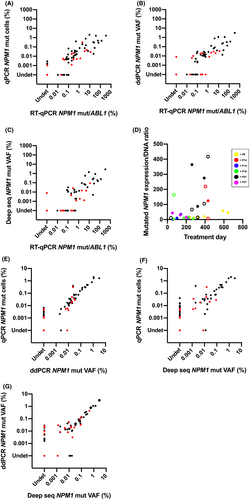
Because of its high sensitivity, RT-qPCR is the recommended method for MRD analysis in NPM1-mutated AML. In order to determine the performance of different DNA-based MRD detection methods as compared to RT-qPCR, we analyzed 110 follow-up samples from 30 patients with NPM1 type A mutation with RT-qPCR, qPCR, ddPCR and deep seq. There was a statistically significant correlation between all three DNA-based methods and RT-qPCR (Figure 1A-C). Among the DNA-based methods, qPCR showed the highest correlation with RT-qPCR (Rs = 0.903 in BM, Rs = 0.936 in PB, Figure 1A), followed by ddPCR (Rs = 0.881 in BM, Rs = 0.774 in PB, Figure 1B) and deep seq (Rs = 0.826 in BM, Rs = 0.743 in PB, Figure 1C), all P < .001. In BM, the DNA results agreed with those from the RT-qPCR analysis in 84% (56/67) of the samples analyzed by qPCR (Cohen's κ 0.640, 95% CI; 0.448-0.832), 81% (54/67) by ddPCR (κ 0.618; 0.445-0.790), and 73% (49/67) by deep seq (κ 0.496; 0.324-0.668) (Table 1a). In PB, the agreement with RT-qPCR for MRD detection was higher, with 95% (41/43) for qPCR with κ 0.890 (0.741-1.000), 88% (38/43) for ddPCR with κ 0.705 (0.468-0.943), and 86% (37/43) for deep seq with κ 0.638 (0.379-0.896) (Table 1a). To assess diagnostic accuracy of the DNA-based MRD methods, sensitivity, specificity, PPV and NPV were calculated across all of them (Table 1b). In BM, the DNA methods showed good specificity and PPV. In PB, all DNA methods proved highly specific (>96%), but qPCR showed superior sensitivity, PPV and NPV (Table 1b). The DNA-based methods failed to detect leukemic signals in a considerable proportion of RT-qPCR-positive BM samples (Table 1c), demonstrating superior sensitivity of RT-qPCR. On the other hand, leukemic DNA was detected by qPCR (and to a lesser extent ddPCR/deep seq) in a minor fraction (~10%) of samples without detectable transcripts with RT-qPCR, despite high RNA quality (Table 1c). To further dissect the relationship between expression levels and the amount of leukemic DNA, that is, leukemic cells, we analyzed the ratio between RT-qPCR and qPCR results in six patients with several (≥3) follow-up samples. The ratio between transcripts and mutated DNA varied both between individuals and within individuals (Figure 1D). Together, these results suggest that transcript analysis is more complex than just a simple measurement of leukemic cell burden.
| a) | |||||||
|---|---|---|---|---|---|---|---|
| RT-qPCR | qPCR | ddPCR | Deep seq | ||||
| Detectable | Undetectable | Detectable | Undetectable | Detectable | Undetectable | ||
| BM (n = 67) | Detectable | 38 | 7 | 32 | 13 | 27 | 18 |
| Undetectable | 4 | 18 | 0 | 22 | 0 | 22 | |
| PB (n = 43) | Detectable | 12 | 1 | 9 | 4 | 8 | 5 |
| Undetectable | 1 | 29 | 1a | 29 | 1a | 29 | |
| b) | ||||
|---|---|---|---|---|
| DNA-based MRD method | Sensitivity / PPA (%) | Specificity / NPA (%) | PPV (%) | NPV (%) |
| BM Detectable leukemic transcripts by RT-qPCR | ||||
| qPCR (95% CI) | 84.4 (71.2-92.3) | 81.8 (61.5-92.7) | 90.5 (77.9-96.2) | 72.0 (52.4-85.7) |
| ddPCR (95% CI) | 71.1 (56.6-82.3) | 100 (93.1-100) | 100 (89.3-100) | 62.9 (46.3-76.8) |
| Deep seq (95% CI) | 60.0 (45.5-73.0) | 100 (93.1-100) | 100 (87.6-100) | 55.0 (39.8-69.3) |
| PB Detectable leukemic transcripts by RT-qPCR | ||||
| qPCR (95% CI) | 92.3 (66.7-98.6) | 96.7 (83.3-99.4) | 92.3 (66.7-98.6) | 96.7 (83.3-99.4) |
| ddPCR (95% CI) | 69.2 (42.4-87.3) | 96.7 (83.3-99.4) | 90.0 (59.6-98.2) | 87.9 (72.7-95.1) |
| Deep seq (95% CI) | 61.5 (35.5-82.3) | 96.7 (83.3-99.4) | 88.9 (56.5-98.0) | 85.3 1(69.9-93.6) |
| c) | ||||
|---|---|---|---|---|
| qPCR | ddPCR | Deep seq | RT-qPCR | |
| False neg. rate (%) | False neg. rate (%) | False neg. rate (%) | False neg. rate (%) | |
| Detectable leukemic cDNA (n = 58) (95% CI) | 13.8% (7.2-24.9) | 29.3% (19.2-42.0) | 39.7% (28.1-52.5) | n/a |
| Detectable leukemic DNA (n = 58) (95% CI) | n/a | n/a | n/a | 10.3% (4.8-20.8) |
- Abbreviations: BM, bone marrow; cDNA, complementary DNA; CI, confidence interval; n/a, not applicable; Neg., negative; NPA, negative percent agreement; NPV, negative predictive value; PB, peripheral blood; PPA, positive percent agreement; PPV, positive predictive value.
- a Same sample.
3.2 Comparison between the different DNA-based methods
In order to determine if the different DNA-based methods are interchangeable, we compared results from qPCR, ddPCR and deep seq. All three DNA methods correlated significantly: Rs = 0.885 for qPCR vs ddPCR and Rs = 0.797 for qPCR vs deep seq for type A mutation analyses, n = 110, P < .001 for both (Figure 1E-F). For ddPCR vs deep seq, type A, type B and type DD5 were analyzed together, n = 132, also resulting in a significant correlation Rs = 0.794, P < .001 (Figure 1G). The excellent agreement between the different DNA-based methods was further visualized with Bland-Altman plots (Supplementary Figure 2A-C). Despite this agreement, qPCR proved most sensitive since it detected mutant DNA in a higher fraction of samples than did ddPCR and deep seq (Table 2). The higher sensitivity was further shown by serial dilution (1:10-107) of the heterozygous NPM1 type A mutated leukemia cell line OCI-AML3 (Supplementary Figure 1B-D). For qPCR and ddPCR, the leukemic signal was completely lost at the 105 dilution, and for deep seq at the 2 × 104 dilution (Supplementary Figure 1B-D). Based on these results, the LOD was at least 0.005% mutated cells for qPCR and at least 0.005% VAF (corresponding to 0.01% mutated cells) for ddPCR and deep seq, respectively.
| n = 110 | ddPCR | Deep seq | |||
|---|---|---|---|---|---|
| Detectable | Undetectable | Detectable | Undetectable | ||
| qPCR | Detectable | 40 | 15 | 34 | 21 |
| Undetectable | 2 | 53 | 2 | 53 | |
| Deep seq | Detectable | 32 | 4 | ||
| Undetectable | 10 | 64 | |||
3.3 Ability of DNA-based methods to classify bone marrow samples according to RT-qPCR level
To evaluate if the DNA-based methods despite their lower sensitivity could classify BM samples regarding MRD levels of relevance for response to treatment, we tested the methods against cutoffs published for the gold standard RT-qPCR. The following definitions of MRD analyzed with RT-qPCR were applied: MRDhigh was defined as a less than 3 log10 reduction of NPM1 mutant transcripts as compared to the diagnostic level9 or as >200 mutant NPM1 copies/104 ABL copies (>2%).14 Both definitions classified all samples the same way (data not shown). MRDlow/undet was defined as positive but below both these cutoffs, or negative. To select the corresponding DNA-based cutoffs in BM, ROC analyses were performed for each DNA method demonstrating excellent diagnostic accuracy (AUC qPCR = 0.974, AUC ddPCR = 0.990, AUC deep seq = 0.987). Based on the ROC analysis and supported by previously published prognostic value of reduction to <0.1% mutated cells,8 the cutoff 0.1% was chosen for qPCR. Consequently, for ddPCR and deep seq, VAF 0.05% (reflecting the heterozygous NPM1 mutation) was chosen. When applying the chosen cutoffs for DNA-based MRD classification and comparing with the RT-qPCR MRD classification, concordant classification was obtained in 93% (62/67) of BM samples with qPCR (Cohen's κ 0.769, 95% CI; 0.577-0.961), 96% (64/67) with ddPCR (κ 0.861; 0.709-1.000), and 97% (65/67) with deep seq (κ 0.910; 0.787-1.000) (Table 3a). Sensitivity, specificity, PPV and NPV for MRD classification are presented in Table 4. Other cutoffs (0.5 and 0.05 for qPCR) were tested with inferior results (data not shown). To assess if the reduction of leukemic DNA in relation to the diagnostic sample would be of value, we compared 3 log10 reduction as analyzed with qPCR vs RT-qPCR (Table 3b). Concordance regarding 3 log10 reduction was found in 94% 61/65 of samples, κ = 0.827 (95% CI; 0.664-0.990). This strategy displayed slightly higher sensitivity and NPV and slightly lower specificity and PPV than direct measurement of leukemic DNA by qPCR, ddPCR or deep seq (Table 4).
| a) | |||||||
|---|---|---|---|---|---|---|---|
| RT-qPCR | qPCR | ddPCR | Deep seq | ||||
| Above cutoff (c) | Below cutoff (c) | Above cutoff (d) | Below cutoff (d) | Above cutoff (d) | Below cutoff (d) | ||
| BM n = 67 | MRDhigh (a) | 11 | 4 | 12 | 3 | 13 | 2 |
| MRDlow/undet (b) | 1 | 51 | 0 | 52 | 0 | 52 | |
| b) | ||
|---|---|---|
| RT-qPCR, BM, n = 65 | 3 log10 reduction of NPM1-mutated DNA | |
| Above cutoff (e) | Below cutoff (e) | |
| MRDhigh (a) | 13 | 1 |
| MRDlow/undet (b) | 3 | 48 |
Note
- a, <3 log10 reduction of leukemic transcripts as compared to the diagnostic level or >2% NPM1 transcripts /104 ABL transcripts; b, ≥3 log10 reduction of leukemic transcripts or ≤2% NPM1 transcripts /104 ABL transcripts as compared to the diagnostic level; c, 0.1% leukemic DNA; d, 0.05% VAF; e, 3 log10 reduction of leukemic DNA as compared to the diagnostic level. BM, Bone marrow; det, detectable; PB, peripheral blood; undet, undetectable.
| RT-qPCR cutoff 2% transcripts or 3 log10 reduction (a) | ||||
|---|---|---|---|---|
| DNA-based MRD method | Sensitivity / PPA (%) | Specificity / NPA (%) | PPV (%) | NPV (%) |
| qPCR cutoff 0.1% (95% CI) | 73.3 (48.1-89.1) | 98.1 (89.9-99.7) | 91.7 (64.6-98.5) | 92.7 (82.7-97.1) |
| ddPCR cutoff 0.05% (95% CI) | 80.0 (54.8-93.0) | 100 (93.1-100) | 100 (75.8-100) | 94.5 (85.2-98.1) |
| Deep seq cutoff 0.05% (95% CI) | 86.7 (62.1-96.3) | 100 (93.1-100) | 100 (77.2-100) | 96.3 (87.5-99.0) |
| Log10 reduction of leukemic DNA (b) cutoff 3log10 (95% CI) | 92.9 (68.5-98.7) | 94.1 (84.1-98.0) | 81.3 (57.0-93.4) | 98.0 (89.3-99.6) |
- Abbreviations: BM, bone marrow; CI, confidence interval; NPA, negative percent agreement; NPV, negative predictive value; PPA, positive percent agreement; PPV, positive predictive value.
- a 2% NPM1 transcripts/104 ABL transcripts or 3 log10 reduction of transcripts as compared to the diagnostic level. b determined by qPCR of the NPM1 type A mutation as compared to the diagnostic level.
4 DISCUSSION
Monitoring MRD in AML patients has strong clinical relevance since accurate MRD detection can identify those at high risk of relapse. In AML with mutated NPM1, RNA-based MRD assessment using RT-qPCR is most established although several DNA-based methods, which all display certain advantages, are available. In this study, we examined the correlation for mutated NPM1 detection in both PB and BM follow-up samples between the gold standard method RT-qPCR and the DNA-based methods qPCR, ddPCR and deep seq. The results show that although the RNA-based method is more sensitive, DNA-based techniques may add relevant information that can be missed by RT-qPCR. Also, for detection of BM levels above an RT-qPCR cutoff of relevance for prognosis, the DNA-based methods showed positive and negative predictive values above 90%.
Since most laboratories have only one method for each MRD marker, published comparisons between RNA and DNA are limited, but support our findings of strong associations between results of RT-qPCR and DNA-based MRD methods. In an early study on NGS-based MRD assessment, including 38 samples from 10 patients, Thol et al demonstrated a 95% concordance between MRD results obtained by sequencing and RT-qPCR of type A mutated NPM1.30 Also, Patkar et al demonstrated a significant correlation between RT-qPCR and deep seq targeting the type A NPM1 mutation in 71 samples, taken at the end of induction and consolidation therapy.37 In AML with t(8;21)(q22;q22), Duployez et al showed a strong correlation between RUNX1-RUNX1T1 levels measured as fusion transcripts by RT-qPCR and as fusion gene copies by break-point specific qPCR.38 In children with AML, we have shown good agreement between MRD levels obtained with patient-tailored deep seq of leukemia-specific mutations and RT-qPCR of RUNX1-RUNX1T1 and KMT2A-MLLT10 transcripts, respectively.22 When we compared detectability of MRD between the DNA-based methods and RT-qPCR in BM, the agreement was rather low, except for qPCR. In PB, the agreement was better. Taken together, these results imply that a proportion of transcript-positive samples will be missed with DNA-based techniques. This is probably of highest relevance when the aim of monitoring is early detection of pending relapse, a situation where RT-qPCR may be superior, at least in cases with fast relapse kinetics. However, in patients with NPM1 mutation without FLT3-ITD, molecular progression often occur over several months before hematological relapse.40, 41
Despite a good correlation between RT-qPCR and DNA-based methods in this study, we noticed that the relation between leukemic transcripts and mutated DNA (RNA copy number per leukemic DNA molecule) fluctuated considerably between different follow-up samples for a number of patients. This indicates that transcript analysis is more complex than a frank translation of residual leukemic cell burden. A high leukemic transcript load may reflect either many leukemic cells or a few cells with high expression. Whether the expressing cells have leukemia-initiating potential or constitute differentiated cells can actually not be determined. Interestingly, we found 10% samples with convincing leukemic DNA signals, but devoid of leukemic transcripts. Thus, interpreting results from MRD assessment may be more complicated than meets the eye, and sensitive DNA-based analyses have the potential to add important information for some patients.
When comparing the three different DNA-based methods with each other, we found a high correlation. Previous studies comparing DNA-based methods for NPM1 MRD detection have also demonstrated a good correlation between the methods. However, those studies were all based on significantly fewer samples compared to this study, often with the aim to verify a new analysis method. We have in different such studies on NPM1 mutation type A shown significant correlations between qPCR and ddPCR in 34 samples28 and between qPCR and deep seq in 8 samples.25 Similar results have been obtained by others in analyses of other mutations in AML, for example, between NGS and ddPCR for mutated IDH1/2.27 Here, qPCR showed detectable levels of mutated NPM1 in more samples than ddPCR and deep seq and detection of diluted OCI-AML3 at lower levels, probably reflecting the higher DNA input in the qPCR assay (DNA inputs were most often 500 ng for qPCR, 100 ng for ddPCR, and 100 ng for deep seq). It cannot be ruled out that the performance of the methods would be similar if equal DNA input is used. One drawback with qPCR is its requirement of both a standard curve and a reference gene – as in RT-qPCR – factors not needed in ddPCR or deep seq. Both qPCR and ddPCR, like RT-qPCR, require separate primers/probes for each specific mutation of interest but have simple workflows and short turn-around-times. For AML with mutated NPM1, most clinical laboratories using one of these methods will therefore not offer MRD analysis for patients with the rarest NPM1 mutations. Deep seq on the other hand simultaneously covers all different mutation types of NPM1 in a single assay, without the need for standard curve, mutation-specific primer/probe or reference gene, but to a higher cost.21 Thus, the different MRD methods all have their advantages and disadvantages, and the appropriate choice of method will depend on the specific mutation, the locally available technique and the purpose of the MRD analysis.
To shed light on the potential of DNA-based techniques for determination of MRD, we compared results from qPCR, ddPCR and deep seq with an RT-qPCR cutoff of high relevance for response to treatment; 3 log10 reduction of NPM1 transcripts as compared to the diagnostic level and 200 mutant NPM1 copies/104 ABL copies.9, 11, 14, 15, 32 Hubmann et al showed that a 3 log10 reduction in NPM1/ABL1 mutation ratio in BM after induction therapy was the most appropriate threshold to identify patients at high risk of relapse.9 The 3 log10 reduction in BM was confirmed by Ivey et al, who also demonstrated that an even stronger predictor of relapse and death is persistence of mutated NPM1 transcripts in PB after two cycles of chemotherapy.15 We aimed especially for high specificity for calculation of relevant DNA cutoffs, in order not to inflict a risk of overtreatment when risk stratifying with DNA dependent MRD methods. Based on the ROC analyses and available literature, we chose 0.1% leukemic cells for qPCR8 and consequently for a heterozygous mutation 0.05% VAF for ddPCR and deep seq. The high specificity at these cutoffs was confirmed for all three methods. Considering sensitivity, the DNA-based methods correctly classified most, but not all samples defined as MRDhigh. Conversely, almost all MRD results above the cutoff level for the DNA-based methods were also classified as MRDhigh for RT-qPCR, reflecting their high ability to predict clinically relevant MRD. The proposed cutoffs are also in line with the ELN recommendations for flow cytometry, where 0.1% leukemic cells is considered critical for risk stratification of AML patients.7 A limitation of our approach is that we were not able to investigate whether our RT-qPCR assay gave similar results to the assay originally described by Kronke et al,14 which may bias the interpretation. The DNA assays will require validation of their prognostic power in an independent cohort of uniformly treated patients before they can be used to inform treatment decisions.
So far there is no single assay, threshold or time point where MRD analysis can confidently predict the course for the individual patient. Relying on single measurements of MRD with whatever method confers a risk of oversimplification, since some MRD-negative patients will relapse anyway, and conversely, some patients will remain in remission despite detectable levels of MRD.19, 39 Molecular MRD methods are still unavailable for the majority of AML patients that lack NPM1 mutation, RUNX1-RUNX1T1 or CBFB-MYH11 but harbor other mutations. Whether our results on DNA-based methods can be translated to other mutations remains to be tested with the advent of more extensive DNA-based MRD analyses. Important aspects to consider for such translation is whether the mutations of interest reflect clonal hematopoiesis.42
In conclusion, this study shows that DNA-based methods offer high positive and negative predictive values with respect to residual leukemic NPM1 transcripts at levels of importance for response to treatment. This suggests that DNA-based MRD techniques have the potential for determination of clinically relevant MRD, although offering lower sensitivity than RT-qPCR.
ACKNOWLEDGMENTS
This work was supported by the Swedish state under the agreement between the Swedish Government and the County Councils, the ALF-agreement (ALFGBG-720681), Skåne University Hospital Research Grants, Region Skåne UFo grants, Västra Götalandsregionen FoU grants, Swedish Cancer Society, Swedish Research Council, Lund University Medical Faculty, the Olle Engkvist Foundation, and Wilhelm och Martina Lundgrens Vetenskapsfond. LP was supported by the Regional Scientific Council of Halland and SJA by the Sahlgrenska University Hospital.
The authors would like to thank Per Levéen, Annika Billing, Morten Grauslund and Angela Cheng Pettersson for technical support, the staff at Genetic Laboratory, Center of Genomic Medicine, Sahlgrenska University Hospital for assistance with biobanking and routine analyses and Ulf Strömberg and Anders Holmén for statistical support.
5 ETHICS APPROVAL STATEMENT
The study was approved by the Regional Ethical Review Boards at Lund University (diary number 2014/505 and diary number 2017/850) and at the University of Gothenburg (diary number 2017/1138).
CONFLICT OF INTEREST
YC and LHS have ownership interest (including stock, patents, etc) in SAGA Diagnostics AB. There are no other conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
LP, SJA, ME and LF conceived and designed the study. VL, GJ, ME and LF provided samples and clinical information. LP, SJA, GSB, HK, KV, LZ, JA, YC and LHS performed the analyses. AA and GSB created the script for deep seq. LP, SJA, ME and LF analyzed data and wrote the report. All authors approved the final version of the report for publication.
PATIENT CONSENT STATEMENT
Patients consented to biobanking of samples for research purposes.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




