FACSorting help to analyze a rare case of acute myeloid leukemia with concurrent AML1-ETO and PML-RARA
Acute myeloid leukemia (AML) is the most common heterogeneous hematopoietic malignancy in adults, accounting for 80% of acute leukemia cases.1 Chromosomal translocation is the most explored in hematological malignancies, which results in rearrangement of proto-oncogenes or key transcription sites often forming new fusion proteins. The AML1-ETO and PML-RARA, respectively, result from the chromosomal translocation t(8;21)(q22;q22) and t(15;17)(q24;q21), and they are the common chromosomal translocations in AML; however, simultaneous occurrence of both translocations in one patient is rare. Here, we first report a case of concurrent AML1-ETO and PML-RARA using flow cytometry sorting (FACSorting) flowed by morphology, immunophenotyping, fluorescence in situ hybridization (FISH), and molecular technique.
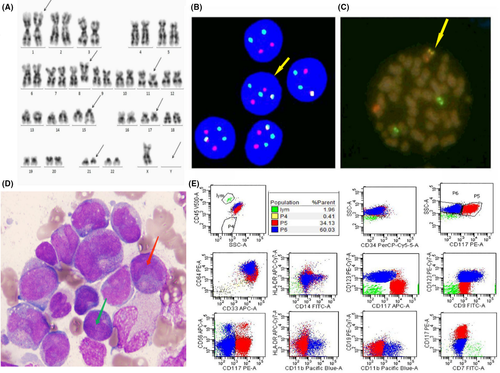
A 17-year-old boy was admitted to our hospital with fever, vertigo, and scattered bleeding petechiae on the skin and mucous membranes over the whole body in April 2018. The patient had been diagnosed with AML by another hospital. Routine blood examination revealed white blood cells count of 14.68 × 109/L, hemoglobin concentration of 79.5 g/L, and platelet count of 34.1 × 109/L. The karyotype 2 showed 45,X,-Y,add(1)(p36.1),der(8)ins(8;21)(q22;q22q22)inv(8)(p23q22),del(11)(p13p14-15),t(15;17)(q24;q21),der(21)ins(8;21)(q22;q22q22) (Figure 1A). Among the 500 interphase nuclei analyzed, nuc ish (ETO, AML1)×3(ETO con AML1×1)[474]/(ETO×2, AML1×3)(ETO con AML1×1)[25]/(ETO, AML1)×2[1] (Figure 1B,C). Combined with the chromosome results of Figure 1A, it is suggested that insertion translocation of chromosome 8 and chromosome 21 was occurred followed by inversion (Figure 1B,C). Combined with the chromosome results of Figure 1A, it is suggested that insertion translocation of chromosome 8 and chromosome 21 was occurred followed by inversion (Figure 1B,C). Bone marrow smears observed 90% immature myeloid cells with two kinds of morphological characteristics. The majority AML patients possessed the features of PML-RARA (Red arrow), with irregular nuclei, visible distortion or folding, many azurophilic granules, and visible internal and external plasma (Figure 1D). The minority AML patients had the morphological features with AML1-ETO fusion gene (Green arrow), containing many azurophilic granules, and obvious superficial staining areas in the nuclear folds (Figure 1D). PML-RARA and AML1-ETO fusion genes were detected by RT-qPCR of 15.3% and 228.33%, respectively. WT1 and ASXL2 gene mutations were found by next generation sequencing. Flow cytometric analysis revealed two distinct aberrant cell populations: (a) CD117- immature myeloid cells and (b) CD117+ myeloid blasts (Figure 1E). These results together confirmed the previous AML diagnosis, and the co-expression of AML1-ETO and PML-RARA fusion genes.
Moreover, the patient was treated with 10 mg arsenious acid for 2 days, 10 mg NIT for 3 days, 4 mg omacetaxine for 3 days, 100 mg cytarabine for 7 days, 3.25 g refined realgar for 7 days, and 20 mg tretinoin for 28 days. One month later, reexamination revealed 50% of bone marrow involvement; PML-RARA and AML1-ETO fusion genes were detected at 221.9% and 27.3%, respectively, indicating that chemotherapy was ineffective. Transplant was indicated for the patient; however, the patient died prior to transplant due to severe infection.
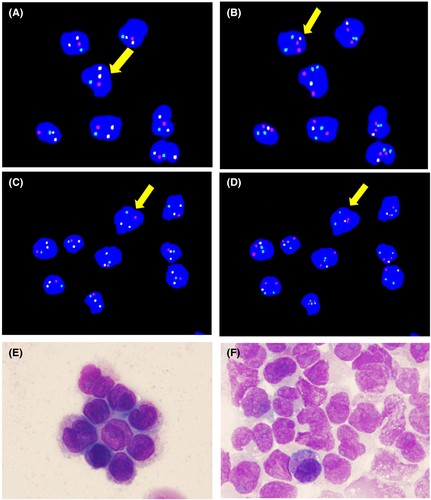
After FACSorting, the CD117+/CD123− and CD117−/CD123+ malignant cells were sent to detect morphology, FISH, and PCR. Hybridization showed yellow signals for both PML-RARA and AML1-ETO fusion genes in malignant myeloblasts, as well as in leukemic promyelocytes (Figure 2A-D). In aberrant promyelocytes, PML-RARA and AML1-ETO fusion transcripts were detected at 150.00% and 37.49%, respectively (Figure 2A-D). Additionally, 44.73% PML-RARA and 205.40% AML1-ETO fusion transcripts were detected in malignant myeloblasts. In terms of cell morphology, the leukemic promyelocytes possessed the characteristic morphological features of PML-RARA (Figure 2E). Malignant myeloblasts had the characteristic morphological features of AML with AML1-ETO fusion gene (Figure 2F). The cell characteristic morphological features of PML-RARA and AML1-ETO were consistent with the description in Figure 1D.
Up to now, there are few reports of AML with the coexistence of AML1-ETO and PML-RARA fusion genes. Charrin et al3 first reported such a case in 1992, wherein three distinct clones were observed in the bone marrow of a patient with leukemia: t(15;17), t(8;21), t(15;17)/t(8;21). Other cases of coexistence of t(8;21) or t(15;17) in leukemia have been described.4, 5 Although cases of PML-RARA co-expression with AML1-ETO are rare, studies on the proximity of nonrandomly associated translocation genes involved in leukemia subtypes to interphase genomic location suggest that PML and RARA genes are relatively close to each other in hematopoietic cells, as are AML1 and ETO,6 suggesting increased probability of AML1-ETO and PML-RARA fusion gene occurrence. Researchers examined AML cases defined as acute leukemia with malignant myeloblasts accompanied by leukemic promyelocytes and found that both PML-RARA and AML1-ETO fusion genes coexisted in a few cases.7 In this study, the cells in the bone marrow sample were divided into significant difference two cell populations and the expression level of the PML-RARA fusion gene in leukemic promyelocytes was higher than that in malignant myeloblasts, while the reverse was true for the AML1-ETO fusion gene. Therefore, a correlation between differentiation and related fusion gene expression could not be made.
A single effect does not directly contribute to the development of AML in patients with AML1-ETO fusion gene, but may require a second-hit factor like the appearance of the PML-RARA fusion gene or other relevant gene mutations.8 The study found that the AML1-S291fs300X mutation was introduced in CD34 stem/progenitor cells and human induced pluripotent stem cells, confirming that the AML1 mutation induced bone marrow differentiation arrest at the myeloblasts stage.9 Among patients with leukemia having both the AML1-ETO fusion and C-KIT gene mutations, most possessed both phenotypic changes, whereas in three patients in complete remission, only the AML1-ETO fusion gene was expressed.8 Therefore, we suspected the C-KIT gene mutation may develop subsequently from the event of the t(8;21), indicating that a stepwise model occurs in leukemogenesis. That is, AML1-ETO represents the first genetic hit to initiate leukemia, while the C-KIT gene mutation may be the second hit for overall leukemia development. Our results indicated that the C-KIT receptor (CD117) was highly expressed in malignant myeloblasts, while the C-KIT gene mutation was negative, ruling out the possibility of a second genetic hit in the C-KIT pathway. Previous reports have implicated ASXL2 gene mutations in AML1-ETO fusion gene pathogenesis,10 but failed to detect WT1 and ASXL2 gene mutations in sorted cells due to the limited sample size. Charrin et al3 asserted that t(8;21) may be the first mutation in early bone marrow stem cells, while t(15;17) is acquired by the patient during the course of the disease. Whether the PML-RARA fusion gene is a secondary genetic hit for the simultaneous occurrence of PML-RARA and AML1-ETO fusion genes in AML needs to be investigated in greater depth.
In summary, the coexistence of PML-RARA and AML1-ETO fusion genes is rare in AML. Although further studies with a greater number of patients are needed, this case is the first one applying FACSorting technique combining with morphology, FISH, and PCR techniques to accurately define the leukemogenesis in patients with concurrent AML1-ETO and PML-RARA fusion genes in AML.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHOR CONTRIBUTIONS
Hui Wang, corresponding author, designed the research and revised paper. Man Chen, first author, analyzed data and wrote the paper. Minjing Fu, Qing Du, and Meiwei Gong involved in test samples and gather clinical data. Junyi Zhen, Ping Wu, Tong Wang, and Hongxing Liu reported results. Xueying Wu and Aixian Wang drew the figure.
ETHICAL APPROVAL
All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Written informed consent was obtained from the patient for publication of this case.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




