Platelets: "multiple choice" effectors in the immune response and their implication in COVID-19 thromboinflammatory process
Abstract
Although platelets are traditionally recognized for their central role in hemostasis, the presence of chemotactic factors, chemokines, adhesion molecules, and costimulatory molecules in their granules and membranes indicates that they may play an immunomodulatory role in the immune response, flanking their capacity to trigger blood coagulation and inflammation. Indeed, platelets play a role not only in the innate immune response, through the expression of Toll-like receptors (TLRs) and release of inflammatory cytokines, but also in the adaptive immune response, through expression of key costimulatory molecules and major histocompatibility complex (MHC) molecules capable to activate T cells. Moreover, platelets release huge amounts of extracellular vesicles capable to interact with multiple immune players. The function of platelets thus extends beyond aggregation and implies a multifaceted interplay between hemostasis, inflammation, and the immune response, leading to the amplification of the body's defense processes on one hand, but also potentially degenerating into life-threatening pathological processes on the other. This narrative review summarizes the current knowledge and the most recent updates on platelet immune functions and interactions with infectious agents, with a particular focus on their involvement in COVID-19, whose pathogenesis involves a dysregulation of hemostatic and immune processes in which platelets may be determinant causative agents.
1 INTRODUCTION
Platelets are small, anucleated blood cellular fragments, traditionally known for their physiological role in hemostasis. In physiological conditions, they circulate in a resting state as small discs but, in the presence of vascular injury, they are rapidly activated and aggregate with one another to form a hemostatic plug on the vessel wall, preventing vascular leakage.1, 2 In particular, platelets contain two main types of secretory organelles, alpha (α)-granules and dense bodies (δ-granules), and most effector functions depend on their secretion. As a consequence of granule fusion with the platelet plasma membrane, several granule molecules may be expressed on the platelet surface or released as soluble molecules (eg, coagulation factors, mitogenic factors, angiogenic mediators, and chemokines) acting either locally at sites of vascular injury or even systemically (Figure 1). In addition, platelet activation is also essential for the initiation of many other innate defense mechanisms, acting by promoting the recruitment, migration, and interaction of immune cells.
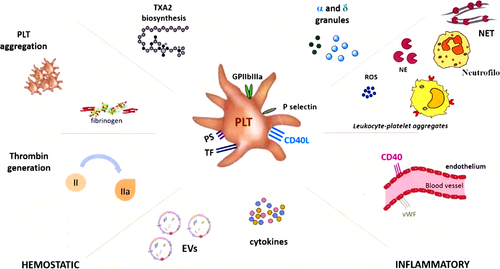
Proteomic studies indicate that α-granules release more than 300 soluble proteins acting in processes such as blood coagulation, inflammation, immunity, cell adhesion and growth, and possibly other less known activities2, 3 (Table 1). Moreover, platelets express a broad range of key receptors involved in the immune response and release a huge amount of extracellular vesicles (EVs) carrying multiple molecules capable to modulate the immune response, and it is possible that different biological activities might be exerted by partially differing platelet populations. This narrative review will examine the role of platelets as “multiple-choice immune effectors” involved in recognition of microbial invaders, with a final focus on their involvement in COVID-19.
| Particle | Biological function | Mediators | Target process/cell |
|---|---|---|---|
| α-granules | Coagulant proteins | Factor V, factor IX, factor XIII | Activation of coagulation |
| Anticoagulant proteins | Antithrombin, protein S, tissue factor pathway inhibitor | Inhibition of coagulation | |
| Fibrinolytic proteins | Plasminogen, plasminogen activator inhibitor 1, α2 macroglobulin | Control of blood coagulation | |
| Adhesion proteins | Fibrinogen, von Willebrand factor, thrombospondin | Clot formation | |
| Integral membrane proteins | GPαIIbβ3, GPIbα-IX-V, GPVI, TLT-1, P-selectin | Interaction with endothelial cells, immune cell recruitment | |
| Chemokines | GRO-α, PF4, ENA-78, MCP-1, MIP-1α, TARC and RANTES | Immune cell recruitment | |
| Costimulatory molecule | CD40L, TREM1 ligand | Interaction with endothelial cells, immune cell recruitment | |
| Growth factors | PDGF, CTGF, stromal-derived factor-1 alpha, VEGF/VEP, TGFβ, TGFα, TNFα, FGF-1 | Recruitment of immune cells, stem cells, endothelial cells, osteoblasts, fibroblasts, and epidermal cells | |
| Angiogenic inhibitors | Tissue inhibitors of metalloproteinases, angiostatin, endostatin | Endothelial cells, endothelial progenitor cells, fibroblasts, and epidermal cells | |
| Microbicidal proteins | Thymosin-β4, thrombocidins 1 and 2 (from NAP-2) | Microbes | |
| Immune mediators | Complement C3 precursor, complement C4 precursor, β1H, Globulin, factor D, factor H, C1 inhibitor | Regulation of immune activation/ switching off | |
| δ-granules | Bioactive amines | Serotonin, histamine | Clot formation and coagulation |
| Nucleotides | ADP, ATP, UTP, GTP | ||
| Phosphates | Polyphosphate, pyrophosphate | ||
| Cations | Ca2+, Mg2+, K+ | ||
| Extracellular Vesicles | Cell cross talk | Proteins, lipids, metabolites, miRNA and nucleic acids | Coagulation, thrombin generation and blood clotting, monocyte activation, cytokine release |
2 EXTRACELLULAR VESICLES DERIVED FROM PLATELETS
EVs are cell-derived membrane particles, ranging from 30 to 5000 nm in size, that have been highly conserved during evolution. Under physiological conditions, EVs circulate in the blood and other body fluids and are important mediators of intercellular communication: They act as shuttle vectors and signal transducers, both locally and at a distance from their site of origin.4 Accumulating evidence shows that EVs are also emerging as biomarkers of several human disorders, including cancer, infections, and autoimmune diseases.3, 4 The most abundant circulating EVs, accounting for 70%-90% of all EVs, are derived from platelets.4 Since EVs transfer proteins, lipids, metabolites, miRNA, and nucleic acids, platelet-derived EVs (PDEVs) may influence various physiological and pathological functions of platelets themselves. Platelets mainly circulate in the blood, whereas PEDVs can reach also other tissues such as the lymph and lymph nodes tissues and might therefore influence both the afferent and efferent arms of the immune response.5
Circulating PDEVs may originate from either megakaryocytes or platelets.6 Those derived from megakaryocytes express on their surface the integrin subunits αIIb (GPIIb, CD41), and GPIb (CD42b), and contain filamin A, whereas those derived from activated platelets express markers of granule fusion with the plasma membrane, like CD62P.6 During their activation and maturation platelets release two different types of EVs, that is, exosomes and microvesicles, respectively,7 but it is not well known their potential extracellular function than the anticoagulant activity attributed to the latter. In general, PDEVs derived from activated platelets may be involved in the coagulation process, either directly or indirectly. Direct exposure of phosphatidylserine on PDEV surface leads to thrombin generation and blood clotting. Conversely, exposure of P-selectin, which binds glycoprotein ligand-1 on monocytes, leads to monocyte activation and production of tissue factor (CD142).
The cargo of PDEVs includes a variety of molecules ranging from miRNAs, mRNA, non-coding RNA, to cytokines and surface proteins. Pro-inflammatory cytokines are released on activated endothelium, promoting leukocyte recruitment, and thus contributing to inflammation during infection. Alternatively, they can directly affect endothelial cells (EC), participating in the development and progression of atherosclerosis.8 In vitro, PDEVs promote the adhesion of neutrophil to EC even when P-selectin is not expressed on EVs surface.9 Moreover, PDEVs released from activated platelets carry IL-1β and caspase-1, capable to promote platelet-neutrophil aggregation both in vivo and in vitro.10 In Dengue infection, PDEVs released from activated platelets activate neutrophils and macrophages through the CLEC5A receptor, induce formation of neutrophil extracellular trap (NET, see below) and release of pro-inflammatory cytokines.11 In systemic sclerosis and septic shock, PDEVs express HMGB1 (high mobility group B1), that can induce neutrophil activation by interacting with TLR412 leading to autophagy and NET production.13 Moreover, HMGB1 expressed on the surface of PLT-derived EVs can be internalized by neutrophils and alter their function. PDEVs can bind also to classical and intermediate monocyte subsets, supporting their activation leading to secretion of inflammatory mediators such as IL-1β, IL-6, and TNF-α, and adhesion to EC through GPIbα.14
Regarding the miRNA content, it has been shown that the expression of miR-223 in PDEVs released from activated platelets regulates ICAM-1 expression via the NF-κB and MAPK pathways.15 Moreover, they carry miR-142-3p enhancing endothelial cell proliferation and dysfunction via Bcl-2-associated transcription factor 1 (BCLAF1).16 Two other miRNAs, that is, miR-15b-5p and miR-378a-3p, induce NET formation.13 Another study showed that PDEVs can be internalized by primary human macrophages and deliver the functional miR-126-3, which modifies their transcriptome and reprograms their function toward a phagocytic phenotype.17 Moreover, PDEVs are also involved in vascular and metabolic diseases, through upregulation of several miRNAs, for example, miR-144-3p, miR-486-5p, miR-142-5p, miR-451a, miR-25-3p, miR-145-5p, and let-7f-5p, having as targets mRNAs involved in vascular remodeling. Platelet-derived exosomes induce NET formation via miR-15b-5p and miR-378a-3p. Lastly, circular RNA, which is a novel class of non-coding RNA, has been identified in PDEVs derived from activated platelets.18
3 PLATELETS AS MODULATORS OF THE IMMUNE RESPONSE
Platelets play a role in the innate immune response, through the expression of Toll-like receptors (TLRs), and in the adaptive immune response by directly modulating lymphocyte activation, mainly by CD40 ligand (CD40L) and major histocompatibility complex (MHC) molecules. Platelets play a sentinel role at sites of vascular injury, which may be crucial in regulating both the inflammatory and the adaptive immune responses, taking part in pathogen clearance and tissue repair (Figure 2). This also involves switching off the immune response, through FasL-mediated triggering of Fas on lymphocytes.
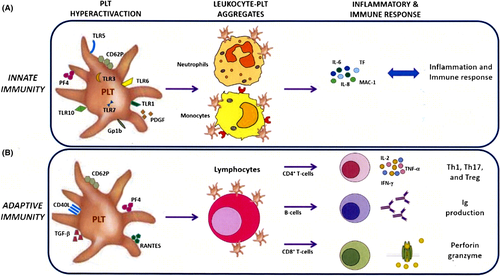
3.1 Platelets as innate immune activators
TLRs play key roles in the innate immune response, which subsequently leads to the activation of antigen-specific adaptive immunity. They are expressed mainly on the membrane of sentinel cells such as macrophages and dendritic cells. They belong to the pattern recognition receptor (PRR) family, recognizing molecules or portions of molecules expressed by pathogenic microorganisms, named pathogen-associated molecular patterns (PAMPs).19
Recently, both human and murine platelets, as well as megakaryocytes, have been shown to express TLR4, which recognizes lipopolysaccharides (LPS) expressed on the surface of Gram-negative bacteria. In damaged infected tissues, pathogens can be recognized directly by platelet TLR4, inducing platelet activation, by the release of pro-inflammatory mediators, and by the recruitment of leukocytes at sites of infection, and improving their ability to remove pathogens. Moreover, circulating pathogens may be bound and sequestered by platelet TLR4, which decreases their systemic dissemination.20
Other platelet receptors that can interact with bacteria are integrin αIIbβ3, FcgRIIa, and glycoprotein Ib-IX. These trigger platelet activation, followed by the release of pro-inflammatory mediators and activation of the coagulation cascade, with positive feedback activity.21
3.2 Platelets as pan-immune activators
Platelets are considered as silos of CD40L: They express CD40L on their surface within minutes from activation, and they are the main source of the soluble circulating form of CD40L (sCD40L). sCD40L can be produced by metalloprotease-dependent cleavage of the membrane form, or, alternatively, by splicing of the CD40L mRNA. Besides, CD40L can be released associated with platelet-derived microparticles. Plasma levels of sCD40L are routinely used as a marker of platelet activation and may be associated with clinical adverse outcomes, such as cardiovascular events.22
CD40L induces inflammatory and pro-thrombotic responses by interacting with CD40. CD40L and CD40 are surface receptors, belonging to the tumor necrosis factor (TNF) and TNF receptor superfamilies, respectively. CD40 was initially identified on B cells, but it is also expressed on other cell types, such as T cells, natural killer (NK) cells, dendritic cells, monocytes, macrophages, as well as on non-hematopoietic cells such as fibroblasts and endothelial cells. Moreover, CD40L can bind the integrins αIIbβ3, α5β1 (CD49e/CD29), and macrophage-1 antigen (MAC-1, or CD11b/CD18, or αMβ2), and CD40L-mediated triggering of integrin αIIbβ3 expressed on platelets is involved in platelet activation in an auto-amplification loop.23
Alongside platelet activation, CD40/CD40L interaction also triggers bidirectional signals, regulating a broad spectrum of cellular and molecular processes involved in activation and progression of the innate and adaptive immune responses. In endothelial cells, CD40 triggering stimulates the expression of adhesion molecules and chemokines, enhancing recruitment of immune cells. In monocytes, macrophages, and dendritic cells, CD40 stimulates cell activation, expression of cytokines, and release of procoagulant tissue factor. In B cells, it co-stimulates activation, isotype switching, and germinal center development. T cells express CD40L and CD40, both displaying costimulatory activity for T-cell activation. Similarly, platelets can co-express CD40L and CD40, and thus support T-cell activation both directly and indirectly, since stimulation of CD40L and, to a lesser extent, CD40 on T cells co-stimulates T-cell activation. Conversely, stimulation of CD40 on antigen-presenting cells potentiates their antigen-presenting cell activity for T-cell activation.24
sCD40L may trigger activating stimuli on CD40, thanks to its ability to oligomerize, mimicking the activity of membrane-bound CD40L. However, sCD40L can also act as a competitive antagonist in the interaction between the membrane-bound forms of CD40 and CD40L, playing a role in down-modulating the immune response.25
3.3 Class I MHC molecules
Platelets also express class I major histocompatibility complex (MHC I), of which two distinct pools can be expressed. Resting platelets express partially denatured MHC I molecules adsorbed from the plasma. They are generally unable to activate CD8 + T cells, and most of them contribute to their inhibition, possibly by triggering negative signals through the CD8 co-receptor. Conversely, activated platelets express functional MHC I molecules associated with α-granules, which can present not only endogenous antigens by the standard cytosolic pathway but also exogenous antigens, by cross-presentation.26 Moreover, they can express key costimulatory molecules for T cells, such as CD86 binding CD28 expressed on T cells and involved in delivery of the “second signal” needed for T-cell activation together with the “first signal” delivered by TCR engagement by the MCH/peptide complex.27 As a result, platelets may act as a double-edged sword, capable of inhibiting or activating CD8 + T cells depending on their activation state and the pool of MHC I used.
3.4 FasL
Activated platelets also express FasL, which triggers the death receptor Fas expressed by several cell types, including activated immune cells. FasL and Fas are surface receptors, belonging to the TNF and TNF receptor superfamilies, respectively. Fas triggering by FasL induces apoptosis of activated lymphocytes and inflammatory cells and plays a key role in shutting down the immune response. FasL expressed by activated platelets might thus be involved in down-regulating the inflammatory and adaptive immune responses. Inherited defects of the FAS or FASL gene cause autoimmune lymphoproliferative syndrome (ALPS), due to defective switching down of the immune response. ALPS is characterized by autoimmunity (chiefly autoimmune thrombocytopenia), lymphadenopathy, and splenomegaly due to accumulation of lymphocytes in the secondary lymphoid tissues, and expansion of T-cell receptor (TCR) αβ + T cells lacking both CD4 and CD8, known as double-negative T cells. Intriguingly, defective Fas function can frequently be detected in patients with immune thrombocytopenia, even in the absence of the clinical features of ALPS or mutations of the FAS and FASL genes.28
FasL expressed on platelets may also play a role in hemostasis and thrombosis, by interacting with Fas expressed on red blood cells. Fas triggering induces externalization of phosphatidylserine on the red blood cell membrane, promoting hemostasis and thrombosis. Inhibition or genetic deletion of either FasL or Fas results in decreased thrombin generation and thrombus formation in vitro, and in reduced protection from arterial thrombosis in vivo.29
Another shutting down system of the immune response, which may be recruited by platelets, is the programmed cell death protein-1 (PD-1) system. PD-1 is a key negative receptor expressed by activated and exhausted T cells and acts as an immune checkpoint to down-regulate the immune response.
4 ROLE OF PLATELETS IN INFECTIONS
The normal blood platelet count is between 150 × 109 and 450 × 109 platelets/L; however, only a small proportion of them (10 × 109 platelets/L) is normally necessary to prevent bleeding, although severe injury or major surgery may consume larger numbers. This has always suggested that the numerous platelets in circulation have other functions besides hemostasis. Recent studies indicate that platelets may play a key role in the immune response against pathogens, by inhibiting their dissemination through the circulation, which would increase the severity of the infection. The thrombocytopenia that may develop during infections can thus be understood as the consequence of platelet direct involvement in defensive mechanisms, and their consequent consumption.
4.1 Neutrophil extracellular traps
Neutrophils not only are key early responders to tissue injury and infection, but are also able to release their nuclear content into the vascular system in order to deceive and capture circulating pathogens. They form networks of chromatin, histones, and degradation enzymes, known as neutrophil extracellular traps (NETs), which are able to capture microbes in the bloodstream, thus limiting their capacity to colonize distant sites.
The classical process of NET generation, named netosis, may be accompanied by a peculiar type of cell death, called suicidal netosis, different from necrosis and apoptosis, whose mechanism is not yet fully understood. Vital and suicidal netosis differ in the triggering stimulus, timing, and mechanisms of DNA release. Suicidal netosis is induced by PMA, requires hours to occur, and releases DNA upon cell lysis. By contrast, vital netosis is induced by the interactions of PAMPS expressed by microbes with specific host receptors, is relatively rapid, and releases DNA upon vesicular trafficking from the nucleus to the extracellular space.
To trigger NET generation, neutrophils must interact with activated platelets. Platelets can be activated by TLR-4 ligands such as LPS, HMGB-1, and flagellin.30
Once activated, platelets can interact with neutrophils through platelet P-selectin directly engaging PSGL-1 (P-selectin glycoprotein ligand-1) on neutrophils and platelet GPIbα directly binding to neutrophil MAC-1. The latter interaction is particularly important to support the interactions between platelets and neutrophils at low shear rates such as those found in arteries. Platelets can also bind to neutrophils through the activated αIIbβ3 integrin that, on neutrophils, can bind either directly the newly identified receptor SLC44A2 or indirectly MAC-1 through fibrinogen bridging.31 The complex formed by platelets plus NETs leads to the formation of “immuno-thrombi,” which appear to function as focal points for microbial elimination. Immuno-thrombi act not only as three-dimensional networks trapping microbes, but also as platforms concentrating leukocytes and lytic enzymes, actively contributing to the elimination of pathogens, and actively modulating the immune response.
Furthermore, the negatively charged nucleotides of NETs are able to trigger the intrinsic coagulation pathway, whose activation leads to the formation of thrombin that, in turn, amplifies platelet activation being a potent agonist of protease-activated receptors (PAR).3
4.2 Platelets and bacterial infections
Bacteria can indirectly bind platelets through fibrinogen, which can form a bridge between platelets and bacteria expressing fibrinogen-specific receptor. In addition, some bacteria, such as Staphylococcus aureus, directly bind integrin αIIbβ3 through iron-regulated surface determinant (Isd), normally used to catch and internalize the heme group of hemoglobin for an appropriate iron supply.32 Finally, some bacterial toxins, such as Streptococcus pyogenes streptolysin O, Streptococcus pneumoniae pneumolysin, and Staphylococcus aureus protein A, can bind to von Willebrand factor (vWF) potentiating attachment to GPIb, even in the absence of shear forces that would be normally required.33
Activated platelets increase their expression of C1qR and CD62P, which may recognize bacteria opsonized by C1q or C3, respectively, and even lead to internalization of bacteria as detected by electron microscopy.32, 34
Platelets activated by thrombin or bacteria can also secrete platelet microbicidal proteins (PMPs), also known as thrombocidins, including platelet factor-4 (PF4), platelet basic protein (PBP), and fibrinopeptides. These proteins are activated by thrombin-mediated cleavage and act by both chemoattracting leukocytes and targeting bacterial membranes. Intriguingly, PF4 binds with high affinity LPS lipid A expressed by Gram-negative bacteria and, then, exposes a neo-epitope recognized by anti-PF4 antibodies. Since PF4 binds many different bacteria, these antibodies will be able to “aspecifically” opsonize all of them allowing their clearance by phagocytes expressing Fc receptors.35
4.3 Viral infections
Several platelet receptors mediate direct binding to viruses; they include integrin αIIbβ3, collagen receptor GPVI, complement receptor 2 (CR2), TLRs 2 and 4, lectins like DC-SIGN or C-type lectin-domain family 2 (CLEC-2), several chemokine receptors. Some of them are known to work as co-receptors for viral entry, such as CCL5 for human immunodeficiency virus (HIV), and enable multiple and complex virus-platelet interactions that may be involved in viral entry into platelets or viral neutralization. Interestingly, these surface molecule are also inducible by platelet agonists such as thrombin, resulting in the amplification of platelet response to viral pathogens.33
Moreover, indirect binding is mediated by the immunoglobulin receptor FcγRIIA recognizing IgG bound to viral particles and triggering platelet activation.33
As for bacteria, viral trapping within platelets has been documented by electron microscopy. Youssefian et al observed platelet endosomes containing HIV particles, likely captured through a dynamin and chlathrin-mediated engulfment.34 Moreover, adenoviruses can be taken up through the membrane-associated open canalicular system,.36 Upon entry, viruses may take advantage of the platelet translational apparatus to synthetize viral proteins and assembly new virions, so that platelets may become virus reservoirs. By contrast, the DNA transcription incompetence of platelets does not allow assembly of DNA viruses.
From a clinical viewpoint, the thrombocytopenia often detected during viral infection can be explained by two mechanisms: downregulation of bone marrow thrombocytopoiesis and peripheral platelet consumption. Thrombocytopoiesis may be decreased either by impairing hepatic production of thrombopoietin (TPO), the main growth factor involved in megakaryopoiesis, by decreasing the sensitivity of the TPO receptor c-Mpl to TPO, or by directly infecting and disrupting bone marrow hematopoietic stem cells.37 Platelet consumption may be increased by direct infection or by their activation either directly or indirectly through the coagulation cascade. To cite just a few examples, TF release is a key mechanism. Platelets are exquisite sensors of endothelial dysfunction and immediately reactive to subendothelial molecules exposed by a disrupted endothelium or by products of endothelial secretion. TF release can also be induced by viruses in lymphnode and circulating macrophages. In turn, TF initiates the extrinsic pathway of the coagulation cascade, again leading to thrombin generation and consequent platelet activation.38 Furthermore, induction of microparticle release (both exosomes and microvesicles) has been described for HIV and Dengue virus as a hallmark of platelet activation. Dengue virus can also activate caspases, resulting in platelet apoptosis, or induce P-selectin exposure of procoagulant phospholipids on the platelet surface, stimulating the coagulation cascade and leading to thrombin generation, which in turn further stimulates platelets.38
Finally, thrombocytopenia and inflammation are also associated with parasitic infections, in particular with malaria. Platelets have been shown to be infected with intact Plasmodium and contain structures suggestive of degraded parasite material in electron microscopy.27
On the whole, platelet role in body defense against pathogens involves plenty of bidirectional and sometimes even redundant signaling pathways able to build up what has been elegantly termed an “immune syntax,” that is, a coordinated strategy whose primary aim is the optimization of host defense.
5 PLATELETS AND CORONAVIRUS DISEASE 2019 (COVID-19)
Platelets play key roles in viral infections, and the recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has demonstrated how the interplay between hemostasis and the immune response is particularly evident. Coronavirus disease 2019 (COVID-19) first emerged in December 2019, originating from a cluster in the seafood market in Wuhan, China, and was declared a pandemic on March 11, 2020. It severely challenged health systems worldwide, making it urgent not only to identify effective treatments and to develop a vaccine but also to understand the pathogenic mechanisms of this insidious and unpredictable disease.
Coronaviruses are a large family of enveloped single-stranded RNA viruses with a nucleocapsid. In humans, they generally cause mild upper respiratory tract infections, but three of them, that is, SARS-CoV, Middle-East respiratory syndrome-CoV (MERS-CoV), and SARS-CoV-2, can cause severe lower respiratory tract infections leading to a severe acute respiratory syndrome (SARS), with high lethality. Angiotensin-converting enzyme 2 (ACE2) is the main receptor used by SARS-CoV-2 to invade several types of host cells. The main targets are lung cells, but others can be the liver, heart, kidney, intestine, brain, and blood vessels. The infection impacts not only on the host immune defenses, but also on hemostasis, metabolisms, and the global plasma lipidome.39 Moreover, proteomic analysis revealed a proteomic signature in COVID-19 patients, which involved proteins associated with the immune response, coagulation, and inflammation.39
Severe cases develop pneumonia with acute respiratory distress syndrome (ARDS) that, in some patients, is associated with a cytokine storm resembling the sepsis-like picture of macrophage activation syndrome (MAS) and progressing to multi-organ failure. A key role in lung damage is played by thrombosis of the perialveolar capillaries, triggered by severe endothelial damage and potentiated by inflammation, a picture known as thromboinflammation. Since platelets play a significant role in thrombosis and inflammation, they may be key players in COVID-19. Indeed, typical hematological features of COVID-19 are thrombocytopenia, lymphopenia, and neutrophilia. Lymphopenia and thrombocytopenia are often already present at admission and are more prominent in severe cases.40
5.1 Thrombocytopenia
A recent meta-analysis reported a significant association between thrombocytopenia and both COVID-19 severity and its mortality in 1 779 COVID-19 patients.41 This finding is supported by other studies, showing that platelet count is an independent predictor of disease progression, with a high negative predictive value (NPV). Interestingly, a relevant predictive role was detected not only for the baseline platelet count but also for its evolution during the clinical course of the infection, so that in-hospital mortality is positively correlated with the magnitude of the decrease.42 Even more specifically, an initial increase in platelet counts when the infection begins has been reported, followed by a rapid drop after the occurrence of severe illness, probably reflecting an initial acute phase reaction, followed by massive platelet activation and consumption.43
COVID-19 thrombocytopenia is likely to be multifactorial. Firstly, it may be related to very high platelet consumption originating from the vasculopathy induced by generalized inflammation. Endothelial damage promotes platelet activation, by increasing the expression of adhesion molecules and receptors like CD40 and at the same time exposing collagen and tissue factor, the latter also being able to trigger the coagulation cascade.44 Moreover, platelet activation results in a vicious circle, since the chemotactic function of active platelets recruits leukocytes and increases endothelial inflammation. This process can be considered part of a more systemic phenomenon, occurring as a result of the cytokine storm following massive immune activation in response to the viral infection.45, 46 In the worst cases, this defense mechanism may end up causing disseminated intravascular coagulation (DIC), a well-known complication of severe COVID-19.41
Exposure of endothelium, platelets, and leukocytes to PAMPs and damage-associated molecular patterns (DAMPs), and to pro-inflammatory cytokines (primarily IL-6), initiates a cascade of events leading to dysregulated thrombin generation and a thromboinflammatory process, in which pro-thrombotic phenomena prevail over hyperfibrinolysis. It seems that in COVID-19 this sepsis-induced coagulopathy (SIC), despite its potential to act systemically, especially targets the lungs and, in particular, the pulmonary microvasculature. Following the induction of primary and secondary hemostasis, microthrombi develop, gas exchange is impaired, and the patient eventually develops ARDS.47 To further worsen the clinical scenario, mechanical ventilation, used in patients with respiratory failure, can also promote damage of alveolar epithelial and endothelial cells (ventilation-induced lung injury, or VILI),48 thus initiating a self-amplifying process challenging patient survival.
A role in thrombocytopenia may also be played by a reduced production of platelets by the bone marrow. This possibility was already described for SARS but it might be true also for SARS-CoV-2, which shows almost 80% of nucleotide identity with SARS-CoV,49 even though this hypothesis has not been proven yet. The lung is known to be the primary site where megakaryocyte fragmentation into platelets occurs since it is the first vascular bed encountered after leaving the bone marrow.50 A study to elucidate the possible pathogenesis of late neonatal thrombocytopenia using mouse models pointed to lung injury as a likely cause, since endothelial damage could impair the ability of pulmonary capillaries to retain megakaryocytes and favor platelet release. Thus not only do platelets have a reduced half-life due to their activation and consumption caused by the vasculopathy, but they also fail to be released from their precursors while in the pulmonary circulation.51 Thus, the loss of the lung's function as "platelet manufacturer” after virus-induced microvascular damage provides an alternative explanation of the platelet count reduction also found in COVID-19.
Another cause of decreased thrombocytopoiesis is direct bone marrow infection by SARS-CoV-2, or induction of an autoimmune response against blood cells including megakaryocytes.41 A role might also be played by liver dysfunction, due to direct liver infection (hepatocytes express ACE2), hyperinflammation, thrombotic microangiopathy, drug-mediated toxicity, and hemodynamic alterations, with hepatic ischemia due to cardiac failure, and hepatic venous congestion due to increased central venous pressure52 (Figure 3A).
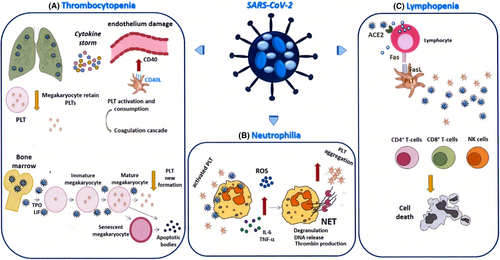
5.2 Neutrophilia
COVID-19 neutrophilia might be ascribed to the cytokine storm that may promote neutrophil production and release from the bone marrow, and cytokine release from the marginated pools of the lungs, liver, and spleen. Neutrophils may play a role in COVID-19 hemostatic dysfunction, by producing NETs capable of interacting with platelets. Platelet activation by pro-inflammatory cytokines and adhesion molecules, expressed by damaged endothelium, sets off a mutual interaction with neutrophils, enhancing platelet degranulation and DNA release to form NETs.53 In addition, the interplay between neutrophils and platelets may partly explain the induction of platelet aggregation and coagulation, a relevant issue especially in an atypical viral infection like COVID-19, characterized by prevailing neutrophilia, rather than lymphocytosis, in which coagulopathy is a possible adverse outcome.54 Indeed, NETs released by activated neutrophils are able to trigger the coagulation cascade, through both platelet-independent and platelet-dependent mechanisms. In the first case, the cell-free DNA (cfDNA) of NETs activates the intrinsic pathway, by interacting with contact factors XII and XI,55 while neutrophil elastase and cathepsin G inhibit tissue factor pathway inhibitor,56 and histones block the thrombomodulin-mediated protein C activation.57 All these effects converge to generate thrombin. Conversely, if NETs come into contact with platelets, the cationic histones forming the chromatin structure of NETs induce platelet activation, likely by interacting with TLR2 and TLR4, or via electrostatic contact with phospholipids,58 leading to aggregation but also to the release of polyphosphates, and triggering the coagulation cascade. This may explain why DNase can, paradoxically, lead to advanced sepsis, since DNA digestion exposes previously hidden histones, which in turn have a greater chance of interacting with platelets.59 In this connection, histone-targeting drugs, but also polyphosphate-targeting substances such as alkaline phosphatase, might be considered as possible future therapeutic strategies also in COVID-19 patients developing coagulopathy (Figure 3B).
5.3 Lymphopenia
In the initial phases of the infection, blood lymphocyte counts are either normal or mildly decreased; however, when the “cytokine storm” blows up, substantial lymphopenia becomes evident, which is an atypical feature for viral infections. Lymphopenia involves a marked reduction of T cells, involving both CD4 + T helper and especially CD8 + T cytotoxic cells, and NK cells, whereas B-cell numbers are reported to be either reduced or normal.42 An increased number of T cells express activation markers, such as CD69, CD38, CD44, OX40, 4-1BB, and exhaustion markers such as PD1.60
Several mechanisms have been suggested to explain lymphopenia. On the one hand, lymphocytes express ACE2 and might thus be infected by SARV-CoV2, with a possible cytolytic effect. On the other hand, hyperinflammation induced by the infection may favor lymphocyte apoptosis, by activation-induced cell death or triggering of death receptors. Indeed, COVID-19 patients display increased numbers of T cells expressing the death receptors Fas and PD-1,60 which can be triggered by their ligands, FasL and PD-L1/L2, respectively: These are widely expressed in inflamed tissues and can also be expressed by platelets themselves. Moreover, the COVID cytokine storm includes the production of high levels of TNFα triggering TNF receptors, which, too, may work as death receptors under certain circumstances (Figure 3C).
5.4 PDEVs and COVID-19
Recent studies reported the emerging role of PDEVs in SARS-CoV-2 infection. We showed that the PDEV count is higher in SARS-CoV-2+ hospitalized patients compared to SARS-CoV-2- ones and healthy controls. PDEVs showed also a good performance as diagnostic biomarkers in discriminating SARS-CoV-2+ from SARS-CoV-2+ patients, and might be possibly involved in the thromboembolism and vascular leakage, which are clinical hallmarks of SARS-CoV-2 infection.61 A recent study showed that activation of the platelet inflammasome leads to release of PDEVs carrying IL-1β and caspase-1 that bind to neutrophils and promote platelet-neutrophil aggregation in lung arterioles.10 Another study showed that, in COVID-19 patients, PDEVs are increased and platelets carry the SARS-CoV-2 RNA. The authors hypothesized that the presence of viral RNA in the endosomal compartment may activate platelet TLR-7, as reported also in influenza and encephalomyocarditis virus infections.62 Moreover, we detected the SARS-CoV-2 RNA in the exosomal cargo from both critical and not critical SARS-CoV-2+ patients, and we suggested that the virus might use this route to spread the infection.63
6 CONCLUSIONS
Platelets are true sentinels of our organism, the front line of a large army that quickly and efficiently coordinates the immune response. Once the pathogen is revealed, platelets are rapidly activated and guide the inflammatory response, producing a wide spectrum of immunomodulatory cytokines, chemokines, and other mediators. Platelets can simultaneously coordinate multiple cells with different functions, such as the endothelium (through adhesion molecules and chemokines), neutrophils (promoting phagocytosis and oxidative burst), and lymphocytes. Thanks to their wide range of adhesion molecules and preformed chemokines, platelets can also adhere directly to leukocytes and facilitate their recruitment to sites of tissue damage or infection, and they participate directly in the capture and sequestration of pathogens within the vascular system. Moreover, thanks to platelet-neutrophil interactions, platelets induce the release of NETs in response to bacterial or viral infection and are capable of trapping pathogens within platelet aggregates. Lastly, platelets provide an abundance of growth factors, cytokines, chemokines, and EVs, with local and systemic effects. All these features may play key roles in COVID-19 pathogenesis.
CONFLICT OF INTEREST
All authors disclose no financial or personal relationships with other people or organizations that could inappropriately influence (bias) this work. No study sponsors were involved in this manuscript.
AUTHOR’S CONTRIBUTION
All Authors participated in the collection of available information from the literature and in the writing of this review. In particular, Alessandra Bertoni wrote the section about NETs and contributed to the final revision of the manuscript. Elena Boggio, Casimiro Luca Gigliotti, Annalisa Chiocchetti, and Giuseppe Cappellano were involved in the sections dedicated to the role of platelets in the innate and adaptive immune response and to platelet-derived extracellular vesicles, also providing data from their own studies in the field. Chiara Puricelli contributed to the section regarding platelet involvement in infections and to the final focus on COVID-19. Roberta Rolla and Umberto Dianzani supervised the whole writing process and gave a substantial contribution to the entire structure of the manuscript. All authors have read and agreed to the published version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data used for this review are available from the corresponding author.




