Current and emerging approaches for assessing von Willebrand disease in 2016
Summary
von Willebrand disease (VWD) is the most common inherited bleeding disorder and is due to a deficiency and/or abnormality of von Willebrand factor (VWF). VWD is inherited in an autosomal dominant or recessive pattern, but women are apparently more symptomatic. Diagnosis of VWD is still difficult in most countries due to the multiple activities of VWF and the heterogeneity of the disease. VWD is mainly associated with mild mucosal bleeding although gastrointestinal and joint bleeds may occur in severe VWD forms. This review describes the most recent clinical and laboratory procedures for the correct diagnosis of VWD. Assays for the evaluation of the platelet-dependent VWF activity (PD-VWFact) with or without ristocetin as well as VWF collagen binding (VWF:CB) are currently in use. However, other tests such as VWF antigen (VWF:Ag), factor VIII procoagulant (FVIII:C), ristocetin-induced platelet agglutination (RIPA), multimeric analysis (VWF:MA), VWF propeptide (VWFpp), VWF:FVIII binding assay (VWF:FVIIIB), and the assessment of biological response to desmopressin (DDAVP) are necessary to characterize VWD types. Levels of VWF activities <30 U/dL have been associated with a bleeding phenotype and the presence of mutations in the VWF gene.
Learning Objectives
At the conclusion of this presentation, participants should be able to:
- Identify patients at risk for VWD according to their history of bleeding using a specific questionnaire to calculate bleeding score (BS)
- Use appropriate laboratory test to identify patients with VWD
- Classify VWD types by interpreting the results of laboratory assays.
- Predict the bleeding phenotype of VWD and the need for replacement therapy using BS and baseline levels of VWF activities.
Introduction
von Willebrand disease (VWD) is due to quantitative and/or qualitative defects of von Willebrand factor (VWF), a multimeric glycoprotein synthesized by endothelial cells and megakaryocytes that mediates platelet adhesion/aggregation and stabilizes factor VIII (FVIII) in the circulation 1-5. VWD has been always considered the most common inherited bleeding disorder, even though its prevalence varies considerably according to the setting of diagnosis 1-5. In population-based studies, prevalence was estimated to be as high as 0.6–1.3% 6, 7, about two orders of magnitude higher than in specialized centers (0.005–0.01%) to which symptomatic patients with VWD are usually referred 8-12. In VWD, bleeding events are caused not only by impaired platelet–VWF interactions, usually assessed in plasma by platelet-dependent VWF activity (PD-VWFact) in the presence or absence of ristocetin and by VWF collagen binding (VWF:CB), but also by reduced FVIII levels that often accompany the VWF defect 1-12.
The current classification of VWD has proposed six different types: VWD1, VWD3, VWD2A, VWD2B, VWD2M, and VWD2N 1. A partial quantitative defect marks VWD1, whereas VWD3 is characterized by the nearly total absence of VWF in plasma and platelets. VWD2A and VWD2B are marked by the absence of high molecular weight VWF multimers in plasma, but in VWD2B, there is also an increased affinity of VWF for its platelet receptor, the glycoprotein Ibα (GpIbα). The identification of qualitatively abnormal variants with decreased platelet-dependent function and a normal multimeric structure marks VWD2M. VWD2N shows a full array of multimers, the defect being in the N-terminal region of the VWF where the binding domain for FVIII is located. The pathophysiology, inheritance, and VWF gene defects of the six different VWD types are summarized in Table 1. Correct classification of different types by clinical and laboratory parameters is important for management of patients with VWD 1-12.
| Type | Pathogenetic mechanisms | Inheritance | Most frequent VWF gene defects |
|---|---|---|---|
| VWD1 | Partial quantitative deficiency of VWF | Autosomal dominant | Missense mutations (85%), null alleles (15%), variable penetrance |
| VWD2A |
Decreased VWF-dependent platelet adhesion due to a loss of HMW VWF multimers |
Autosomal dominant Autosomal recessive |
Missense mutations, mainly in D3, A2, and CK domains Missense mutations in propeptide |
| VWD2B | Increased affinity of VWF for platelet GPIbα | Autosomal dominant | Missense mutations in A1 domain |
| VWD2M |
Decreased VWF-dependent platelet adhesion without a loss of HMW VWF multimers |
Autosomal dominant | Missense mutations in A1 domain |
| VWD2N | Decreased binding affinity of VWF for factor VIII | Autosomal recessive | Missense mutations in D’ and D3 domains |
| VWD 3 | Virtually complete deficiency of VWF | Autosomal recessive | Mainly null alleles, Large–small deletions |
Clinical and Laboratory Approaches for VWD Diagnosis
Three main criteria are required for correct diagnoses of VWD: (i) positive bleeding history since childhood; (ii) reduced VWF activity in plasma; and (iii) history of bleeding in the family with autosomal dominant or recessive inheritance. The clinical, laboratory, and molecular parameters useful for VWD diagnosis and classification are listed in Table 2 and described in a proposed flowchart (Figure 1).
| Clinical parameters for VWD |
| Clinical history: lifelong mucosal, cutaneous, and postoperative bleeding, to be collected with appropriate questionnaires to calculate the Bleeding Score (BS). |
| Family history positive for bleeding and/or other affected VWD (not always) |
| Laboratory parameters for correct diagnosis of VWD types |
| First level |
| Platelet-related VWF activity (PD-VWFact) with different methods (Table 3) |
| VWF collagen binding (VWF:CB) |
| VWF antigen (VWF:Ag) |
| Factor VIII procoagulant (FVIII:C) |
| PD-VWFact/Ag, VWF:CB/Ag, and FVIII:C/VWF:Ag |
| Second level |
| Ristocetin-induced platelet agglutination (RIPA)* |
| VWF multimeric analyses (VWF:MA) on low- and high-resolution gels |
| VWF propeptide measured as ratio with VWF antigen (VWFpp/VWF:Ag) |
| Infusion test with desmopressin (DDAVP) |
| Factor VIII binding assay (VWF:FVIIIB)** |
| Molecular parameters for confirmation of VWD |
| Search for large deletion in VWD3 |
| Search for mutations clustered within specific VWF domains |
| D2-D3-C2-A2-CK (VWD2A); D3 (VWD1/2M Vicenza) D’-D3 (VWD2N) |
| A1 (VWD2B and VWD2M) |
- For the use of these tests see the diagnostic flowchart in Figure 1.
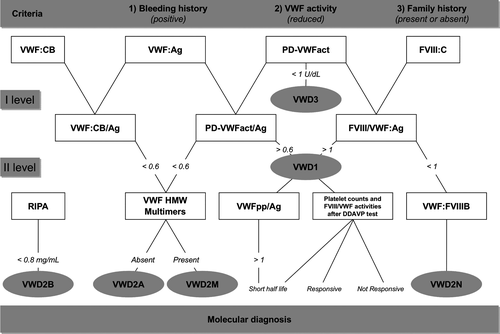
The clinical parameters include both personal and family history of bleeding; the presence of other affected members within the family is important to determine whether the inheritance is autosomal dominant or recessive. Clinical manifestations are excessive mucosal and cutaneous bleeding with prolonged oozing after surgical procedures. In women, menorrhagia may be the only clinical manifestation. Soft tissue and joint bleeding are rare, except in patients with VWD3, characterized by severe deficiencies of both VWF and FVIII. The clinical expression of the disease is usually mild in most patients with VWD1 and VWD2N, whereas severity increases in VWD2M, VWD2B, VWD2A, and particularly in VWD3. While in classical hemophilia there is an excellent relationship between plasma levels of FVIII, frequency, and severity of clinical bleeding, such a relationship is less clear and straightforward in VWD. A plasma VWF level of 30 IU/dL has been suggested as a threshold to distinguish patients with a bleeding tendency from healthy subjects with low-borderline plasma levels of VWF 13. Usually, the bleeding history is an essential criterion for the diagnosis of inherited bleeding disorders, including VWD 14, 15. A bleeding score (BS) based upon bleeding symptoms and calculated using the questionnaire proposed by Tosetto et al. 16 was used to confirm the diagnosis in a large cohort of European families with type 1 VWD 17; it was subsequently applied with some modifications to other clinical studies on VWD 18, 19. More recently, in a limited number of patients with some VWD types (1, 2A, 2B, 2M), attempts were made to use the BS, not only for diagnostic purposes, to evaluate the patients’ tendency to bleed 20-22. It has been suggested that BS > 3 and BS > 5 in males and females, respectively, constitute useful cutoffs to identify individuals for whom measuring VWF activities is worthwhile. Pediatric cases should be evaluated using less stringent criteria 23, 24. However, since a young child may have had no hemostatic challenges at all, the correct diagnosis of VWD requires in most cases repeated assessment of VWF activities and an accurate family history. More recently, a more comprehensive bleeding assessment tool (ISTH-BAT) has been recommended by the International Society on Thrombosis and Haemostasis 25.
In contrast to hemophilia A that requires only two parameters for diagnosis, namely the prolonged partial thromboplastin time (PTT) with low levels of FVIII, several laboratory tests are always necessary to diagnose VWD types (Table 2). Among other general diagnostic tools used in the past, the bleeding time (BT), the original hallmark of the disease, is not always prolonged and may be normal in patients with mild forms, such as those with VWD1 and VWD2N 1-5. Hence, it is not particularly useful for diagnosis. Evaluation of closure time (CT) with the Platelet Function Analyzer (PFA-100) gives a rapid and simple measure of VWF-dependent platelet activity at high shear stress; it can be performed on whole blood and therefore can be employed instead of the BT in children or when the BT is not feasible. This system is sensitive and reproducible for VWD screening, but the CT is normal in VWD2N and cannot be modified in VWD3 after the administration of VWF/FVIII concentrates 26. Based on these observations, BT and CT are not introduced in the flowchart proposed for the differential diagnosis of VWD types (Figure 1).
First Level Laboratory Tests
In patients with VWD, the severity of bleeding correlates with the degree of reduction in VWF activities. The most appropriate tests to identify patients with VWD are those that measure in plasma the interaction between VWF A1 domain and its specific platelet receptor, Glycoprotein Ibα (GPIbα). As there are many assays currently available for assessing this VWFA1–GPIbα interaction, a panel of experts has recently updated the correct nomenclature to be used to identify the type of assay (Table 3). In this Ms, all the assays assessing VWFA1–GPIbα interaction will be generally indicated as platelet-dependent VWF activity (PD-VWFact) following the recommendations of the Sub-Committee on VWF for the Scientific Standardization Committees (SSC-SC on VWF) of the International Society on Thrombosis and Haemostasis 27.
| Assay | Activator | Description of activity |
|---|---|---|
| VWF:RCo | Ristocetin | Ristocetin-induced VWF binding to GPIbα on platelets |
| VWF:GPIbR | Ristocetin | Ristocetin-induced VWF binding to recombinant wild-type GPIbα fragment |
| VWF:GPIbM | – | Spontaneous VWF binding to recombinant gain-of-function mutant GPIbα fragment |
| VWF:Ab | – | Binding of monoclonal antibody to the GPIbα binding site in VWF (A1 domain epitope) |
- Additional information published by Bodo et al. 27.
The VWF ristocetin cofactor (VWF:RCo) assay, originally developed in mid 1970s, is based on the property of the antibiotic ristocetin to agglutinate formalin-fixed normal platelets in the presence of VWF. This method is specific for VWF abnormalities, but it is not very sensitive (values < 15 U/dL not reliable) and not always reproducible (inter- and intra-assay CV of 8–15%). A number of modifications to the original VWF:RCo assay have been published. Several diagnostics companies have produced more reliable reagents and assays that can be automated on common photo-optical coagulation analyzers. This allows turbidimetric measurements and faster availability. The first commercially available automated VWF:RCo was produced by Siemens (BC VWF reagent) using Siemens BCS analyzers. Instrumentation Laboratory has developed an improved version of the VWF:RCo assay; recombinant wild-type GPIb has been coupled with uniform beads making the assay completely platelet free. The assay is available in two versions based on turbidimetric or chemiluminescence detection; both systems are precise and suitable for VWD diagnosis 28-31. More recently, automatic VWF:GPIb-binding assays independent of ristocetin have been developed and introduced into general use with promising results 32, 33.
The VWF collagen binding assay (VWF:CB) is particularly sensitive to VWD variants characterized by the absence of the larger VWF multimers 34, 35 Therefore, VWF:CB is often used as an alternative to multimeric analysis and VWF:CB/Ag ratios are useful in distinguishing VWD2A from VWD2M. However, in rare patients with mutations in the A3 domain (W1745C and S1783A) with normal multimeric structure, the VWF:CB/Ag is abnormal in the presence of normal VWF:RCo 36. Both PD-VWFact and VWF:CB activities are important to identify patients with VWD as levels of these activities <40 U/dL are usually associated with bleeding. However, to establish the VWD types both activities must be always compared with the concentrations of the protein measured as VWF antigen (VWF:Ag). In fact, in patients with normal VWF structure (VWD1 and VWD2N), PD-VWFact values are similar to VWF:Ag (PD-VWFact/Ag ratio >0.6). PD-VWFact/Ag ratios <0.6 are characteristic of VWD2A, VWD2M, and most cases with VWD2B 35. In the past, VWD1 was reported to be the most frequent form of VWD, accounting for approximately 70% of cases. A reappraisal of VWD diagnoses after 10 years (1998–2008) in 1234 Italian patients showed only 671/1234 (55%) patients with VWD1, because many cases previously diagnosed as VWD1 were re-diagnosed as VWD2A or VWD2M due to discrepant VWF:RCo/Ag ratios 2. The presence of qualitative defects of VWF in previously diagnosed VWD1 has been also reported in 154 families evaluated prospectively by the European Study 20; in this cohort of patients, a VWF:RCo/Ag ratio <0.6 was predictive of structural abnormalities and mutations within specific regions of VWF gene 17. After these observations, the cutoff level of 0.6 for the PD-VWFact/Ag and VWF:CB/Ag ratios has been introduced in the flowchart diagnosis (Figure 1).
The procoagulant activity of factor VIII (FVIII:C) is usually very low (1–5 U/dL) in patients with VWD3, who are characterized by undetectable levels of VWF:Ag. In patients with VWD2A, VWD2B, and VWD2M, FVIII:C is normal in most cases. VWF is the carrier of FVIII; in normal individuals, the proteins are found in the circulation as the FVIII/VWF complex with a FVIII:C/VWF:Ag ratio of 1. The FVIII:C/VWF:Ag ratio can be a useful laboratory marker, because a ratio >1 suggests VWD1 and <1 suggests VWD2N 1-5. However, additional tests should be always performed to confirm the diagnosis of patients with VWD1 and VWD2N.
Second-Level Laboratory Tests
Ristocetin-induced platelet agglutination (RIPA) is measured by mixing different concentrations of ristocetin and patient platelet-rich plasma (PRP) in the aggregometer. Results are expressed as the concentrations of ristocetin (mg/mL) able to induce 30% agglutination. Most VWD types show a low response to ristocetin (>1.2 mg/mL of ristocetin concentration), but an important exception is VWD2B, where there is hyper-responsiveness to ristocetin (<0.8 mg/mL) due to a higher than normal affinity of VWF for platelet GPIbα 20. A similar enhanced RIPA can be found in platelet-type VWD (PT-VWD) 1. Both VWD2B and PT-VWD can be associated with thrombocytopenia.
Normal VWF is composed of a complex series of multimers with molecular weights ranging from 800 to 20 000 kDa, which can be analyzed by agarose gel electrophoresis. Low-resolution agarose gels distinguish VWF multimers, which are conventionally indicated as high, intermediate, and low molecular weight. In VWD1, VWD2M and VWD2N, all multimers are present, whereas in VWD2A the high and intermediate molecular weight multimers are missing. Most VWD2B show the loss of high molecular weight multimers, although there are patients with relatively normal multimers 20. VWF multimeric analysis with high-resolution agarose gels can be useful to further characterize patients with VWD2A (VWD2A, subtypes IIC, IID, IIE, IIF, IIG, IIH), as reported 1.
The VWF propeptide (VWFpp) and VWF proteins remain noncovalently associated and stored in alpha-granules of megakaryocytes/platelets or Weibel–Palade bodies in endothelial cells for regulated release. In plasma, VWFpp and mature multimers dissociate and circulate independently. VWFpp circulates in plasma as a homodimer with a half-life of 2–3 h, while mature VWF circulates with a half-life of 8–12 h 3, 4. For these reasons, the ratio between VWFpp and VWF:Ag has been proposed to identify VWD1 patients with reduced VWF survival 37, 38.
Desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) is a synthetic analogue of vasopressin that is relatively inexpensive and carries no risk of transmitting bloodborne infectious agents. DDAVP, infused intravenously at a dose of 0.3 μg/kg diluted in 50 mL saline over 30 min, usually increases plasma VWF and FVIII 3–5 times above baseline levels within 30–60 min; in general, high VWF and FVIII levels last for 6–8 h 39, 40. A test dose of DDAVP is recommended in patients with VMD at the time of diagnosis to establish the individual patterns of biological response and to predict clinical efficacy during bleeding, as the responses in a given patient are consistent on different occasions 39, 40. A DDAVP challenge test is an important tool for VWD management, because patients with VWD can be divided according to their biological response into three different groups: short half-life, responsive, not responsive 40. An increased ratio of VWFpp/VWF:Ag usually correlates with a short half-life of VWF activity after DDAVP 34, 35. Knowledge of the biological response after such an infusion at the time of diagnosis is important, because patients with VWD can be identified as being unresponsive or having a short-lived responsive to DDAVP. Such patients should be shifted to the use of VWF/FVIII concentrates 1-5. The use of DDAVP challenge test at the time of diagnosis with the assessment before and after DDAVP injection of platelet counts and all the VWF activities, with the calculation of their ratios with VWF:Ag, namely VWF:PLAct/Ag, VWF:CB/Ag, VWF:PP/Ag, and FVIII/VWF:Ag, may allow the identification of VWD types (Figure 1). Indeed, in case of reduced platelet counts 1–2 h after DDAVP together with PD-VWFact/Ag, VWF:CB/Ag <0.6, VWD2B can be identified without using RIPA. On the other hand, the measurements of VWF:PLAct and VWF:CB after DDAVP with the ratios of VWF:PLAct/Ag and VWF:CB/Ag may identify abnormal structure of VWF typical of VWD2A and VWD2M patients.
The VWF binding assay to FVIII (VWF:FVIIIB) measures the affinity of VWF for FVIII. In this assay, anti-VWF antibody is coated on wells of a microtiter plate and test plasma is added to the wells. The FVIII/VWF complex in plasma is bound by the antibody after which FVIII is removed from the complex by a high ionic strength buffer. Excess recombinant FVIII (rFVIII) is then added and, after removal of unbound rFVIII, the VWF and the bound rFVIII are assayed 1-5. This assay allows VWD2N to be distinguished from mild-to-moderate hemophilia A.
The Role of Genotype in VWD Diagnosis
Molecular diagnosis can be useful to confirm specific VWF defects in VWD families, especially those with VWD2A, VWD2B, VWD2M, and VWD2N as mutations are clustered in specific exons of VWF gene 1-5. In VWD3 patients, no specific mutations can be used as molecular markers for the disease as gene defects are spread throughout the entire VWF gene. However, large deletions should be sought because they can be associated with the appearance of alloantibodies against VWF. In VWD1, the probability of finding mutations within the entire VWF gene is high only when VWF levels are below 30 U/dL. It is still not clear whether most mild VWD1 patients really have a mutation in the VWF locus and the possibility of external modifiers of VWF levels should be considered 1-5.
Clinical and Laboratory Definition of Severe vs. Mild VWD
VWD3 is always classified as severe by definition as VWF levels are undetectable in both plasma and platelets with relatively low amounts of FVIII:C (<20 U/dL) in plasma 1-5. Conversely, VWD1, VWD2A, VWD2B, VWD2M, and VWD2N can be very heterogeneous and their clinical presentation is strictly correlated with the circulating levels of functional VWF activity. Utilizing such a definition of ‘clinical severity’ based on the levels of defective VWF:RCo and FVIII:C, three different groups of VWD could be identified in the 796 Italian patients according to the inclusion criteria of RENAWI-2 5. Considering the extreme heterogeneity of VWD, the ‘severe forms of VWD’ might represent the ‘tip of the iceberg’ overlying a large number of patients with moderate–mild VWF defects. While no diagnostic problems occur in moderate–mild VWD with levels of VWF:RCo <30 U/dL, a definite diagnosis of VWD is often difficult to make in patients with very mild VWD forms and VWF:RCo levels >30 U/dL. In fact, it is well known that the physiological changes of VWF levels and the variability of the VWF:RCo assays can obscure mild defects of VWF. In the very mild VWD, the limit between ‘disease’ and ‘reduced levels of VWF in a normal individual’ can be difficult in the absence of bleeding history in other members of the family, as discussed previously 13. By contrast, many mild VWF defects remain undiagnosed in the absence of well-documented personal and family bleeding histories. The use of the analytic approach suggesting that all the three major criteria (bleeding, reduced VWF activity and affected family members) should be satisfied might help to distinguish mild VWD from normal individuals with low VWF 13. BS together with threshold levels of VWF:RCo and/or FVIII has not only been useful to confirm the diagnosis, but also should be considered a predictor of clinical outcomes as recently observed in a large cohort of Italian patients with different VWD types 5. Indeed, in RENAWI-2 the bleeding rates (any, mucosal and/or nonmucosal) were different according to different BS, VWF:RCo and FVIII:C levels. BS>10 was associated with the highest incidence of both mucosal [20.63 per 100 patient-years (95% CI: 12.20–29.06)] and nonmucosal bleeding [12.54 per 100 patient-years (95% CI: 6.90–19.95)].
Conclusions and Future Perspectives
VWD is the most common inherited bleeding disorder due to the heterogeneity of VWF defects. The clinical diagnosis and classification of VWD can be difficult because of the widely variable phenotype. The likelihood of diagnosing VWD correctly improves when the clinical assessment of bleeding is correlated with appropriate laboratory studies. Molecular diagnosis can be useful to confirm specific VWF defects in VWD families. It is still not clear whether most mild VWD1 patients really have a mutation in the VWF locus. Despite its complex and heterogeneous nature, VWD can now be efficiently diagnosed and classified in most Western countries. A correct diagnosis and classification of VWD provides the best therapeutic approach to patients with VMD.
Acknowledgements
We wish to thank all the members of the Italian Association of Hemophilia Centers who participated in the Italian Registries of VWD (RENAWI1 and RENAWI2). We acknowledge the work of Luigi Flaminio Ghilardini, who prepared the figures reported in this manuscript.
Conflicts of Interest
ABF has been involved in advisory boards and received honoraria as a speaker at educational meetings organized by Baxalta, Csl-Behring, Grifols, Kedrion Biopharma, LFB, Octapharma, Werfen-Instrumentation Laboratory.




