Laboratory diagnosis of thalassemia
Summary
The thalassemias can be defined as α- or β-thalassemias depending on the defective globin chain and on the underlying molecular defects. The recognition of carriers is possible by hematological tests. Both α- and β-thalassemia carriers (heterozygotes) present with microcytic hypochromic parameters with or without mild anemia. Red cell indices and morphology followed by separation and measurement of Hb fractions are the basis for identification of carriers. In addition, iron status should be ascertained by ferritin or zinc protoporphyrin measurements and the iron/total iron-binding capacity/saturation index. Mean corpuscular volume and mean corpuscular hemoglobin are markedly reduced (mean corpuscular volume: 60–70 fl; MCH: 19–23 pg) in β-thalassemia carriers, whereas a slight to relevant reduction is usually observed in α-carriers. HbA2 determination is the most decisive test for β-carrier detection although it can be disturbed by the presence of δ-thalassemia defects. In α-thalassemia, HbA2 can be lower than normal and it assumes significant value when iron deficiency is excluded. Several algorithms have been introduced to discriminate from thalassemia carriers and subjects with iron-deficient anemia; because the only discriminating parameter is the red cell counts, these formulas must be used consciously. Molecular analysis is not required to confirm the diagnosis of β-carrier, but it is necessary to confirm the α-thalassemia carrier status. The molecular diagnosis is essential to predict severe transfusion-dependent and intermediate-to-mild non-transfusion-dependent cases. DNA analysis on chorionic villi is the approach for prenatal diagnosis and the methods are the same used for mutations detection, according to the laboratory facilities and expertise.
Introduction
Thalassemia syndromes are a heterogeneous group of hemoglobin disorders due to a decreased or absent production of normal globin chains. They are the most common recessive diseases worldwide, with an estimation of 1–5% of the global population carriers of a genetic thalassemia mutation 1. The thalassemias are most frequent in southeastern and southern Asia, in the Middle East, in the Mediterranean countries, and in North and Central Africa; however, in view of continued migration, thalassemias are now also becoming increasingly common in North Europe and North America 2, 3. These changes have challenged health professionals and policymakers throughout the region in providing equitable access to quality services for prevention, diagnosis and adequate treatment of thalassemias 4.
The thalassemias can be broadly characterized as α- or β-thalassemias, depending on the defective globin chain and on the underlying molecular defects; they are recessive trait; thus, the clinical relevant phenotypes result from homozygosity or double heterozygosity for different globin gene defects. They can also manifest from co-inheritance of thalassemia trait and structural hemoglobin variants such as hemoglobin S, C, and E. Several forms of hemoglobin E/β-thalassemia, S/β-thalassemia, and hemoglobin C/β-thalassemia are common, yet these forms need molecular analysis as they have unique characteristics and management peculiarities 5. Without treatment, the hallmark of thalassemia syndromes is the imbalance in the α/β-globin chain ratio leading to ineffective erythropoiesis. The unstable free globin chain tetramers precipitate in erythroid cells leading to premature cell death inside and outside (peripheral hemolysis) the bone marrow. The resulting clinical manifestations are bone marrow expansion, increase function of the spleen (splenomegaly) and a chronic hemolytic anemia 6, 7.
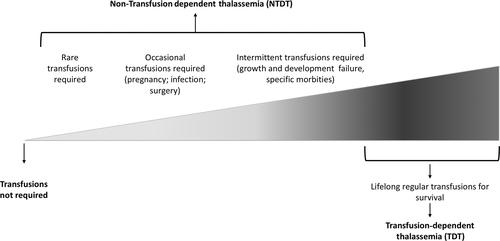
Transfusion therapy remains the standard treatment for the severe forms of thalassemia, and the frequency and transfusion requirement indirectly reflects the underlying disease severity. Moreover, transfusion therapy is able to control the majority of underlying pathophysiologic mechanisms while also contributing to a great deal to secondary morbidity 8-11. Today, for management purposes, the thalassemia patients are commonly categorized into transfusion dependent (TDT: those who are not capable to produce sufficient amount of Hb to survive without blood transfusion) or non-transfusion-dependent (NTDT) 12, 13 (Figure 1). A proper laboratory diagnosis is crucial for characterizing the different forms of thalassemia with important implications for prevention and treatment.
Laboratory diagnosis of thalassemias: hematological tests
Being recessive condition, recognition of carriers, is essential and possible by hematological tests. Both α- or β-thalassemia carriers (heterozygotes) present with microcytic hypochromic parameters with or without anemia, which requires a differential diagnosis to exclude iron-deficient anemia. After ferritin or zinc protoporphyrin measurement 14, family history and ethnicity may provide useful information in approaching the laboratory diagnosis of thalassemias. The hematological parameters including red cell indices and morphology, followed by separation and measurement of Hb fractions are the basis for the identification of thalassemia carrier. A flowchart for detecting thalassemia carrier is reported in Figure 2.
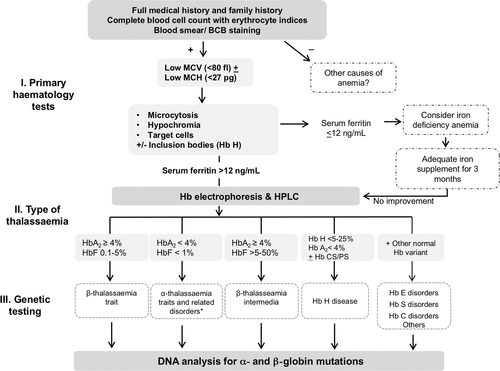
In some high-risk areas, strategies have been implemented for the identification of carriers based on a primary prescreening with red cell indices separation and measurement of Hb fractions for those with reduced mean corpuscular volume (MCV) and/or MCH; however, the screening strategies are very much depending on frequency of the disease, heterogeneity of population and genetic defects, resources available and social, cultural and religious factors. In our multi-ethnic societies, preselection based upon MCV and MCH is not advisable. Not to miss severe normocytic traits, separation and measurement of the Hb fractions should be performed together with blood count 15.
Moreover, the screening can be targeted at different age groups being the newborn screening for thalassemias too late for prevention and therefore not advisable. Numerous carrier screening programs are conducted around the world at the premarital or early pregnancy level, and they can be divided into mandatory or voluntary programs. Recently, in some Islamic high-risk countries, such as Iran, Saudi Arabia, and Palestinian territories, the hemoglobinopathy screening has became mandatory for all couples before having the approval to get married 16, 17.
Beta-thalassemia carriers
At present, there are more than 200-point mutations and deletions of different severity described for the β-globin gene (http://globin.cse.psu.edu/hbvar/menu.html). The different types of beta-thalassemia mutation produce clinical and hematological phenotypes of variable severity even in beta-carriers (the identification of thalassemia major or intermedia will be discussed later in this paper). The red blood cell count (RBC) and the derived indices are extremely important in the diagnosis of asymptomatic carriers. Their determination is the most common laboratory test even in poor countries, and it is usually carried out by automated electronic cell counters, which need to be calibrated daily with appropriate materials in order to obtain accurate results. The carriers of either β° or severe β++ mutations have relatively high red blood cell count (RBC), while (MCV = hematocrit/RBC number) and mean corpuscular hemoglobin (MCH = Hb/RBC number) are markedly reduced (MCV: 60–70 fl; MCH: 19–23 pg). The Hb levels vary widely and they can be from normal to up to 2 g/dL. Carriers of mild β-mutations have usually higher values of MCV and MCH than β° carriers although lower than normal. The most widely used cutoff values of MCV and MCH for indicating thalassemia are 79 fl and 27 pg, respectively 14. Reticulocytes are normal or slightly increased, but they do not have diagnostic value. In carriers of very mild-or-silent β-mutations, the minimal deficit of β-globin production is not associated with any consistent hematological change. The iron status should always be taken into account when evaluating MCH and MCV values of an individual screened for thalassemia. Iron deficiency is the most common condition responsible for microcytosis and eventually microcytic anemia. Although zinc protoporphyrin is increased in case of iron depletion, its specificity is low, thus the transferrin saturation associated with ferritin remains the recommended parameters to confirm iron deficiency. Transferrin saturation is the ratio of serum iron to iron-binding capacity and is the most accurate indication of iron supply to the bone marrow. Normal values are higher than 16% in adults and higher than 10% in children. The RDW, a measure of variation in the size of the RBC (anisocytosis), tends to be above the reference interval in iron deficiency anemias and other microcytic anemias. The RDW in thalassemias is usually within or very close to the reference interval, reflecting the uniformity of red cell size (microcytes). Several formulas, based on parameters from the CBC, may be used to calculate a thalassemic index and have been used to differentiate iron deficiency from thalassemia. Although none have proved to be totally satisfactory in all clinical situations or to add significant information over the use of MCV alone in selecting cases for further investigation, many laboratories use these calculations as an adjunct to RBC parameters. The most commonly used formulas are the following: Mentzer index (MI) = MCV/RBC; discriminant factor (DF) = MCV × (RDW/Hb × 100); Shine and Lal Index (S&L) = MCV × (MCH/100); Srivastava Index (SI) = MCH/RBC; RDW index (RDWI) = (MCV × RDW)/RBC.
In using these formulas, to distinguish iron deficiency from thalassemia, it is recommended to use at least two cutoff points because some overlapping has been noted 18, 19.
Whenever iron deficiency is detected, before a definitive diagnosis, the hematological parameters must be repeated after iron supplementation.
Morphology changes of red cells can be detected in most thalassemia carriers: Microcytosis, hypochromia, and anisopoikilocytosis (variation in the size and shape of red cells) are the most typical changes. Other less common findings are basophilic stippling and the presence of some target cells.
Quantitative HbA2 determination is the most valuable test for β-thalassemia carrier detection. Several methods are available: The most accurate, fast and simple are the michrochromatography and the cation exchange HPLC and capillary electrophoresis 20. The expected normal range for HbA2 is between 2.4 and 3.2% in normal subject, while in typical β-thalassemia carriers, it is between 3.6 and 7% (Figure 3); values between 3.2 and 3.6% are considered borderline and they need further investigation, especially in young subjects or couple at risk. The normal range for HbF in adult life is usually <1.5% of total hemoglobin.
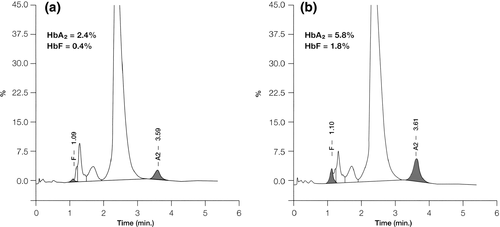
Despite the large amount of accumulated knowledge, several problems in carrier identification remain. Some β-thalassemia carriers have normal or borderline HbA2 levels but reduced MCV and MCH: These include heterozygotes for mild-or-very mild β-mutations (IVS1-6, -87, 101) or double heterozygotes for β- and δ-thalassemia, or co-inheritance of α-thalassemia 21. To differentiate these atypical β-thalassemia carriers from pure α-thalassemia heterozygotes, it is necessary to perform family study or further molecular analysis 21, 22. The recognition of such condition may have important implication for counseling couple at risk.
HbE carrier
HbE is an abnormal globin due to a substitution of lysine for glutamic acid at position 26 of the β-globin chain; however, the mutation causes an alternative splice site and is therefore characterized by a substantial reduction of ΗbΕ chain production. HbE is very common in Thailand and in some areas of South East Asia and the carrier, recognition is important because the interaction with β- or α-thalassemia may cause different phenotypes ranging from a condition similar to that of thalassemia major to a mild form of thalassemia intermedia. HbE carrier is asymptomatic with minimal changes in blood counts and erythrocytes indices; Hb is slightly reduced as well as MCV (84 + 5 fl). Red cell morphology is similar to that observed in β-thalassemia carriers with the presence of few target cells. The detection of HbE is essential for the diagnosis. In simple HbE trait, the HbE levels are 25–30% of the total Hb; the values of HbE vary when co-inherited with α- or β-traits 23, 24 (Table 1).
| β-TM | β-TI | HBE/β-Thal | ||
|---|---|---|---|---|
| Hb levels | <7 g/dL | ̴ 7–10 g/dL | Mild | 9–12 g/dL |
| Moderately severe | 6–7 g/dL | |||
| Severe | 4–5 g/dL | |||
| Hemoglobin study | β0/β0 | HbF 10–50% | HbE (40–60%) | |
| HbF up to 95% | (up to 100%) | HbF (60–40%) | ||
| HbA2 >5% | HbA2 >4% | ±HbA (with β+) | ||
| β0/β+ | HbA2 ↑ | |||
| HbF 70–90% | ||||
| HbA up to 30% | ||||
| Transfusion requirements | TDT | Variable: rare, occasional, or intermittent transfusions depending on the clinical features (e.g., infections, pregnancy, growth failure) | ||
- TDT, transfusion dependent.
Beta-thalassemia major and intermedia
Clinical presentation of β-thalassemia major usually occurs between 6 and 24 months of life, with severe microcytic/normocytic anemia, mild jaundice, and hepatosplenomegaly. The hematological diagnosis is based on reduced hemoglobin level (<7 g/dL) and very low MCH (<20 pg). The peripheral blood smear shows severe erythrocyte morphologic changes with marked poikilocytosis (speculated tear-drop cells), target cells, and numerous erythroblasts. The number of erythroblasts is related to the degree of ineffective erythropoiesis and is markedly increased after splenectomy. In the classical form of β-thalassemia major (homozygotes β°), at hemoglobin analysis, HbA is absent and HbF represents the 92–95% of the total hemoglobin. In thalassemia major forms due to double heterozygosity of β°/β+, the HbA levels can be variable between 10 and 30% and HbF between 70 and 90%. In such condition, both parents usually have typical hematological parameters of β-carriers.
Beta-thalassemia intermedia should be suspected in subjects who present at a later age with similar but milder clinical findings than thalassemia major. The clinical spectrum of thalassemia intermedia is very wide as well as the hematological phenotype. Patients with milder forms may have moderate-to-mild anemia, and the levels of HbA and HbF are very much dependent of the underlying molecular defects and the degree of ineffective erythropoiesis. These patients are usually capable of surviving without regular blood transfusions. Even in patients with more severe forms of thalassemia intermedia, Hb levels are >7 g/dL (Table 1) and they may drop in case of intercurrent events such infection, surgery, pregnancy. Family study, whenever possible, is extremely helpful allowing to evaluate hematological variations in carriers. Molecular analysis remains the definitive diagnostic tool for thalassemia intermedia phenotype 4, 25.
Alpha-thalassemia carriers
Alpha-globins production is regulated by four α-genes. Alpha-thalassemia is usually caused by a reduction (α+) or completely abolished (α°) globin chains production by the affected allele. The carrier state can either be α+ trait (α-thalassemia 2) or be α°-trait (α-thalassemia 1). α-Thalassemia 2 is an asymptomatic carrier state in which only one α-globin gene is dysfunctional. RBC is mildly microcytic or even normal; HbA2 and HbF levels are always normal. It is worth to note that in the neonatal period small amount (1–3%) of Hb Bart's (γ4) may be detected. α-Thalassemia 1 results when 2α-globin genes are dysfunctional. It is usually characterized by mild anemia, slight reduction of red cell indices (MCV, MCH), hypochromia, microcytosis, and anysopoichilocytosis. HbA2 are low-to-low normal range (1.5–2.5%). During neonatal period, moderate amounts of Hb Bart's hydrops fetalis syndrome (3–8%) can be detected. Low number (1:1000/10 000 RBC) of Heinz inclusion bodies (intracellular hemoglobin precipitates) may be detected by supravital stains 26. Both these conditions require a differential diagnosis with iron deficiency and in case hematological parameters must be reevaluated after iron supplementation. It should be noted, as previously mentioned, that the presence of β-thalassemia trait may mask the simultaneous presence of the α°-thalassemia trait; therefore, in some ethnic group where α-thalassemia is very common (e.g., Chinese), a detailed investigation is indicated if one partner has the β-thalassemia trait and the other is a probable carrier of α°-thalassemia trait 27.
Alpha-thalassemia: symptomatic forms
HbH disease, enclosed into the NTDT, occurs when α-globin synthesis is reduced to about one quarter of normal level and it is characterized by the presence of HbH (homotetramers of β-globin chains) which can be detected by HPLC or by electrophoresis. HbH amount is usually between 3 and 30%, and it is associated with mild-to-severe microcytic/normocytic anemia and increased bilirubin due to a moderate hemolytic component. Both parents are carrier of α-thalassemia trait.
The most severe form of α-thalassemia is the homozygous state for α°-thalassemia, known as Hb Bart's hydrops fetalis syndrome. In this condition, the fetus cannot synthesize any α-globin chains to make HbF or HbA. Fetal blood contains only Hb Bart's hydrops fetalis syndrome (γ4) and a small amount of embryonic Hb Portland. In these cases, prenatal diagnosis is indicated; therefore, the diagnosis of the carrier parents is mandatory 26.
Molecular diagnosis of thalassemia
Before the DNA era, the globin chain synthesis analysis, introduced more than 30 years ago 28, was utilized to identify the severity of globin chain imbalance and consequently to predict the clinical severity. This method has been used in the late 1970s for prenatal diagnosis; at present, it remains a sensitive diagnostic tool limited to define some complex or atypical forms of thalassemia.
The molecular diagnosis was applied in hemoglobinopathies and thalassemias an early stage of DNA era, and these diseases have been used as a prototype for the development of new techniques for molecular abnormalities. At present, there are many different PCR-based techniques used to diagnose the known globin gene mutations as well as other methods to identify the unknown mutations.
There are more than 200 known β-thalassemia mutations, the majority being single nucleotide substitutions, insertions, or short deletions. Few large β-gene deletions have been identified and most of them can be diagnosed by Gap-PCR 29. Fortunately, a limited number of point mutations are prevalent in different ethnic group; therefore, for any given ethnic region, a PCR method designed to detect the common-specific mutation simultaneously is initially used. With this approach, more than 80% of cases for most ethnic groups could be identify. For β- thalassemia mutations, the reverse-dot-blotting technique, in which amplified DNA is hybridized to a panel of mutation-specific probes fixed to a nylon strip has been widely used. The amplification refractory mutation system is also used in some laboratories: It is rapid, cost-effective, and convenient to test multiple mutations simultaneously. Denaturing gradient gel electrophoresis is an alternative approach to the above-mentioned methods, and it is convenient in countries where a very large spectrum of β-mutations occur. Almost 90% of β mutations may be detected by a shifted band pattern to normal pattern. However, the improvement and cost reduction of DNA sequencing rendered the direct sequence of β-gene the most widely use in many laboratories allowing the analysis of the exact nucleotide sequence of the gene or the gene area under evaluation. The original method described by Sanger et al. in 1977 has been modified and today several type of automatic sequencer is available. Direct sequencing allows also the identification of unknown mutations 14, 30, 31.
Alpha-thalassemias are mainly due to deletions of different length and they can be detected preferentially by reverse dot blot and Gap-PCR. Primer sequences have been published for the diagnosis of several α+ or α° 32-34. The amplification of sequences in the α -globin gene cluster is more difficult than that of the β-globin gene cluster due to a considerable sequence homology within the α-globin gene cluster. For the unknown α-deletion, the Southern blotting technique is still used in some laboratories; however, more recently, the multiplex ligation-dependent probe amplification have been introduced. This method is now used instead of Southern blot due to its sensibility and reliability, without the needs of using radioactive detection of the first one 35.
After Sanger sequencing era, next-generation sequencing (NGS) represents a new principle of sequencing technology; it increases the sequencing capacity from a few hundred base pairs to several thousands in a single analysis. NGS is based on massively parallel sequencing of clonally amplified DNA molecules, coupled with sufficient computational power and appropriate software for efficient data analysis, has brought genetic diagnostics into clinical practice at an affordable price 36. It can be applied to whole genome, whole exome and to specific targeted regions of the genome. At present in the literature, no data are available about sequencing of globin genes with this approach. Only one commercial sequencing panel (TruSight Inherited Disease, Illumina Inc.) is available reporting that HBB and HBA1 genes are included, but the sequencing of only two genes does not justify the cost of the entire procedure for a single patient.
The most critical step of NGS is the experimental design of the probe set to be used for DNA capture: the high level of homology between the genes in the alpha and beta clusters makes the NGS approach to thalassemia very difficult, time-consuming and expensive, even though not impossible. Another critical point of NGS is that it is useful for the detection of single nucleotide substitutions and insertions or small deletions (depending on the type of study) but it is less accurate for other types of genomic variation. Although bioinformatics’ studies are now also working on tools to check for the presence of complex rearrangements, large deletion or triplications are at now missed and especially in the case of alpha-thalassemia, it make the entire procedure totally useless.
To conclude, it is important to underline that the DNA analysis for globin gene mutations is not required to diagnose most of the carrier status; it is advisable to confirm the diagnosis of TDT, knowing the parents mutations and in NTDT cases were the coinheritance of different globin gene defects may be responsible for different phenotype expression.
Some cases are hematologically and clinically difficult to be diagnosed even with the family hematological evaluations due to the presence of mild mutations or α- and β-interaction; therefore, in such cases, the molecular analysis is convenient before starting the treatment.
The DNA analysis on chorionic villi is the approach for prenatal diagnosis and the methods used are the same used for mutations detection according to the laboratory facilities and expertise. The essential step in prenatal diagnosis is the removal of any maternal tissue as the presence of maternal residual tissue may result in diagnostic error. To rule out maternal contamination, the short-tandem repeats or variable number of tandem repeats in fetal and parental DNA samples by PCR is essential 37.
Details for the methods commonly utilized for molecular diagnosis of thalassemias are reported in Thalassemia International Federation publications ‘Prevention of Thalassemias and other Hemoglobin Disorders’ 14.
Acknowledgements
This work was supported by grants from Ministero dell'Istruzione, Università e Ricerca (PRIN 2012: 20128PNX83_002), by grants from Ministero della Salute (RF-2010-2303934) and from Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico.




