Diagnosis, risk stratification and management of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma
Summary
Monoclonal gammopathy of undetermined significance (MGUS) is one of the most common premalignant disorders. IgG and IgA MGUS are precursor conditions of multiple myeloma (MM), whereas light-chain MGUS is a precursor condition of light-chain MM. Smoldering MM (SMM) is a precursor condition with higher tumor burden and higher risk of progression to symptomatic MM compared to MGUS. Assessment of the risk of progression of patients with asymptomatic monoclonal gammopathies is based on various factors including clonal burden, as well as biological characteristics, such as cytogenetic abnormalities and light-chain production. Several models have been constructed that are useful in daily practice for predicting risk of progression of MGUS or SMM. Importantly, the plasma cell clone may occasionally be responsible for severe organ damage through the production of a M-protein which deposits in tissues or has autoantibody activity. These disorders are rare and often require therapy directed at eradication of the underlying clone. Importantly, recent studies have shown that asymptomatic patients with a bone marrow plasma cell percentage ≥60%, free light-chain ratio ≥100, or >1 focal lesion on MRI (myeloma-defining events) have a 80% risk of developing symptomatic MM within 2 years. These patients are now considered to have MM requiring therapy, similar to patients with symptomatic disease. In this review, we provide an overview of the new diagnostic criteria of the monoclonal gammopathies and discuss risk of progression to active MM. We also provide recommendations for the management of patients with MGUS and SMM including risk-adapted follow-up.
Introduction
Nearly all symptomatic plasma cell disorders are preceded by an asymptomatic precursor state 1, 2, which is the most common form of the plasma cell dyscrasias. Progression of an asymptomatic plasma cell disorder involves a multistep transformation process occurring as a consequence of the sequential acquisition of genetic events such as chromosomal abnormalities, mutations, and epigenetic alterations leading to altered gene expression 3-6. Multiple myeloma (MM) development is also accompanied by altered interactions of the tumor cells with various components of their bone marrow microenvironment such as osteoclasts, endothelial cells, and cells of the immune system 6. Recent data suggest that progression from monoclonal gammopathy of undetermined significance (MGUS) to smoldering multiple myeloma (SMM) and then to MM is also mediated via competition between subclones and outgrowth of the fittest 3.
IgG or IgA MGUS patients typically progress to MM, and IgM MGUS patients progress to Waldenström's macroglobulinemia (WM) or other lymphoproliferative disorders 7. Patients with a light chain only asymptomatic monoclonal gammopathy are at risk of developing light-chain MM or immunoglobulin light-chain amyloidosis (AL amyloidosis) 2, 8, 9.
In this review, we will not discuss IgM MGUS, smoldering WM, or symptomatic WM, but we will focus on the diagnosis and risk stratification of myeloma and its asymptomatic precursor states.
Definitions
Monoclonal gammopathy of undetermined significance
Monoclonal gammopathy of undetermined significance affects approximately 3.5% of the population older than 50 years 8, 10, 11. IgG and IgA MGUS are defined by a M-protein <30 g/L, bone marrow (BM) plasma cell percentage <10%, and absence of signs or symptoms related to MM (CRAB criteria: hypercalcemia, renal insufficiency, anemia, or bone lesions; Table 1) or other lymphoproliferative disorders 11, 12. Light-chain MGUS is defined by an abnormal κ/λ free light-chain (FLC) ratio, increase in concentration of the involved light chain, and absence of expression of a monoclonal peak of immunoglobulin heavy chain in the serum on immunofixation 8. In case of renal disease or polyclonal B-cell activation, increased levels of κ and λ chains may be observed, but with a normal ratio. In persons aged 50 years and older, light-chain MGUS has a prevalence of ~0.7–0.8% 8, 10. Obesity and personal history of autoimmune diseases, inflammatory conditions, and infections are associated with increased risk of MGUS 4. Furthermore, occupational studies have demonstrated that exposure to radiation or pesticide is associated with the development of MGUS 4. Also, Vietnam war veterans exposed to the herbicide agent orange had a 2.4-fold increased risk for MGUS 13.
| International Myeloma Working Group (IMWG) consensus diagnostic criteria 18 | |
|---|---|
| Non-IgM MGUSa | Serum M-protein <30 g/L and |
| Clonal bone marrow plasma cells <10% and | |
| Absence of end-organ damageb,c | |
| IgM MGUS | Serum IgM M-protein <30 g/L and |
| Bone marrow lymphoplasmacytic infiltration <10% and | |
| Absence of end-organ damagec,d | |
| Light-chain MGUS | Abnormal FLC ratio (<0.26 or >1.65) and |
| Increased level of the involved light chain and | |
| No immunoglobulin heavy-chain expression on immunofixation and | |
| Clonal bone marrow plasma cells <10% and | |
| Urinary monoclonal protein <500 mg/24 h and | |
| Absence of end-organ damageb,c | |
| Smoldering MM | Serum M-protein ≥30 g/L and/or |
| Clonal bone marrow plasma cells 10–60% and | |
| Absence of myeloma-defining eventse or amyloidosis | |
| Idiopathic Bence Jones proteinuria | Urinary M-protein ≥500 mg/24 h and/or |
| Clonal bone marrow plasma cells 10–60% | |
| No immunoglobulin heavy-chain expression on immunofixation | |
| Absence of myeloma-defining eventse or amyloidosis | |
| Symptomatic MM | Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma and any one or more of the myeloma-defining events (end-organ damage or biomarker of malignancy)e |
- a Almost all patients have IgG or IgA MGUS. IgD isotype is found in only ~0.04–0.1% of MGUS patients. Very rarely, cases of IgE MGUS have been reported.
- b End-organ damage includes hypercalcemia [serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL)], renal insufficiency [Creatinine clearance <40 mL/min or serum creatinine >177 μmol/L (>2 mg/dL)], anemia [Hemoglobin value of >20 g/L (>1.25 mm) below the lower limit of normal, or a hemoglobin value <100 g/L (<6.2 mm)], and lytic bone lesions (one or more osteolytic lesions on skeletal radiography, CT, or PET-CT. If bone marrow has <10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal bone marrow involvement) (CRAB) that can be attributed to the plasma cell proliferative disorder.
- c In particular, in elderly persons, other causes should be considered such as deficiencies of vitamin B12, folic acid or iron for anemia; primary hyperparathyroidism for hypercalcemia; diabetes and hypertension for renal insufficiency; metastatic carcinoma for lytic bone lesions 75.
- d End-organ damage includes anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly that can be attributed to the underlying lymphoproliferative disorder.
- e Myeloma-defining events: evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically hypercalcemia, renal insufficiency, anemia, or bone lesions, or any one or more of the following biomarkers of malignancy [clonal BM plasma cell percentage ≥60%, involved:uninvolved serum free light chain ratio ≥100 (the involved free light chain must be ≥100 mg/L), or >1 focal lesions on MRI studies (each focal lesion must be 5 mm or more in size)].
Smoldering multiple myeloma
Smoldering MM (SMM) is a premalignant plasma cell disorder with a higher tumor burden (M-protein ≥30 g/L and/or BM plasma cells 10–60%) and higher risk of progression compared to MGUS (Table 1) 14. Similar to MGUS, SMM is characterized by the absence of CRAB features. Importantly, SMM patients with a BM plasma cell percentage ≥60%, FLC ratio ≥100, or >1 lesion on MRI (either with whole-body MRI 15 or axial MRI 16) have approximately an 80% risk of developing CRAB features within 2 years 17, 18. Using these biomarkers of malignancy, a small subgroup of ultra-high-risk SMM patients is identified, which according to the revised IMWG criteria are now considered to have MM requiring therapy irrespective of the presence or absence of CRAB features. Treatment should be considered in these patients to prevent serious end-organ damage such as bone fractures or renal failure, which can have long-term effects on the quality of life of patients. However, two recent studies showed that SMM patients with a FLC ratio ≥100 have a 2-year progression risk of 30% and 64% 19, 20, which is lower than reported in two previous studies (progression risk at 2 years: ~80%) 21, 22 that were used for these updated IMWG criteria 18. The authors from these studies conclude that FLC ratio ≥100 as a sole criterion for treating as active MM may not be justified, but that very close monitoring is warranted for this group.
The light-chain equivalent of SMM (light-chain SMM or idiopathic Bence Jones proteinuria) is defined by complete loss of immunoglobulin heavy-chain expression, plus either urinary light-chain excretion of ≥0.5 g/24 h or BM plasma cells 10–60% 23. These patients are at increased risk of developing light-chain MM or light-chain amyloidosis 23. The FLC assay is easier to obtain than 24-h urine M-protein measurements. However, at this moment, it is unknown which FLC threshold can be used to differentiate between light-chain MGUS and light-chain SMM 23.
Multiple myeloma
As described in the previous section, the IMWG criteria for multiple myeloma were updated in November 2014 and now include also validated biomarkers in addition to existing requirements of attributable CRAB features (hypercalcemia, renal failure, anemia, and bone lesions) 18. As discussed in the section on SMM, these biomarkers are BM plasma cell percentage ≥60%, FLC ratio ≥100, or >1 focal lesion on MRI 15, 16, 24 (Table 1).
Progression of MGUS
Progression of MGUS to MM or other related malignancies occurs at a rate of approximately 1% per year 12, 25, 26. Even after >25 years of follow-up, the risk of progression does not decrease. As many MGUS patients have advanced age and comorbidities, they will often die from unrelated diseases.
The risk of progression for light-chain MGUS is lower when compared to the risk of progression in conventional MGUS. In a population-based cohort study, only 3 of 133 light-chain MGUS patients experienced progression to MM (all three developed light-chain MM) during 1100 patient-years of follow-up (progression rate: 0.27% per year) MM 8. Similarly, in another study, none of 34 light-chain MGUS cases had progression during a median observation time of 5 years 10. Importantly, it is possible that a small proportion of the patients with apparent light-chain MGUS do not have a true clonal disorder but rather renal dysfunction or polyclonal activation 8.
Predictors of malignant transformation in MGUS
Various presenting features are helpful in predicting risk of progression of MGUS to symptomatic disease and therefore in individualizing follow-up. The size of the MGUS clone as determined by BM plasma cell percentage 25-30 or M-protein level 12, 25-29, 31-37 is an important risk factor for progression of MGUS.
In addition, several biological characteristics of the clone have predictive value in conventional MGUS including heavy-chain isotype (IgA/IgM > IgG) 12, 25, 28, 31, 32, 34, 35, 38; light-chain production as determined by abnormal serum FLC ratio 31, 39 or presence of Bence Jones proteinuria 25, 27, 37; detection of circulating plasma cells 34 or clonal B cells 40; increased bone resorption in bone biopsy 41; clonal heterogeneity 42; DNA aneuploidy determined by flow cytometry 27; and abnormal metaphase cytogenetics 42. Gene expression profiling of purified plasma cells has recently been demonstrated to have prognostic value 43. It is currently unknown whether specific chromosomal abnormalities, including del(17p) and t(4;14), are predictive of malignant progression in MGUS.
Furthermore, suppression of nonclonal BM plasma cells, based on multiparameter flow cytometric analysis, has been identified as a risk factor for progression 27, 44, which explains the predictive value of reduced levels of polyclonal serum immunoglobulins 25, 27, 35, 37, 45. Moreover, several imaging techniques may be useful in predicting progression of MGUS. In this respect, detection of focal lesions by MRI at baseline 36, 43, 46 and development of focal lesions by MRI or PET-CT are predictive for progression to active MM 46.
Not only baseline characteristics but also the dynamics of the plasma cell clone during the first years of follow-up is helpful in predicting the risk of transformation to a malignant plasma cell disorder. Indeed, Rosinol et al. 28 showed that a progressive increase of the M-protein (evolving MGUS) predicted progression.
Until now, no predictive factors for progression have been identified for light-chain MGUS. It is currently unknown whether higher levels of the involved light chain result in a higher risk of malignant transformation in light-chain MGUS.
Prediction models in MGUS
The Mayo Clinic MM group constructed a model for predicting risk of progression of MGUS based on three parameters: the size of the M-protein (≥15 g/L), type of M-protein (non-IgG), and the presence of an abnormal FLC ratio 31. In this model, the absolute risk of progression at 20 years was 5% for patients without risk factors (low-risk MGUS), 21% for patients with 1 risk factor (low-intermediate-risk MGUS), 37% for patients with two risk factors (high-intermediate-risk MGUS), and 58% for patients with three risk factors (high-risk MGUS) (Table 2). Another prognostic stratification system was proposed by Perez-Persona et al. 27 which is based on the percentage of aberrant plasma cells and DNA aneuploidy, which were both assessed by flow cytometric analysis. Also, the combination of the presence of an evolving M-protein and percentage of aberrant plasma cells identifies three different risk groups of MGUS patients 44 (Table 2).
| Rajkumar et al. 31 (n = 1148) | Perez-Persona et al. 27 (n = 276) | Perez-Persona et al. 44 (n = 311) | ||||
|---|---|---|---|---|---|---|
| Serum M-protein ≥15 g/L | ≥95% aberrant bone | ≥95% aberrant bone | ||||
| Non-IgG subtype | marrow plasma cells | marrow plasma cells | ||||
| Abnormal FLC ratio | DNA aneuploidy | Evolving MGUSa | ||||
| Number of risk factors | Risk of progression at 20 years (%) | % Of total | Risk of progression at 5 years (%) | % Of total | Risk of progression at 7 years (%) | % Of total |
| 0 | 5 | 39 | 2 | 46 | 2 | 49 |
| 1 | 21 | 37 | 10 | 48 | 16 | 45 |
| 2 | 37 | 20 | 46 | 6 | 72 | 6 |
| 3 | 58 | 5 | – | – | – | – |
- a Evolving MGUS is defined as an increase of M-protein of at least 10% by the third year, confirmed by two consecutive measurements separated by at least 1 month.
Progression of SMM
Smoldering multiple myeloma represents an intermediate stage between MGUS and MM and has a risk of progression of approximately 10% per year for the first 5 years and decreases thereafter. This contrasts with MGUS, in which the rate of progression is constant over time 47. The risk of progression of patients with SMM is dependent on tumor mass, as reflected by M-protein level and bone marrow plasma cell percentage 25, 27, 47, 48.
Other factors that predict progression to symptomatic disease include abnormal plasma cell phenotype in ≥95% of cells, high plasma cell proliferative rate, hypogammaglobulinemia, gene expression profile of the purified MM cells, abnormal free light-chain ratio, increasing M-protein, presence and development of MRI abnormalities, or peripheral blood circulating plasma cells 27, 43, 44, 49-54. Also, hyperdiploidy, del(17p), t(4;14), and ampl(1q) are adverse prognostic factors in SMM, independent of tumor load 55, 56 (Figure 1).
Newer imaging techniques have a higher sensitivity compared to conventional X-rays for the detection of MM bone lesions. The IMWG also recently stated that evidence of osteolysis on CT or 18-F-FDG PET-CT meets the definition of bone disease in MM 18. However, PET-CT also identifies patients with SMM who present with focal areas of visually detectable increased tracer uptake (hypermetabolic lesions) or diffuse BM hypermetabolism in the absence of underlying osteolysis. Two studies showed that SMM patients with an increased focal uptake or BM hypermetabolism without underlying osteolysis on PET-CT (8–16% of the patients with SMM) have a higher probability of progression, compared to patients without these abnormalities 57, 58. Risk of progression to symptomatic MM within 2 years was 58–61% 57, 58.
Risk progression in light-chain SMM is approximately 5% per year during the first 5 years and decreases thereafter 23. This risk of progression is lower when compared to conventional SMM, but higher when compared to light-chain MGUS. In light-chain SMM, the risk of progression was higher in patients with 24-h M-protein levels ≥1.0 g/24 h compared to patients with urinary M-protein levels 0.5–1.0 g/24 h (5-year risk of progression: 39% vs. 19%; 10-year risk of progression: 58% vs. 32%) 23.
Prediction models in SMM
Several prognostic models have been developed to predict risk of progression in SMM, but the Mayo Clinic model and PETHEMA classification are most widely used. The Mayo Clinic Model identified three groups of patients based on the percentage of bone marrow plasma cells (≥10% or <10%), the M-protein level (≥30 or <30 g/L), and the FLC ratio (<0.125 or >8) 49. Patients with three risk factors have a risk of progression toward symptomatic MM at 10 years of 84%, while this was 50% and 65% with 1 or 2 risk factors 49. The Spanish PETHEMA group uses the percentage of abnormal plasma cells in multiparametric flow cytometry (≥95% or <95%) and absence or presence of immunoparesis 27. Risk of progression to symptomatic disease at 5 years was 4% in patients without risk factors, and 46% and 72% for patients with 1 or 2 risk factors, respectively 27.
Several other prognostic models have been published, including a recent Danish model derived from a population-based study, in which M-protein ≥30 g/L and immunoparesis were markers for time to progression to MM in SMM (Table 3) 20. In addition, the Arkansas group generated a risk-stratification model based on expression levels of only four genes combined with serum M-protein (≥30 g/L) and albumin levels (<35 g/L). The high-risk, intermediate-risk, and low-risk groups had a 2-year progression probability of 5.0%, 44.8%, and 85.7%, respectively 54.
| Dispenzieri et al. 49 (n = 273) | Perez-Persona et al. 27 (n = 106) | Sorrig et al. 20 (n = 297) | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of risk factors |
|
|
|
|||||
| Risk of progression at 5 years (%) | Risk of progression at 10 years(%) | % Of total | Risk of progression at 5 years (%) | % Of total | Risk of progression at 2 years (%) | Risk of progression at 5 years (%) | % Of total | |
| 0 | – | – | – | 4 | 36.8 | 4.8 | 9 | 30.3 |
| 1 | 25 | 50 | 29.7 | 46 | 36.8 | 18.1 | 24 | 55.6 |
| 2 | 51 | 65 | 41.8 | 72 | 26.4 | 38.4 | 55 | 14.1 |
| 3 | 76 | 84 | 28.6 | – | – | – | – | – |
M-Protein-Related Disorders
Although patients with MGUS and SMM are at increased risk of developing active MM, the vast majority of these patients has no symptoms that can be attributed to the plasma cell clone. However, sometimes the MGUS/SMM plasma cell clone is responsible for the development of severe organ damage through the production of a M-protein with autoantibody activity. This may result in rare hematologic conditions such as immune thrombocytopenic purpura (ITP) 59, acquired von Willebrand's disease 60, or acquired deficiency of FVIII 61. Also, several renal diseases are caused by toxic M-proteins produced by the plasma cell clone: monoclonal immunoglobulin deposition disease (MIDD), light-chain proximal tubulopathy, immunotactoid glomerulopathy, proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID), and type 1 and type 2 cryoglobulinemic glomerulonephritis 62-64. In these disorders, the M-protein is the direct cause of the kidney disease and these diseases are characterized by the presence of monoclonal deposits in kidney biopsies. Also, several skin disorders, neurologic disorders, and metabolic disturbances have been identified in which a M-protein with auto-antibody activity plays an important role in the pathogenesis of the disease 4.
The plasma cell clone may also produce monoclonal antibodies or misfolded immunoglobulin light chains that deposit in tissues leading to M-protein-related disorders with systemic manifestations (e.g., AL amyloidosis and type I cryoglobulinemia) 65. The understanding of the pathogenesis of some of these associated disorders is limited and may be related to both M-protein and growth factors produced by the underlying clone, such as in POEMS syndrome.
Diagnostic Considerations
Patients with symptoms or laboratory abnormalities, which may be related to the underlying MGUS or SMM clone
Monoclonal gammopathy of undetermined significance or patients with SMM are frequently identified when serum protein electrophoresis is ordered as part of a diagnostic assessment for various symptoms including back pain, fatigue, and recurrent infections. Similarly, serum protein electrophoresis is often requested in case of laboratory abnormalities such as anemia, hypercalcemia, elevated total protein, or renal failure, as well as when osteolytic bone lesions are detected. In these cases, the European Myeloma Network (EMN) recommends to exclude the presence of a symptomatic plasma cell disorder (MM, WM, AL amyloidosis, or CLL) by laboratory tests (complete blood count with differential, blood chemistry [including calcium, albumin, and creatinine], serum and urine protein electrophoresis with immunofixation, and measurement of FLCs), BM biopsy and aspiration, and imaging studies. The IMWG recommends to evaluate bone disease by whole-body X-ray, whole-body CT, or PET-CT 18.
In addition, fat, BM, or rectum biopsy with Congo red staining should be performed in case AL amyloidosis is suspected. A kidney biopsy is often indicated to exclude a monoclonal gammopathy of renal significance, when a patient has significant proteinuria or renal insufficiency. To demonstrate monoclonal deposits and their pattern of organization, immunofluorescence and electron microscopic studies should part of the diagnostic evaluation 62, 64.
Asymptomatic patients
A large Italian study showed that the risk of detecting a plasma cell infiltration ≥10% in patients without bone pain with a M-protein ≤15 or ≤10 g/L is very low (7.3% and 5.0%, respectively). But this risk is dependent on heavy-chain isotype (4.7% and 3.5% for IgG isotype; 20.5% and 14.0% for IgA) 66. This study also showed that the likelihood of finding bone lesions at skeletal survey is very low for IgG (1.7% and 2%) as well as IgA isotypes (6.4% and 0.0% for M-protein ≤15 and ≤10 g/L, respectively) 66. Therefore, most specialists do not routinely recommend BM examination in asymptomatic patients with apparent IgG MGUS if the serum M-protein is ≤15 g/L and without end-organ damage, until there is evidence of progression to symptomatic disease. However, for all IgA M-proteins, BM examination should be part of the diagnostic work-up. The EMN does not routinely recommend imaging when patients have a serum IgG M-protein ≤15 g/L or IgA M-protein ≤10 g/L without bone pain, while for all other asymptomatic patients imaging should be considered. Importantly, in case of limited life expectancy, it can be justified to exclude bone marrow investigation and imaging from the diagnostic work-up.
Concerning asymptomatic patients with light chain only disease, there are currently no data available that guide diagnostic work-up. The EMN does not recommend to routinely perform BM examination and imaging in asymptomatic patients with apparent light-chain MGUS 4. However, in patients with high levels of the involved light chain (e.g., FLC ratio >10 or <0.10), BM evaluation and imaging should be considered.
Additional considerations in SMM
At the time of SMM diagnosis, similar tests have to performed as in MGUS including bone marrow evaluation and a skeletal survey (preferably by whole-body CT). In case of absence of osteolytic lesions on CT, one could consider to perform a MRI of the spine and pelvis to identify patients who have >1 focal lesion and therefore should be considered for treatment. Also, assessment of cytogenetic abnormalities may be helpful to estimate risk of progression 67.
Follow-up
Follow-up of MGUS patients
Current guidelines recommend lifelong follow-up of MGUS patients to diagnose malignant transformation before the onset of serious complications. This may avoid hospitalizations and costs and also preserve quality of life. Indeed, two recent population studies have shown benefits of MGUS follow-up 68, 69. First, a Swedish study showed improved survival of MM patients with prior knowledge of MGUS, suggesting that earlier treatment of MM leads to better survival 69. Secondly, the SEER database analysis showed that MGUS follow-up was an independent predictor of lower rates of complications at the diagnosis of malignancy 68. Furthermore, patients with MGUS follow-up examination had improved OS calculated from the time that they developed an active malignancy, compared to those without MGUS follow-up 68. In contrast to these two studies, a smaller retrospective analysis from the Mayo Clinic of symptomatic myeloma patients with preceding MGUS (n = 116) demonstrated that follow-up (at least every 3 years) did not lead to reduced hospitalizations, decreased myeloma-related complications, or improved survival from the time of myeloma diagnosis, compared to suboptimal follow-up 70. Progression between screening visits may explain the inadequacy of follow-up in this study 70.
By taking into account the patient's risk of progression and life expectancy, follow-up can be individualized. The recent EMN guideline recommends to use the Mayo Clinic risk-stratification model to predict progression 31, because the three prognostic variables in this model can be easily assessed in all MGUS patients (Table 2). Follow-up consists of a careful history, physical examination, and laboratory studies (quantitation of M-protein, complete blood count, calcium, and creatinine). Therapy should be started only when symptomatic disease develops.
The EMN guideline recommends that patients with intermediate risk (risk of progression at 20 years: 21–37% according to Mayo Clinic risk-stratification model 31) or high-risk MGUS (risk of progression at 20 years: 58%) should be monitored more strictly (at 6 months, and annually thereafter) than patients with low-risk MGUS (risk of progression at 20 years: 5%) for whom less frequent follow-up is reasonable (at 6 months, and every 1–2 years thereafter) 4 (Figure 2). Many MGUS patients can receive appropriate follow-up in primary care. On the other hand, patients with low-risk MGUS may not need annual follow-up, but only laboratory investigations, imaging, or BM analysis when symptoms suggestive of MM or related diseases develop. In addition, no follow-up can also be justified in elderly patients or in patients with significant comorbidity with a short life expectancy. Because of competing causes of death, these patients will probably die before progression of MGUS 4.
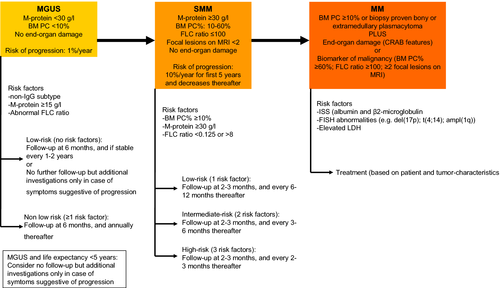
Although the rate of progression in light-chain MGUS is low (~0.3% per year), there is a substantial risk of developing renal disease 8. The EMN therefore recommends that patients with light-chain MGUS should receive follow-up at 6 months, and every year thereafter 4, 8. MGUS patients with elevated free light chains should also be checked for the development of amyloidosis or LCDD by measuring NT-pro-BNP and urine albumin during follow-up. In case of abnormal results, additional investigations should be considered including 24-h urine for total protein, echocardiography, and ultrasound for organomegaly.
Follow-up of SMM patients
A different follow-up strategy is needed in SMM, because risk of progression is markedly higher than in MGUS. Patients should be re-evaluated 2–3 months after diagnosis to determine the stability of laboratory parameters such as serum and urine M-component, hemoglobin, calcium, and creatinine levels 67. Outside the clinical trial setting, additional radiologic examination is only recommended if there is evidence for progressive disease from the routine work-up. Subsequent follow-up should be based on the patient's individual risk of progression. In our clinic, we generally use the Mayo Clinic prognostic scoring system, because it uses three easily assessable markers, but we also take into account all other available information to classify patients with SMM such as MRI findings, evolving nature of the M-protein, and presence of cytogenetic abnormalities.
Low-risk patients should be followed every 6–12 months, while intermediate-risk patients should undergo follow-up every 3–6 months. High-risk patients require a closer follow-up every 2–3 months 6, 67, 71. Alternatively, these high-risk patients may be treated in the setting of a clinical trial (Figure 2). Similar to light-chain MGUS, also in patients with light-chain SMM, we recommend to monitor NT-proBNP and urine albumin.
Treatment of MGUS and SMM
Treatment of SMM
Outside of the clinical trial setting, treatment for SMM or MGUS is not recommended. Treatment is initiated upon symptomatic progression.
In the past attempts at early intervention with alkylating agents, bisphosphonates, interleukin-1beta antagonists, or thalidomide failed to show a significant benefit 71. However, a recent Spanish randomized phase 3 study showed benefit for early intervention in patients with high-risk SMM (BM plasma cells ≥10% and M-protein [defined as ≥30 g/L if IgG; ≥20 g/L if IgA; or Bence Jones proteinuria >1 g/24 h], or only one of the two criteria described above, plus at least 95% phenotypically aberrant plasma cells in the BM plasma cell compartment, with reductions in one or two uninvolved immunoglobulins of more than 25%, as compared with normal values). In this study, early treatment consisting of nine cycles of lenalidomide–dexamethasone induction followed by lenalidomide maintenance was compared with observation only. CR was achieved in 14% of the patients during induction and in 26% during maintenance. There was a significant benefit in terms of time to progression (TTP) and OS for the lenalidomide–dexamethasone group (median TTP: not reached vs. 21 months; 3-year OS: 94% vs. 80%) 72.
Also, the triple combination of carfilzomib, lenalidomide, and dexamethasone (KRd) followed by lenalidomide maintenance was well tolerated and had high efficacy in high-risk SMM (n = 12) with CR achieved in all patients. Deeper responses are expected to result in improved TTP and OS, and although the follow-up of this study is short, up till now no patients experienced disease progression 73. Based on these promising results, the Spanish group is currently evaluating in SMM a more intensive approach with carfilzomib, lenalidomide, and dexamethasone (KRd) in induction, followed by autologous stem cell transplantation, and then consolidation with KRd (NCT02415413).
Furthermore, there are currently multiple trials that are evaluating immunotherapy in patients with high-risk SMM. Monoclonal antibodies that are currently being evaluated include daratumumab (anti-CD38), siltuximab (anti-interleukin-6), and elotuzumab (anti-SLAMF7) as single agent or combined with lenalidomide 67, 74.
Treatment of M-protein-related disorders
Although MGUS and patients with SMM are generally not treated, patients with M-protein-related disorders may benefit from clone-directed therapies.
In case of only mild symptoms, supportive care alone may be sufficient. However, in general, the most effective treatment of M-protein-related disorders is directed to the underlying plasma cell clone. This clone-directed therapy should only be considered in case of aggressive and disabling disease, because this approach is potentially toxic. In addition, therapy directed at eradication of the MGUS or SMM clone should only be instituted when there is a clear causal relationship between MGUS/SMM and the associated disorder 4.
In non-IgM MGUS/SMM-related disorders, antimyeloma agents should be used. In younger patients (≤65–70 years), high-dose melphalan with autologous SCT to induce a durable remission can be considered if the symptoms are severe, progressive, and/or disabling. In case of a small clone, induction therapy preceding autologous SCT is probably not needed. However, induction therapy may be advantageous for patients with a poor performance status due to the MGUS/SMM-associated disorder, and in case of a significant plasma cell clone (M-protein ≥10 g/L). In patients with neuropathy, we consider a lenalidomide-based regimen as the first choice, while bortezomib has the highest efficacy in M-protein-associated renal disorders, because it rapidly reduces tumor load and toxic M-proteins. In addition, bortezomib clearance is not altered in case of renal dysfunction 64.
Conclusions and future prospects
Monoclonal gammopathy of undetermined significance and SMM are asymptomatic plasma cell disorders, which have different risks of progression toward symptomatic MM. Importantly, the updated IMWG criteria for MM recommend to start therapy in patients before end-organ damage appear on the basis of specific biomarkers such as BM plasma cell percentage ≥60%, FLC ratio ≥100, or ≥2 focal lesions on MRI 18. Patients with these myeloma-defining events have a 2-year risk of progression of approximately 80%.
Monoclonal gammopathy of undetermined significance patients have an average risk of progression to MM or, to a lesser extent, other lymphoproliferative disorders of 1% per year. MGUS patients should receive follow-up according to their risk of progression as well as their life expectancy based on age and presence of comorbidities (Figure 2). Similarly, patients with SMM, who have a higher risk of progression compared to MGUS patients (risk of progression of approximately 10% per year for the first 5 years, which decreases thereafter), should also be monitored carefully or enrolled on clinical trials (Figure 2). In this respect, several studies in high-risk SMM are evaluating new therapeutic interventions to delay or prevent disease progression or even eradicate the plasma cell clone.
We expect that in the nearby future increased knowledge of mechanisms underlying the progression of MGUS or SMM to MM results in a further improvement in the identification of patients at high risk of progression, which will hopefully result in an even more tailored follow-up with start of therapy before serious complications develop.
Author Contributions
N.W.C.J.v.d.D. performed literature searches and prepared the first draft of the manuscript; and all other authors reviewed and edited the draft of the report and approved the final manuscript.
Conflict of Interest
All authors declare no conflict of interest.




