Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: analysis of results obtained in the DULCIS study
Summary
Introduction
D-dimer assay, generally evaluated according to cutoff points calibrated for VTE exclusion, is used to estimate the individual risk of recurrence after a first idiopathic event of venous thromboembolism (VTE).
Methods
Commercial D-dimer assays, evaluated according to predetermined cutoff levels for each assay, specific for age (lower in subjects <70 years) and gender (lower in males), were used in the recent DULCIS study. The present analysis compared the results obtained in the DULCIS with those that might have been had using the following different cutoff criteria: traditional cutoff for VTE exclusion, higher levels in subjects aged ≥60 years, or age multiplied by 10.
Results
In young subjects, the DULCIS low cutoff levels resulted in half the recurrent events that would have occurred using the other criteria. In elderly patients, the DULCIS results were similar to those calculated for the two age-adjusted criteria. The adoption of traditional VTE exclusion criteria would have led to positive results in the large majority of elderly subjects, without a significant reduction in the rate of recurrent event.
Conclusion
The results confirm the usefulness of the cutoff levels used in DULCIS.
Introduction
In the last decade, several clinical studies have shown that D-dimer assay can be used to evaluate the individual risk of recurrence after a first idiopathic event of venous thromboembolism (VTE) 1-6. In these studies, D-dimers were measured after stopping anticoagulation, although at different time intervals, using qualitative or quantitative methods calibrated in both cases for VTE diagnosis procedure. A study-level meta-analysis discovered that a negative D-dimer result was associated with a 3.5% annual risk of recurrent disease, whereas a positive D-dimer result was associated with an 8.9% annual risk of recurrence 7. A recent patient-level meta-analysis 8 confirmed that the hazard ratio of D-dimer status for VTE recurrence (positive vs. negative) was 2.59 (95% CI, 1.90–3.52); it also found that the ability of D-dimer assay to distinguish patients with a higher or lower risk for recurrent VTE was not affected by the timing of D-dimer testing after stopping anticoagulation, patient age, or the assay cut point used.
A post hoc analysis 9 of the results of the Prolong study 3found that the proportion of patients with positive D-dimer was much higher in patients aged ≥70 years (54.9%) vs. patients aged <70 years (24.3%) and was slightly lower in men (33.1 vs. 40.5% in men and women, respectively). These results were not unexpected as it is well known that D-dimer levels increase with age 10-12 and are higher in women 13. Furthermore, it was found that the risk of VTE recurrence associated with positive D-dimer was significantly higher only in patients aged <70 years and not in the elderly, probably because of the high proportion of positive results in the elderly. With this in mind, using plasma samples available from patients enrolled in the Prolong study, cutoff values specific for age (<70 or ≥70 years) and gender were calculated for different commercial D-dimer assays, turning out to be much higher than those recommended for VTE exclusion in elderly patients, but similar or even lower in younger patients, especially in men. These cutoffs were then used in the recently published DULCIS study 14, in which the participant centers were allowed to use their routinely adopted quantitative commercial D-dimer assay. As a result, more elderly patients discontinued anticoagulation, whereas the opposite was true for younger patients, especially males.
The increase in age-dependent D-dimer levels has long been seen as a problem for the use of this assay in the work-up for VTE exclusion, and the assay performs poorly in the elderly population when traditional cutoff levels are adopted. Different cutoff systems have therefore been proposed 15-18 and prospectively investigated to rule out PE 19.
The present study, a subanalysis of the DULCIS study results, aimed at seeing how different the results might have been had different cutoff levels from those adopted in the study been used. In particular, the results (in terms of prevalence of positive D-dimer tests and predictivity of negative tests for VTE recurrence) obtained in DULCIS were compared with those that might have been obtained if the following cutoff criteria had been used: (i) conventional cutoff for diagnosis, (ii) different cutoff levels in subjects aged 60 years or more 15, 18, and (iii) age multiplied by 10 16, 17 or by five for assays with results expressed as D-dimer units 18 in subjects aged 50 years or more.
Materials and Methods
As detailed elsewhere 14, subjects who had experienced a first symptomatic VTE episode were screened for participation in the collaborative, prospective DULCIS study and those who did not present predefined criteria for exclusion, or indefinite or short anticoagulation duration were included in the study and followed the management protocol. A total of 1010 patients, whose index VTE event was idiopathic or associated with weak risk factors, were included in the management protocol that was based on (i) a minimal anticoagulation duration of 3 months (12 months in case of persistence of residual thrombus at ultrasonography examination of deep leg veins), and (ii) serial D-dimer measurements (during anticoagulation, and 15, 30, 60, and 90 days after its suspension). Persistently negative D-dimer results were recorded in 528 patients (528%), who permanently interrupted anticoagulation and were followed up for a maximum of 2 years, during which a rate of VTE recurrence of 3.0% patient/year was obtained. In contrast, the remaining 482 patients had positive D-dimer results and, at the moment of their first positive result, were recommended to continue or resume anticoagulant therapy (at that moment, only VKA drugs were available for chronic therapy), and were followed by their anticoagulation clinic. Of these latter subjects with positive D-dimer, 109 refused to resume anticoagulation, and at the end of 2 years of follow-up, their rate of recurrent VTE events was as high as 8.8% patient/years (for details, see Ref. 14).
To measure D-dimer levels, participant centers were allowed to use the quantitative assay customarily used, provided it was one of the following: (i) VIDAS D-dimer Exclusion; bioMerieux, Lyon, France), (ii) Innovance D-DIMER (Siemens, Deerfield, IL, USA), (iii) HemosIL D-dimer HS (Instrumentation Laboratory, Milan, Italy), (iv) HemosIL D-dimer (Instrumentation Laboratory), and (v) STA Liatest D-dimer (DiagnosticaStago, Asnieres-sur-Seine, France). As cutoff values for negative/positive results, the centers used those specific for the assay [distinguishing those with results expressed as fibrinogen equivalent units (FEU) or non-FEU (D-dimer units)] and for age and gender, as calculated elsewhere 9 (Table 1).
| Commercial D-dimer assay (manufacturer) ng/mL | Cutoff levels used in the DULCIS study | VTE exclusion | Age correction 1a | Age correction 2b | |||
|---|---|---|---|---|---|---|---|
| Males ≤70 years | Males >70 years | Females ≤70 years | Females >70 years | ||||
| VIDAS D-dimer Exclusion (BioMerieux) | 490 | 1050 | 600 | 1300 | 500 | 750 | Years × 10 |
| Innovance D-DIMER (Siemens) | 500 | 950 | 550 | 1150 | 500 | 750 | Years × 10 |
| HemosIL D-dimer HS (Instrumentation Laboratory) | 170 | 345 | 215 | 430 | 230 | 375 | Years × 5 |
| HemosIL D-dimer (Instrumentation Laboratory) | 205 | 300 | 225 | 330 | 230 | 375 | Years × 5 |
| STA Liatest D-dimer (Roche Diagnostics) | 340 | 700 | 450 | 1050 | 500 | 750 | Years × 10 |
In the present analysis, the prevalence of negative/positive D-dimer results across the patient population, and the ability of negative results to predict recurrent events using the above described cutoff values in patients who did not resume anticoagulation were compared with those that would have been obtained using either (i) VTE exclusion = the traditional diagnostic cutoffs declared by the producers of the assays, or (ii) age correction 1 = 750 ng/mL for assays with results expressed as FEU 15 or 375 ng/mL for non-FEU assays 18 in patients with ≥60 years, or (iii) age correction 2 = age multiplied by 10 for FEU assays 16, 17 or by 5 for non-FEU assays 18 (Table 1).
Statistical analysis
The Fisher's exact test was used for group comparison. A two-sided P value ≤0.05 was considered as statistically significant. The spss statistical software package (version 11.0, Chicago, IL, USA) was used for data processing.
Results
Quantitative D-dimer results were available in 1009 of the 1010 patients included in the DULCIS study (a qualitative result was available for just one patient) and were examined for the present analysis. Significantly more patients presented positive D-dimer in the DULCIS study than with age-corrected criteria (Table 2); this difference was entirely due to the higher prevalence of positive young subjects with the DULCIS criteria (44.1%) than with VTE exclusion (28.4%), age correction 1 (26.0%), or age correction 2 (27.7%; P < 0.0001 for all these results vs. DULCIS), especially males (Table 2). The prevalence of positive D-dimer in elderly subjects was not different with DULCIS (52.6%), age correction 1 (54.5%), or age correction 2 (51.9%) criteria, whereas it was, as expected, much higher with VTE exclusion criterion (71.5%, P = 0.0065 vs. DULCIS).
| Cutoff criteria for positive/negative DD results (see Table 1 for details) | DULCIS | VTE exclusion | Age correction 1a | Age correction 2b |
|---|---|---|---|---|
| Total patients with positive/negative DD (% positive) | 482/527 (47.8) |
515/494 (51.0) NS |
383/626(37.9) P = 0.0050 |
382/627 (37.9) P = 0.0044 |
| Young patients (n. 585, ≤70 years) with positive DD (%) | 259 (44.1) |
194 (33.2) P < 0.0001 |
152 (26.0) P < 0.0001 |
162 (27.7) P < 0.0001 |
| Elderly patients (n. 424, >70 years) with positive DD (%) | 223 (52.6) |
321 (75.7) P = 0.0065 |
231 (54.5) NS |
220 (51.9) NS |
| Males (n. 559) with positive DD (%) | 282 (50.4) |
268 (47.9) NS |
183 (32.7) P < 0.0001 |
183 (32.7) P < 0.0001 |
| Young males (n. 337) with positive DD (%) | 165 (49.0) |
111 (32.9) P = 0.0065 |
86 (25.5) P = 0.0001 |
80 (23.7) P = 0.0001 |
| Elderly males (n. 222) with positive DD (%) | 117 (52.7) |
157 (70.7) P = 0.0648 |
97 (43.7) NS |
103 (46.4) NS |
| Females (n. 450) with positive DD (%) | 199 (44.2) |
247 (54.9) NS |
199 (44.2) NS |
200 (44.4) NS |
| Young females (n. 248) with positive DD (%) | 9 3 (37.5) |
83 (33.5) NS |
76 (30.6) NS |
72 (29.0) NS |
| Elderly females (n. 202) with positive DD (%) | 106 (52.5) |
164 (81.2) P = 0.0073 |
123 (60.9) NS |
128 (63.4) NS |
- a Age correction 1 = 750 ng/mL for assays with results expressed as FEU 15 or 375 ng/mL for non-FEU assays 18 in patients with ≥60 years.
- b Age correction 2 = age multiplied by 10 for FEU assays 16, 17 or by 5 for non-FEU assays 18. AC, anticoagulant therapy; DD, D-dimer; NS, nonsignificant; P, statistical significance vs. results obtained with the DULCIS criteria.
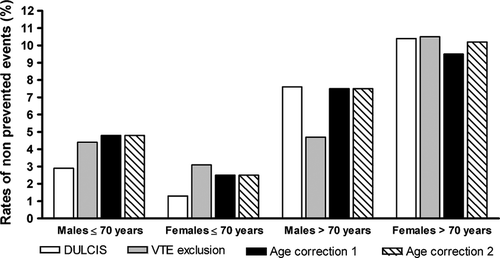
The number and rates of recurrent events in patients who did not resume anticoagulation and presented negative D-dimer with the different criteria are reported in Table 3 and Figure 1. Overall, there was no significant difference between the different cutoff criteria. However, the number and rates of recurrences in young subjects (either males or females, Figure 1) in DULCIS were half those recorded using the other criteria (7 vs. 14 events, respectively), although the difference was not statistically significant. In elderly patients, the number of thrombotic recurrences was lowest using VTE exclusion criterion thanks to the very high prevalence of positive D-dimer results in these patients, who would therefore have resumed anticoagulation.
| Cutoff criteria (Table 1 for details) | DULCIS | VTE exclusion | Age correction 1a | Age correction 2b |
|---|---|---|---|---|
| Young patients (≤70 years) n. events/total patients with negative DD (%) | 7/326 (2.1) | 14/364 (3.8) | 14/370 (3.8) | 14/371 (3.8) |
| Elderly patients (>70 years;) n. events/total patients with negative DD (%) | 18/201 (8.9) | 7/102 (6.9) | 15/181 (8.3) | 16/184 (8.7) |
| Total Events/neg | 25/527 (4.7) | 21/466 (4.5) | 29/551 (5.3) | 30/555 (5.4) |
Discussion
Several studies 1-3, 20 and meta-analyses 7, 21 have proven the predictive value for VTE recurrence of D-dimer results assessed after anticoagulation is stopped in patients with a previous unprovoked VTE event. D-dimer assessment in these studies was performed about one month after anticoagulation withdrawal using either qualitative or quantitative assays calibrated for VTE exclusion. The recently published DULCIS study 14 had important differences in its design vs. the previous studies: (i) the use of the local routinely adopted commercial assay for D-dimer testing was allowed, provided it was one of those included in the protocol, in an attempt to make it easier for clinicians and laboratories to take part in the study; (ii) age- and gender-specific D-dimer cutoff levels were adopted, to increase the sensitivity of the assay for the risk of recurrence in young patients, especially males, and to limit the risk of indefinite anticoagulation in elderly patients who often have nonspecific high D-dimer levels; and (iii) repeated D-dimer measurements during the first 3 months from anticoagulation withdrawal were adopted to detect any late, but no less potentially harmful appearance of hypercoagulability 22.
The present study compared the performance of the cutoff values for negative/positive D-dimer results adopted in DULCIS with the results that would have been obtained if different cutoff criteria had been used. The criteria used for comparison were the following: the D-dimer cutoff points recommended by the manufacturers of assays for VTE exclusion (the same whatever the age and sex of investigated subjects), and two different age-adjusted criteria recently proposed for VTE exclusion based on higher cutoff values in symptomatic outpatients aged 50 years or more 15-18. The most important difference in this comparison was the much higher prevalence of positive D-dimer in young patients in DULCIS (44.1%) than the one calculated with the other criteria (ranging from 26.0% to 28.4%, P < 0.0001). In this way, more young patients had a positive D-dimer assay and were invited to resume anticoagulation, thus limiting to a half the number of recurrences (seven cases) in negative D-dimer subjects vs. the 14 cases of recurrence that would have occurred using the other criteria for negative D-dimer. Conversely, the high cutoff values in DULCIS for patients aged over 70 years led to a prevalence of positive results (52.6%), similar to those calculated using the two age-corrected criteria for VTE exclusion (51.9% and 54.5%), but much lower than those using standard VTE exclusion criterion (71.5%, P = 0.0065). Due to the high prevalence of positive results in this population, VTE exclusion criterion would have led to a rate of recurrence of 5.8% in negative elderly patients, a rate which while not statistically significant is lower than the 8.9% recorded in DULCIS, although obtained at the expense of giving indefinite anticoagulation to much more elderly patients (18.9% more patients than with DULCIS).
We believe that the enhanced protection from recurrent events provided by the DULCIS criteria in young subjects, both men and women, is clinically important. It has been shown that, in contrast with what happens for the first VTE event, the risk of recurrence is higher in young than in elderly patients 23, 24, and in men than in women 25-27. The lower rates of recurrent events in young subjects, including males, achieved with DULCIS vs. the other criteria confirm in our opinion the validity of adopting lower cutoff points than those used for VTE exclusion.
The major limitation of this post hoc analysis is that the clinical management of the patients was performed according to the D-dimer criteria established in the DULCIS protocol while the results using different criteria were calculated only indirectly. Furthermore, most patients with positive D-dimer in DULCIS resumed anticoagulation and were obviously excluded from the analysis of what would have occurred in terms of recurrences if the other criteria had been adopted.
In conclusion, the present analysis shows that the low cutoff levels adopted in the DULCIS study for young subjects resulted in more young patients who resumed anticoagulation with subsequent increased protection from recurrent events in this population that is at high risk of recurrence. In elderly patients, the high DULCIS cutoff points led to results similar to those calculated for the two age-adjusted criteria; the adoption of VTE exclusion criteria would have led to positive results in the large majority of subjects, without a significant reduction in the rate of recurrent event.
Author Contributions
All authors contributed to concept and design the study; C. Legnani and E. Antonucci analyzed the data; all the authors contributed to interpretation of results, suggested correction of the first draft of the article, and approved its final version; G. Palareti wrote the first draft of the article and its final version.
Acknowledgement
We thank Mr. Stephen Jewkes for the revision of English.
Appendix
The following were members of the DULCIS study group (the numbers of patients who participated in the study appear in parentheses):
- Gualtiero Palareti, Benilde Cosmi, Cristina Legnani, Divisione di Angiologia e Malattie della Coagulazione, Azienda Ospedaliero-Universitaria di Bologna, Policlinico S. Orsola-Malpighi, Bologna, Coordinator Center (270)
- Nicoletta Erba, Valeria De Micheli, U.O. Patologia Clinica, Azienda Ospedaliera provincia di Lecco (139)
- Angelo Ghirarduzzi, Maria Rosaria Veropalumbo, Ugolotti Maria Chiara, Angiologia, ASMN-IRCCS - Reggio Emilia, Reggio Emilia (126)
- Daniela Poli, Domenico Prisco, Emilia Antonucci, Malattie Aterotrombotiche, AOU-Careggi, Firenze (114)
- Sophie Testa, Oriana Paoletti, Centro Emostasi e Trombosi, AO Istituti Ospitalieri, Cremona (87)
- Alberto Tosetto, Divisione Ematologia, Ospedale S. Bortolo ULSS 6 – Vicenza (54)
- Anna Falanga, Teresa Lerede SIMT Centro Emostasi e Trombosi Ospedali Riuniti di Bergamo (48)
- Steidl Luigi, Marco Donadini, Elena Rancan, Medicina Interna 1° – Ambulatorio Emostasi e Trombosi, Ospedale Di Circolo - Università dell'Insubria, Varese (42)
- Roberto Quintavalla, Piera Maria Ferrini, Medicina Interna a d indirizzo Angiologico e Coagulativo, Ospedale Maggiore Azienda Ospedaliero-Universitaria di Parma (36)
- Rita C Santoro, Centro Emofilia Emostasi e Trombosi, Az. Osp. “Pugliese-Ciaccio”, Catanzaro (34)
- Francesco Orlandini, Raffaella Benedetti, Medicina Interna, Osp. Civile S.Andrea - ASL 5 presidio Levante Ligure, La Spezia (32)
- Marco Cattaneo, Federico Lussana, Elena Bertinato, Medicina III, A.O. San Paolo - Università di Milano, Milano (28)
- Roberto Cappelli, Medicina Interna 2, Azienda Ospedaliero-Universitaria Senese, Siena (21)
- Attilia Maria Pizzini, Medicina 1° Centro Emostasi e Trombosi, Ospedale Santa Maria Nuova, Reggio Emilia (20)
- Lucia Angeloni, Geriatria, Ospedale Maggiore Bologna (10)
- Armando D'angelo, Luciano Crippa, Ambulatorio Emostasi e Trombosi, Ospedale S. Raffaele, Milano (10)
- Roberta Bortolotti, Medicina,Ospedale di San Giovanni Persiceto, Bologna (8)
- Maria Rita Vandelli, Med. Cardiovascolare – Mod. Organizzativo Angiologia- Centro Trombosi, Nuovo Ospedale Civile S.Agostino Estense, Baggiovara Modena (4)




