Infection risk with JAK inhibitors in dermatoses: a meta-analysis
Conflict of interest: Patrick Ireland, Matthew Verheyden, and Nicholas Jansson have no conflicts of interest to declare. Deshan Sebaratnam has received consulting fees from Galderma, AbbVie, Bristol Myers Squibb, Pfizer, Novartis, Amgen, Janssen, Leo Pharma, Ego Pharmacy, Sun Pharma, Viatris, and material support from Heine Optotechnik, and Candela Medical. John Sullivan has received consulting fees from AbbVie, Novartis, Janssen, and Amgen.
Funding source: None.
Abstract
Evolving evidence suggests that Janus Kinase Inhibitors (JAKi) may predispose to certain infections, including tuberculosis and human herpes viruses. This review aimed to compare the infection risk in patients on a systemic JAKi for a dermatologic indication to a placebo. A systematic review was carried out from inception to June 2023, using the EMBASE, Medline, SCOPUS, and Cochrane Library of Registered Trials databases. Eligible studies included placebo-controlled randomized trials that investigated the incidence of infection in patients with a dermatologic indication. Primary outcomes included the most commonly reported infections pertaining to serious and opportunistic infections, upper respiratory tract infections, nasopharyngitis, herpes simplex, varicella zoster, tuberculosis, neutropenia, and lymphopenia. A meta-analysis of incidence ratios was conducted to determine odds ratios (OR), with a 95% confidence interval (CI) analysis. The meta-analysis found no increased risk of serious (OR: 0.92, 95% CI: 0.61–1.43, P = 0.74) or opportunistic infections (OR: 0.65, 95% CI: 0.32–1.31, P = 0.23). The incidence of varicella-zoster infections was significantly higher in the JAKi cohort (OR: 1.72, 95% CI: 1.08–2.72, P = 0.022). From 25 studies, there was no overall increased risk of herpes simplex infections (OR: 1.43, 95% CI: 0.93–2.23, P = 0.102) to placebo; however, a significantly higher risk in those with atopic dermatitis to alopecia areata was demonstrated (OR: 1.73, 95% CI: 1.13–2.69, P = 0.013). The results of this analysis do not suggest an increased risk of serious and opportunistic infections in those on JAKi compared to placebo. However, they support an increased risk of varicella-zoster infections and a higher risk of herpes simplex infections in those with atopic dermatitis to alopecia areata. The results of this report support these agents' short-term safety but signal that vigilance should be practiced in patients at risk for serious or recurrent herpes virus infections.
Introduction
Many autoimmune diseases are mediated through a secondary messenger pathway that uses intracellular receptors to communicate and amplify signals, known as the Janus Kinase (JAK)-signal transducer and activation of transcription (STAT) pathway.1, 2 Over the past decade, medications have been developed to regulate this pathway, namely the JAK inhibitors (JAKi). They have had widespread demonstrable efficacy against a variety of pathologies.1, 2
Immunocompromise and susceptibility to infection are an inherent corollary of immune-modulating medications, such as JAKi and other disease-modifying anti-rheumatic drugs (DMARD).3 Depending on the pathway modulated, patients are often at an increased risk for serious and opportunistic infections such as tuberculosis (Tb) and human herpes viruses.4, 5
Preexisting literature has demonstrated an increased risk for certain infections, however, often only evaluating individual JAKi in specific pathologies.6 This review sets out to evaluate placebo-controlled randomized trials for all JAKi for all common dermatoses. The aim of evaluating the literature is to discern infection risk associated with the use of these medications in dermatology patients.
Materials and methods
This systematic review followed the reporting guidelines set out by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines and was prospectively registered with PROSPERO (CRD42023480783).
Search strategy
A systematic review of the literature was carried out June 2023, using the EMBASE (Ovid), Medline (Ovid), SCOPUS, and Cochrane Library of Registered Trials (CENTRAL) databases to search for eligible studies. The search included a combination of terms: "Janus Kinase Inhibitor", "Tyrosine Kinase (TYK) 2 Inhibitor", "Eczema", "Vitiligo", "Psoriasis", "Atopic Dermatitis (AD)", "Alopecia Areata (AA)", "Hidradenitis Suppurativa (HS)", "Cutaneous Lupus (CLE)", and "Lichen Planus (LP)"; with synonyms, related terms, and subject headings also utilized (Supplementary Material 1). No time restriction was placed. The initial title and abstract search strategy used no language or species filter. Reference lists of eligible articles were also screened to further identify eligible studies.
Inclusion criteria
The levels of evidence included in the review were randomized controlled trials (RCTs) with a comparator placebo cohort not receiving an active systemic treatment. Studies that compared a systemic JAKi with a placebo and detailed the incidence of infection in patients with dermatoses were eligible for inclusion. RCTs that investigated a systemic JAKi compared with a systemic placebo or an active topical medication (i.e., corticosteroid) were eligible. Studies with a cohort that included adult and pediatric (<18 years) patients were deemed eligible for inclusion. Long-term extension (LTE) studies of RCTs were included, provided the data could be compared to the initial placebo cohort.
Exclusion criteria
The levels of evidence that were excluded from the review were cohort studies, case reports, case series, and other review articles. RCTs that investigated a systemic JAKi compared with an alternative systemic agent (e.g., biologics) were excluded from the analysis. Studies investigating topical JAKi or those evaluating solely pediatric populations were excluded from the analysis.
Outcomes
The primary outcome measures were the most frequently reported infections across studies, in addition to specific infections and laboratory parameters of interest. This pertained to the incidence of serious and opportunistic infections, upper respiratory tract infection (URTI), nasopharyngitis, varicella zoster, herpes simplex, and Tb, in addition to the events of neutropenia (defined as an absolute neutrophil count (ANC) <1.0 × 109/L or grade 3 as per the common terminology criteria for adverse events [CTCAE]) and lymphopenia (defined as an absolute lymphocyte count <0.5 × 109/L). Infections were defined, diagnosed, and reported according to clinician investigators. Varicella zoster infections included events documented as shingles, herpes zoster ophthalmicus, disseminated varicella, or primary varicella infection. Herpes simplex infections included events documented as oral herpes, herpes simplex, genital herpes simplex, eczema herpeticum, or Kaposi varicelliform eruption (KVE).
Data extraction and synthesis
Data extraction was performed by two authors (PI and MV) working independently, utilizing a standardized template with the subheadings: Author, Disease, Medication, Person Exposure Years, Serious Infection, Opportunistic Infection, URTI, Nasopharyngitis, Varicella Zoster, Herpes Simplex, Tb, Neutropenia, and Lymphopenia. Person Exposure Years (PEY) were extracted from published studies or calculated based on the duration and size of treatment arms.
Bias
Two authors (PI and NJ) assessed the risk of bias in all studies that met eligibility for inclusion and were subject to full-text availability. The Cochrane Risk of Bias Version 2 (RoB-2) tool was used to assess the risk of bias.7 Publication bias was evaluated using Harbord's regression analysis and via asymmetry in funnel plot representation.
Statistical analysis
Statistical analysis was performed using STATA 18 (StataCorp, College Station, TX, USA) and SPSS (IBM Corporation, Armonk, New York, United States of America). The pooled incidence of serious and opportunistic infections, URTI, nasopharyngitis, varicella zoster, herpes simplex, Tb, neutropenia, and lymphopenia were analyzed to allow meta-analysis of odds ratio (OR) between PEY. A 95% confidence interval (CI) analysis was used, with a random-effects model presuming heterogeneity due to sampling, methodology, medication, and indication differences between studies. Subgroup analysis was carried out on the specific JAKi (medication), mechanism, and indication. A P < 0.05 was deemed significant, and an I2 > 50% was considered as significant heterogeneity between studies.
Results
Overall, 7,239 abstracts were identified using the described search strategies. The authors PI and NJ screened 5,265 results by title and abstract, evaluating 205 studies for inclusion eligibility. Forty studies were eligible for meta-synthesis and analysis by the authors PI and MV (Table 1).
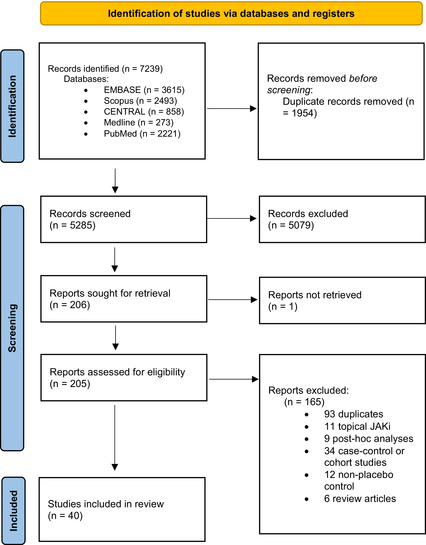
|
Twenty-eight (70%) studies were at low risk of bias across all domains, and 12 had concerns about bias in at least one domain (Appendix 1). There was no evidence of publication bias for any outcomes (Supplementary Material 2).
Forty studies met eligibility criteria and underwent meta-analysis, detailing 1793.24 placebo PEY and 6037.23 JAKi PEY (Appendix 1 and 2). Fourteen unique JAKi agents were evaluated, most commonly abrocitinib (6) and baricitinib (8). Eighteen studies examined the use of these medications in AD, whereas 13 studied psoriasis, and the remainder investigated conditions such as AA (5), chronic hand eczema (1), cutaneous lupus (1), vitiligo (1), and HS (1). The median duration of the placebo-controlled cohort was 16 weeks (range: 2–52).
Serious and opportunistic infections
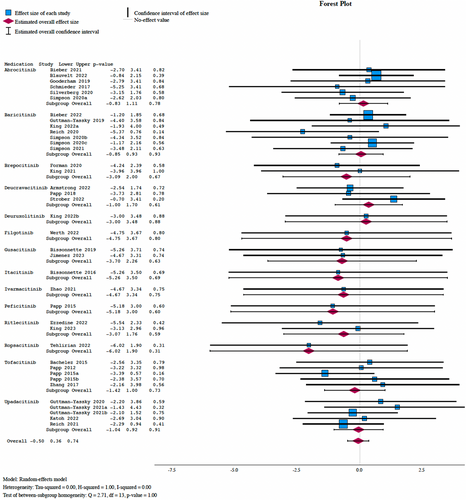
From 41 studies, 99 and 20 were pooled serious infections across the JAKi and placebo cohorts, respectively. A meta-analysis found no significant difference in serious infection incidence between JAKi and placebo arms (OR: 0.92, 95% CI: 0.61–1.43, P = 0.74), with subgroup analysis demonstrating no overall significant difference based on the medication (Q = 2.96, P = 1.00) or mechanism of the JAKi (Q = 0.71, P = 0.87) (Figure 1).
Twenty-three studies reported opportunistic infection incidence, with 38 in the JAKi cohort and 5 in the placebo cohort. A meta-analysis found no increased risk of opportunistic infections with JAKi use compared to placebo (I2 = 0.00%, OR: 0.65, 95% CI: 0.32–1.31, P = 0.23) (Supplementary Material 3). There were no cases of Tb throughout either treatment arm for the duration of the studies.
Herpes virus infections
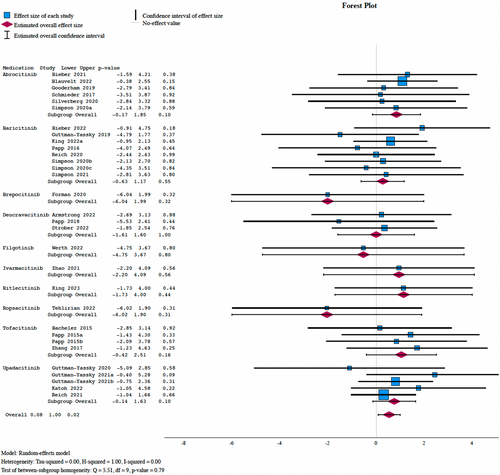
Overall, 33 studies evaluated varicella zoster infection. There were 13 reported cases in the placebo arm and 181 in the JAKi cohort. Meta-analysis demonstrated a significantly higher incidence of varicella zoster in patients on JAKi (I2 = 0.00, OR: 1.72, 95% CI: 1.08–2.72, P = 0.022), with indirect analysis demonstrating no significant difference between agents (Figure 2).
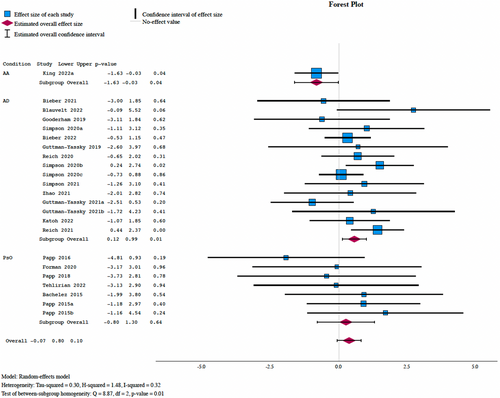
From 24 studies, there were 54 herpes simplex infections in the placebo cohort and 284 in the active JAKi cohort. Meta-analysis demonstrated no significant increase in those on JAKi (I2 = 32.23%, OR: 1.43, 95% CI: 0.93–2.23, P = 0.102) with no significant difference based on the mechanism (P = 0.77), medication (P = 0.97), or class of the JAKi (P = 0.55) (Figure 3). There was a significantly increased risk of herpes simplex infections in patients treated for AD (OR: 1.73, 95% CI: 1.13–2.69, P = 0.013) compared to AA.
Upper respiratory infections and nasopharyngitis
Overall, there were 1,035 URTIs pooled, with 214 and 835 in the placebo and JAKi arms, respectively. From 35 studies, a meta-analysis demonstrated an increased risk of URTI with JAKi use compared to placebo (OR: 1.20, 95% CI: 1.04–1.38, P = 0.012). There was no overall difference based on the medication (Q = 7.75, P = 0.74) or mechanism of JAKi (Q = 6.11, P = 0.41) (Supplementary Material 4). 373 and 1,406 cases of nasopharyngitis were reported in placebo and JAKi cohorts, respectively. A meta-analysis found no significant difference in the incidence of nasopharyngitis between the two groups (OR: 1.07, 95% CI: 0.92–1.25, P = 0.39). There was no difference based on the medication or mechanism (Supplementary Material 5).
Hematology and laboratory parameters
The incidence of lymphopenia and neutropenia was evaluated across the studies. Twenty-seven studies commented on the risk of lymphopenia, whereas 30 studies detailed neutropenia events. There were 10 cases of lymphopenia and 7 cases of neutropenia in the placebo cohorts and 32 cases of lymphopenia and 105 cases of neutropenia in the JAKi cohorts, respectively. A meta-analysis demonstrated no significant difference in risk of lymphopenia between cohorts (OR: 0.68, 95% CI: 0.60–1.11, P = 0.099), with no subgroup differences based on the medication (Q = 2.15, P = 0.95) (Supplementary Material 6). With regards to neutropenia, meta-analysis demonstrated no significant difference in those receiving JAKi compared to placebo (OR: 1.49, 95% CI: 0.86–2.56, P = 0.155) (Supplementary Material 7).
Discussion
While our review found no increased risk of serious, opportunistic, or nasopharyngeal infections, in addition to no increased risk of lymphopenia and neutropenia, the meta-analysis did demonstrate an increased risk of URTI, varicella zoster infection, and herpes simplex infections in patients with AD.
Inflammatory mediators such as tumor necrosis factor (TNF)-alpha, interferon (IFN)-gamma, and interleukin (IL)-12 are postulated to be key drivers of maturation of immune T-cells specific to varicella zoster virus (VZV), which minimize reactivation episodes. IFN-gamma and IL-12 are driven by the JAK1-2 and JAK2-TYK2 pathways, respectively.5, 45, 46 While differentiation of T-Helper (Th)2 and Th17 pathways is possible without JAK signaling, Th1 maturation responsible for intracellular and viral infections is contingent on JAK–STAT pathway activation.47, 48 While the exact mechanism for increased susceptibility has not been completely elucidated, it is suggested that for these reasons, JAKi (and namely medications that modulate the JAK1/2 pathways) may increase the risk of intracellular viral and opportunistic infections, including varicella zoster and Tb. These sentiments have been similarly resonated elsewhere.5, 49 Given the increased risk of varicella-zoster infections, it may be prudent to vaccinate all patients prior to the commencement of a JAKi, preferably with a non-live recombinant vaccine.50
This meta-analysis carried out is not without limitations. The pooling of all infections related to herpes simplex virus was a pragmatic approach to synthesize data across many studies; however, these conditions may not represent identical risk profiles—for example, the risk of herpes labialis may not represent the risk of KVE in patients. Given this, the pooling of results for meta-analysis may skew data. Second, patients with specific dermatoses, namely AD, are at an increased risk of KVE.51 Our meta-analysis demonstrated an increased risk of herpes simplex-mediated infections in patients being treated for AD compared to AA. Given this, it is worth considering that the effect of JAKi in AD may be bidirectional: a reduced risk due to management and improvement in AD while simultaneously increasing the risk of KVE due to herpetic reactivation from JAK inhibition. Several studies have evaluated the risk of herpes viruses (varicella and herpes simplex) infections, comparing incidence in JAKi vs. other biologic agents, such as dupilumab. A head-to-head RCT by Blauvelt et al. in 202152 found that the rates of varicella zoster and eczema herpeticum were numerically higher in the JAKi group compared to dupilumab. This was also reported by Bieber et al.,11 who compared abrocitinib, dupilumab, and placebo and found higher rates of varicella-zoster infections in the JAKi cohort. Limited evidence exists detailing the risk of herpes simplex-mediated infections (KVE and non-KVE), with some studies showing that there may be an equal or reduced incidence of these infections in dupilumab compared to JAKi. Given that there is at least a theoretical risk of herpes simplex reactivation, clinicians should proactively interrogate about the prior history of herpes simplex and facilitate timely access to antivirals should they be required. It may be prudent to consider alternative agents to JAKi in patients who have risk factors for serious or recurrent herpes virus infections or in those who may have experienced these while on JAKi treatment.
While our study demonstrated no increased risk of neutropenia with JAKi, this analysis is not without limitations. Where possible, neutropenia was defined in our analysis in line with the CTCAE grade 3 definition of ANC < 1.0 × 109/L. Unfortunately, many studies either did not provide definitions of thresholds for neutropenia events, did not detail the transiency and duration of these neutropenic states, or if they required treatment interruption. If studies offered no further qualification of the threshold for neutropenia, our review would have included any recorded events of neutropenia, which could have led to inaccuracies. Further standardization of definitions and outcome metrics would serve to improve inter-study reliability.
While the vast majority of studies commented on the possible impacts of JAKi use on laboratory markers, these were not infrequently recorded as continuous data in figures detailing trends against time without tabulated or raw data. Thus, it was not possible to include these measurements in the meta-analysis and relied only on data that was recorded as a binary outcome. In addition, the significance of neutropenia in this setting is unclear; there was no significant difference in the incidence of serious and opportunistic infections and nasopharyngitis between cohorts. When detailing the significance of neutropenia adverse events, one must reflect if the ANC is in itself an important marker or simply a surrogate marker for infection risk. A recent study explored the mechanism of JAKi-mediated neutropenia to be possibly related to the inhibition of anti-apoptotic factors, albeit without a decrease in the production of reactive oxygen species, meaning that neutrophils were still functional despite reduced count.53 Further research is required to investigate these patients' long-term sequelae of such possible neutropenic states.
Another limitation underpinning the analysis was the short follow-up of these studies, with a median placebo-controlled phase of 16 weeks. It is unknown whether the risk of infection requires cumulative immunosuppression from sustained exposure, so this study may provide limited insight into patients who may be on JAKi long-term. While pooling of placebo vs. long-term extension and open-label studies was performed to bolster person exposure data, it is unclear what risk profile JAKi may have at different stages of one's dosing regimen. A combination of these different risk time periods may lead to inaccuracies in true incidence, and as such, interpretation of these results with caution is recommended.
Further analyses based on the agent and dosage of the medication would have clarified the risk profile at certain dosage points. This was not performed in this study, as pool studies and medications were preferred to allow comparative analysis across a large number of studies and medications. The definition of binary outcomes of diseases (present vs. absent) was clinician-dependent, so heterogeneity in diagnostics and reporting is possible across the included studies.
There were no cases of primary mycobacterial infection throughout the studies, and given that negative screening was required for enrolment in these studies, the risk of Tb reactivation was not assessable. There is a gap in the literature detailing the true risk of JAKi and (1) predisposition to primary mycobacterial infection, and (2) risk of Tb reactivation in those with Tb infection (previously known as latent Tb). A study by Song et al. investigated the active Tb occurrence in patients prescribed JAKi vs. biological DMARD (bDMARD), such as infliximab for rheumatoid arthritis (RA).54 They reviewed 16,760 patients in South Korea and found that patients on bDMARDs were at higher risk than those on JAKi for primary mycobacterial infection, with rates of Tb reactivation comparable between the two groups.54 Winthrop et al. evaluated the incidence of Tb in patients on tofacitinib with RA and detailed that the risk of Tb in patients on JAKi was linked to endemicity; the incidence rate was 0.75 per 100 person-years in high endemic areas, compared to 0.02 per 100 person-years in low endemic areas.4 Based on these findings and the low cost of interferon-gamma release assays, the authors recommend screening in all patients prior to commencement. Tuberculin skin test can be additionally evaluated in those who come from high endemic areas due to the risk of false negatives.55, 56
To bolster the current field of literature, long-term placebo-controlled studies would serve to benefit the sphere of understanding by exploring the true risks of infection related to JAKi-mediated immunomodulation. The authors believe it appropriate to recommend vaccination for varicella zoster either before or during treatment with JAKi, to be vigilant with herpes simplex infections, particularly the risk of KVE in AD patients, and to be mindful of the agent being used and its associated mechanism in patients who may be at an increased risk for certain infections.
Conclusion
The results of this meta-analysis do not suggest an increased risk of serious and opportunistic infections in those on JAKi compared to placebo for the corresponding timeframe. It did, however, support an increased risk of varicella-zoster infections and a higher risk of herpes simplex infections in those with atopic dermatitis compared to alopecia areata. The results of this report support the safety of these agents from an infection perspective but do signal that vigilance should be practiced in those who are at high risk for recurrent or serious herpes virus infections.
Appendix 1: Descriptive characteristics of placebo-controlled portion of eligible studies
| Study | Clinical trials no | Disease | Medication | Duration (weeks) | Placebo size (n) | Placebo age (years) | Placebo PEY (years) | JAKi size (n) | JAKi age (years) | JAKi PEY (years) | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alavi 20228 | NCT03607487 | HS | Povorcitinib | 8 | 9 | 40.3 | 1.38 | 36 | 41.62 | 5.54 | SC |
| Armstrong 20229 | NCT03624127 | PsO | Deucravacitinib | 52 | 165 | 47.9 | 46.9 | 531 | 45.9 | 419.1 | Low |
| Bachelez 201510 | NCT01241591 | PsO | Tofacitinib | 12 | 107 | 46 | 24.69 | 659 | 44 | 152.08 | Low |
| Bieber 202111 | NCT03720470 | AD | Abrocitinib | 16 | 131 | 37.4 | 40.31 | 464 | 38.03 | 142.77 | Low |
| Bieber 202212 | NCT03428100 | AD | Baricitinib | 52 | 93 | 38.7 | 93 | 370 | 38.05 | 370 | Low |
| Bissonnette 201613 | NCT01634087 | PsO | Itacitinib | 4 | 12 | 49.1 | 0.92 | 38 | 48.25 | 2.92 | Low |
| Bissonnette 201914 | NCT03139981 | AD | Gusacitinib | 4 | 9 | 29.9 | 0.69 | 27 | 37.9 | 2.08 | SC |
| Blauvelt 202215 | NCT03627767 | AD | Abrocitinib | 40 | 267 | 29 | 205.38 | 531 | 28.5 | 693 | SC |
| Ezzedine 202216 | NCT03715829 | VV | Ritlecitinib | 24 | 66 | 46.1 | 30.46 | 298 | 44.11 | 153.688462 | Low |
| Forman 202017 | NCT02969018 | PsO | Brepocitinib | 12 | 23 | 50.3 | 5.31 | 189 | 44.54 | 43.62 | Low |
| Gooderham 201918 | NCT02780167 | AD | Abrocitinib | 12 | 56 | 42.6 | 12.92 | 211 | 40.37 | 48.69 | SC |
| Guttman-Yassky 201819 | NCT02576938 | AD | Baricitinib | 12 | 49 | 35 | 11.31 | 75 | 37.91 | 17.31 | Low |
| Guttman-Yassky 201920 | NCT02925117 | AD | Upadacitinib | 16 | 40 | 39.9 | 12.31 | 126 | 39.97 | 38.77 | SC |
| Guttman-Yassky 202121 | NCT03569293 | AD | Upadacitinib | 16 | 281 | 34.4 | 86.46 | 566 | 33.84 | 174.15 | Low |
| Guttman-Yassky 202121 | NCT03607422 | AD | Upadacitinib | 16 | 278 | 33.4 | 85.54 | 558 | 33.7 | 171.69 | Low |
| Jimenez 202322 | CHE | Gusacitinib | 16 | 32 | 41.8 | 9.85 | 65 | 45.43 | 20 | Low | |
| Katoh 202223 | NCT03661138 | AD | Upadacitinib | 16 | 90 | 36.3 | 27.69 | 182 | 35.3 | 496.62 | SC |
| King 202124 | NCT04517864 | AA | Brepocitinib/ritlecitinib | 24 | 47 | 38 | 21.69 | 95 | 35.52 | 21.69 | Low |
| King 202225 |
NCT03570749 NCT03899259 |
AA | Baricitinib | 36 | 371 | 37.5 | 905 | 37.92 | 22.15 | Low | |
| King 202226 | NCT03137381 | AA | Deuruxolitinib | 24 | 44 | 37.8 | 243.2 | 105 | 36.31 | 604 | SC |
| King 202327 | NCT03732807 | AA | Ritlecitinib | 24 | 131 | 34 | 20.31 | 587 | 33.66 | 48.46 | Low |
| Papp 201228 | NCT00678210 | PsO | Tofacitinib | 12 | 50 | 43.9 | 11.54 | 147 | 44.43 | 33.92 | Low |
| Papp 201529 | NCT01096862 | PsO | Peficitinib | 6 | 29 | 53.1 | 3.35 | 95 | 46.56 | 10.96 | SC |
| Papp 201530 | NCT01276639 | PsO | Tofacitinib | 16 | 177 | 45 | 54.46 | 723 | 46 | 222.46 | Low |
| Papp 201530 | NCT01309737 | PsO | Tofacitinib | 16 | 196 | 45 | 60.31 | 763 | 45.5 | 234.77 | Low |
| Papp 201631 | NCT01490632 | PsO | Baricitinib | 12 | 34 | 46.7 | 7.85 | 237 | 47.39 | 54.69 | Low |
| Papp 201832 | NCT02931838 | PsO | Deucravacitinib | 12 | 45 | 46 | 10.38 | 222 | 44.4 | 51.23 | Low |
| Reich 202033 | NCT03733301 | AD | Baricitinib | 16 | 109 | 33.7 | 33.54 | 220 | 33.86 | 67.69 | Low |
| Reich 202134 | NCT03568318 | AD | Upadacitinib | 16 | 304 | 34.3 | 93.54 | 597 | 33.97 | 183.69 | Low |
| Schmieder 201835 | NCT02201524 | PsO | Abrocitinib | 4 | 14 | 45.4 | 1.08 | 45 | 45.73 | 3.46 | SC |
| Silverberg 202036 | NCT03575871 | AD | Abrocitinib | 12 | 78 | 33.4 | 18 | 313 | 35.47 | 72.23 | Low |
| Simpson 202037 | NCT03334396 | AD | Baricitinib | 16 | 249 | 35 | 76.62 | 375 | 36.01 | 115.38 | Low |
| Simpson 202037 | NCT03334422 | AD | Baricitinib | 16 | 244 | 35 | 75.08 | 371 | 34.37 | 114.15 | Low |
| Simpson 202138 | NCT03435081 | AD | Baricitinib | 16 | 147 | 39 | 45.23 | 293 | 40 | 90.15 | Low |
| Simpson 202039 | NCT03349060 | AD | Abrocitinib | 12 | 77 | 31.5 | 17.77 | 310 | 32.8 | 71.54 | Low |
| Strober 202240 | NCT03611751 | PsO | Deucravacitinib | 52 | 501 | 47.3 | 194 | 833 | 46.9 | 549.9 | Low |
| Tehlirian 202241 | NCT03895372 | PsO | Ropsacitinib | 16 | 45 | 46.5 | 13.85 | 133 | 44.2 | 112 | SC |
| Werth 202242 | NCT03134222 | CLE | Filgotinib | 12 | 9 | 46 | 2.08 | 17 | 43 | 3.92 | SC |
| Zhang 201743 | NCT01815424 | PsO | Tofacitinib | 16 | 88 | 41.7 | 27.08 | 178 | 40.85 | 54.77 | Low |
| Zhao 202144 | NCT04162899 | AD | Ivarmacitinib | 12 | 35 | 30.3 | 8.08 | 70 | 36.85 | 16.15 | SC |
- AA, alopecia areata; AD, atopic dermatitis; CHE, chronic hand eczema; CLE, cutaneous lupus erythematosus; HS, hidradenitis suppurativa; JAKi, Janus kinase inhibitor; PEY, person exposure years; PsO, psoriasis; SC, some concerns (for risk of bias); VV, vitiligo.
Appendix 2: Summary infection results table
| Study | JAK inhibitor | Placebo | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serious | Opport-unistic | VZ | HSV/KVE | TB | URTI | Naso-pharyngitis | Lympho-paenia | Neutro-paenia | Serious | Opportu-nistic | VZ | HSV/KVE | TB | URTI | Naso-pharyngitis | Lympho-paenia | Neutro-paenia | |
| Alavi 20228 | 0 | NR | NR | NR | NR | 4 | 3 | NR | NR | 0 | NR | NR | NR | NR | 0 | 1 | NR | NR |
| Armstrong 20229 | 6 | NR | 5 | NR | NR | 50 | 96 | 1 | 1 | 1 | NR | 0 | NR | NR | 6 | 7 | 1 | 0 |
| Bachelez 201510 | 4 | NR | 3 | 7 | NR | 10 | 51 | 2 | NR | 0 | NR | 0 | 0 | NR | 0 | 10 | 0 | NR |
| Bieber 202111 | 2 | NR | 6 | 2 | NR | 21 | 37 | 0 | 0 | 0 | NR | 0 | 1 | NR | 6 | 9 | 0 | 0 |
| Bieber 202212 | 11 | 3 | 13 | 38 | 0 | NR | 88 | 3 | 4 | 2 | 0 | 0 | 7 | 0 | NR | 14 | 0 | 0 |
| Bissonnette 201613 | 0 | NR | NR | NR | NR | NR | 7 | NR | NR | 0 | NR | NR | NR | NR | NR | 1 | NR | NR |
| Bissonnette 201914 | 0 | 0 | NR | NR | 0 | NR | 3 | 0 | NR | 0 | 0 | NR | NR | 0 | NR | 1 | 0 | NR |
| Blauvelt 202215 | 13 | NR | 20 | 25 | NR | 79 | 105 | NR | NR | 2 | NR | 2 | 0 | NR | 6 | 5 | NR | NR |
| Ezzedine 202216 | 0 | NR | 3 | NR | NR | 34 | 44 | NR | NR | 0 | NR | 1 | NR | NR | 8 | 14 | NR | NR |
| Forman 202017 | 1 | 0 | 0 | 3 | NR | 14 | 28 | NR | 0 | 0 | 0 | 0 | 0 | NR | 1 | 3 | NR | 0 |
| Gooderham 201918 | 2 | NR | 2 | 2 | NR | 16 | 28 | 1 | 1 | 0 | NR | 0 | 1 | NR | 5 | 5 | 0 | 0 |
| Guttman-Yassky 201819 | 0 | NR | 0 | 1 | NR | 2 | 4 | 0 | 1 | 0 | NR | 1 | 0 | NR | 1 | 1 | 3 | 0 |
| Guttman-Yassky 201920 | 3 | 0 | 0 | NR | NR | 17 | 9 | 1 | 5 | 0 | 0 | 0 | NR | NR | 4 | 1 | 0 | 0 |
| Guttman-Yassky 202121 | 4 | 3 | 11 | 3 | 0 | 63 | 55 | 3 | 19 | 0 | 4 | 0 | 4 | 0 | 20 | 16 | 2 | 2 |
| Guttman-Yassky 202121 | 3 | 3 | 9 | 3 | 0 | 36 | 34 | 1 | 8 | 2 | 0 | 2 | 0 | 0 | 12 | 13 | 0 | 1 |
| Jimenez 202322 | 0 | NR | NR | NR | NR | 7 | 5 | NR | NR | 0 | NR | NR | NR | NR | 3 | 2 | NR | NR |
| Katoh 202223 | 1 | 4 | 4 | 7 | 0 | NR | 79 | 0 | 5 | 0 | 0 | 0 | 2 | 0 | NR | 14 | 0 | 0 |
| King 202124 | 0 | 0 | NR | NR | NR | 11 | 4 | 0 | 2 | 0 | 0 | NR | NR | NR | 5 | 6 | 0 | 0 |
| King 202124 | 0 | 0 | NR | NR | NR | 4 | 6 | 1 | 0 | |||||||||
| King 202225 | 3 | 0 | 9 | 13 | 0 | 57 | 50 | 2 | 6 | 0 | 0 | 2 | 12 | 0 | 21 | 19 | 0 | 0 |
| King 202226 | 1 | NR | NR | NR | NR | 11 | 15 | NR | 2 | 0 | NR | NR | NR | NR | 7 | 1 | NR | 1 |
| King 202327 | 2 | 0 | 8 | NR | NR | 47 | 68 | NR | NR | 0 | 0 | 0 | NR | NR | 10 | 8 | NR | NR |
| Papp 201228 | 1 | NR | NR | NR | NR | 10 | 6 | NR | NR | 0 | NR | NR | NR | NR | 5 | 5 | NR | NR |
| Papp 201529 | 0 | NR | NR | NR | NR | 3 | 5 | NR | 1 | 0 | NR | NR | NR | NR | 0 | 3 | NR | 0 |
| Papp 201530 | 2 | 0 | 8 | 10 | 0 | 41 | 51 | 3 | 1 | 2 | 0 | 0 | 1 | 0 | 5 | 20 | 0 | 0 |
| Papp 201530 | 3 | 0 | 4 | 10 | 0 | 39 | 62 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 11 | 1 | 0 |
| Papp 201631 | NR | 0 | 1 | 1 | NR | 4 | 15 | 3 | 3 | NR | 0 | 0 | 1 | NR | 1 | 4 | 0 | 0 |
| Papp 201832 | 1 | 0 | 0 | 1 | 0 | 10 | 19 | NR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | NR | 0 |
| Reich 202033 | 0 | 0 | 2 | 12 | 0 | 11 | 29 | 2 | 1 | 2 | 1 | 1 | 3 | 0 | 2 | 13 | 1 | 0 |
| Reich 202134 | 3 | 7 | 8 | 40 | 0 | 44 | 77 | 0 | 5 | 3 | 0 | 3 | 5 | 0 | 22 | 34 | 1 | 0 |
| Schmieder 201835 | 0 | NR | 1 | NR | NR | 3 | NR | NR | 4 | 0 | NR | 0 | NR | NR | 0 | NR | NR | 1 |
| Silverberg 202036 | 2 | NR | 2 | NR | NR | 19 | 32 | 0 | 0 | 1 | NR | 0 | NR | NR | 3 | 5 | 0 | 0 |
| Simpson 202037 | 0 | 0 | 2 | 20 | NR | 8 | 46 | 0 | 0 | 0 | 0 | 1 | 3 | NR | 6 | 26 | 0 | 0 |
| Simpson 202037 | 5 | 0 | 0 | 18 | NR | 15 | 39 | 0 | 0 | 2 | 0 | 0 | 11 | NR | 5 | 30 | 0 | 0 |
| Simpson 202138 | 1 | 0 | 1 | 5 | 0 | 20 | 10 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 9 | 11 | 1 | 0 |
| Simpson 202039 | 3 | NR | 4 | 11 | NR | 22 | 41 | 0 | 1 | 1 | NR | 0 | 1 | NR | 5 | 8 | 0 | 0 |
| Strober 202240 | 11 | 0 | 4 | NR | 0 | 74 | 133 | 0 | 0 | 1 | 0 | 1 | NR | 0 | 27 | 47 | 0 | 1 |
| Tehlirian 202241 | 0 | NR | 0 | 0 | NR | NR | NR | 2 | 9 | 0 | NR | 0 | 0 | NR | NR | NR | 0 | 1 |
| Werth 202242 | 0 | 0 | 0 | NR | 0 | NR | NR | NR | NR | 0 | 0 | 0 | NR | 0 | NR | NR | NR | NR |
| Zhang 201743 | 1 | 2 | 5 | NR | 0 | 19 | 17 | 0 | 0 | 0 | 0 | 0 | NR | 0 | 3 | 3 | 0 | 0 |
| Zhao 202144 | 0 | NR | 2 | 3 | NR | 3 | NR | NR | NR | 0 | NR | 0 | 1 | NR | 0 | NR | NR | NR |
- AA, alopecia areata; AD, atopic dermatitis; CLE, cutaneous lupus erythematosus; CHE, chronic hand eczema; HS, hidradenitis suppurativa; HSV/KVE, herpes simplex virus/kaposi varicelliform eruption; JAKi, Janus Kinase inhibitor; NR, not reported; Opportunistic, opportunistic infections; PsO, psoriasis; Serious, serious infections; TB, tuberculosis; URTI, upper respiratory tract infection; VV, vitiligo; VZ, varicella zoster.
Open Research
Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.




