Loss of CELF2 promotes skin tumorigenesis and increases drug resistance
Conflict of interest: Co-author Noah Goldfarb receives research support from Novartis and DeepX Health for unrelated topics. All other authors declare no conflicts of interest.
Funding source: National Institutes of Health, R01CA196639.
Abstract
Background
CELF2 belongs to the CELF RNA-binding protein family and exhibits antitumor activity in various tumor models. Analysis of the pan-cancer TCGA database reveals that CELF2 expression strongly correlates with favorable prognosis among cancer patients. The function of CELF2 in nonmelanoma skin cancer has not been studied.
Methods
We used shRNA-mediated knockdown (KD) of CELF2 expression in human squamous cell carcinoma (SCC) cells to investigate how CELF2 impacted SCC cell proliferation, survival, and xenograft tumor growth. We determined CELF2 expression in human SCC tissues and adjacent normal skin using immunofluorescence staining. Additionally, we investigated the changes in CELF2 and its target gene expression during UV-induced and chemical-induced skin tumorigenesis by western blotting.
Results
CELF2 KD significantly increased SCC cell proliferation, colony growth, and SCC xenograft tumor growth in immunodeficient mice. CELF2 KD in SCC cells led to activation of KRT80 and GDF15, which can potentially promote cell proliferation and tumor growth. While control SCC cells were sensitive to anticancer drugs such as doxorubicin, SCC cells with CELF2 KD became resistant to drug-induced tumor growth retardation. Finally, we found CELF2 expression diminished during both UV- and chemical-induced skin tumorigenesis in mice, consistent with reduced CELF2 expression in human SCC tumors compared to adjacent normal skin.
Conclusion
This study shows for the first time that CELF2 loss occurs during skin tumorigenesis and increases drug resistance in SCC cells, highlighting the possibility of targeting CELF2-regulated pathways in skin cancer prevention and therapies.
Introduction
The CELF (CUGBP and ETR-3-like factors) protein family consists of RNA-binding proteins that regulate target gene expression by post-transcriptional processing of RNAs.1 CELF proteins are found in both the nucleus, where they regulate alternative splicing and editing of pre-mRNA targets, and the cytoplasm, where they control mRNA translation and/or stability.2 The human CELF protein family contains six known members, CELF1 to CELF6. CELF1 and CELF2 are broadly expressed in different tissues,3, 4 whereas CELF3-6 shows restricted neuron expression.4-6
CELF1 is often upregulated in human cancers such as oral SCCs, and its aberrant upregulation is linked to poor prognosis in cancer patients.7, 8 CELF1 might promote tumorigenesis by stabilizing genes involved in the epithelial-mesenchymal transition regulatory networks.9 In contrast, CELF2 exhibits antitumor activity in a variety of tumor models, including breast cancer,10 lung cancer,11 gastric cancer,12 and ovarian cancer.13 CELF2 may also be a more reliable biomarker than PD-1, PD-L1, or CTLA-4 in selecting immunotherapy-sensitive patients.14 To date, the function of CELF2 in nonmelanoma skin cancer has not been investigated. We hypothesized that CELF2 might also act as a tumor suppressor in the skin and that loss of CELF2 function might contribute to the development of SCC.
In this study, we examined the role of CELF2 in the development of squamous cell carcinoma (SCC). Immunofluorescence (IF) staining revealed the loss of CELF2 expression in clinical human SCC tumors and during UV- or chemical-induced skin tumorigenesis in mice. Loss-of-function studies in human SCC cells further demonstrated that CELF2 deficiency significantly increased SCC cell proliferation and anchorage-independent colony growth, as well as resistance to doxorubicin- or UNC0642-induced retardation of tumor growth. These results suggest that CELF2 might play an essential role in skin carcinogenesis.
Materials and methods
Cell culture and lentiviral transduction
Human SCC13 cells were obtained from Columbia University Skin Disease Research Center and were cultured in CnT-07 medium supplemented with the IsoBoost supplement (from CELLnTEC) and 1% penicillin–streptomycin, as reported previously.15, 16 SCC13 cells were infected with lentivirus that expressed scrambled shRNA (shScr) as control and with three different CELF2-targeting shRNA (shCELF2) (Vector Builder). Following lentiviral infection, cells were selected using neomycin (0.7–1 mg/ml) to generate stable CELF2-knockdown (KD) and vector control cell lines for subsequent in vitro and xenograft experiments.
Skin cancer genomic and transcriptomic datasets used for CELF2 expression and mutation analysis
Three different skin cancer genomic datasets, published and available through cBioportal, were used to analyze the rate of CELF2 gene mutation in SCCs.17-19 The mRNA expression of CELF2 in human SCCs and adjacent normal skin tissues was performed using an RNA-seq dataset from a previous transcriptomic profiling study based on five pairs of primary human SCCs and matched adjacent standard skin samples.15
Cell proliferation and wound-healing assays
Cell growth and proliferation were assessed using the IncuCyte® live-cell analysis system (Sartorius), a phenotypic cell-based assay to monitor cell growth over a defined period in a cell culture environment, as previously described.15, 20 Briefly, 5 × 103 cells were seeded in a 96-well plate. To test the impact of CELF2 KD on SCC cell sensitivity to anticancer drugs, cells were treated with doxorubicin (an FDA-approved chemotherapeutic drug; Sigma Aldrich), or UNC0642 (an experimental anticancer drug; Cayman Chemicals), or DMSO as a control. UNC0642 is an epigenetic anticancer drug known to act by inhibiting the G9A histone methyltransferase. Images were acquired and analyzed by using IncuCyte Zoom. Cell proliferation was expressed as the percentage of cell density observed. Cells were seeded in triplicate in 96-well plates for wound-healing experiments and grown to confluence. Wounds were created using the IncuCyte® Wound Maker. After that, cells were washed with PBS to remove cellular debris. Fresh medium was added to the plate for continuous incubation. Wound healing was recorded every 12 hours in the IncuCyte Zoom time-lapse imaging system. The wound-healing rate was expressed as the width of wounds at specific time points.
Soft-agar assay
To assess the colony-forming ability of human SCC cells, 5 × 103 cells were mixed with 0.4% agarose in a growth medium and placed on top of a solidified layer of 0.8% agarose in a growth medium in 6-well plates. The medium was changed every 3 days. After 5–6 weeks, the colonies were stained with crystal violet (0.5% solution), washed with PBS, and imaged with a digital camera. The colonies were counted by using ImageJ software (imagej.org).
Mouse xenograft experiments, UV irradiation, and DMBA treatment
NSG (NOD SCID IL2Rgnull) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). SKH-1 hairless mice were purchased from Charles River Laboratory (Kingston, NY). Mice were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experiments were performed strictly following protocols approved by the Institutional Animal Care and Use Committee of the University of Minnesota (2111-39576A). The study is reported following ARRIVE guidelines.21 Mouse xenograft experiments were performed as previously described.20 SCC13 cells with CELF2 KD or shScr control were injected subcutaneously into SCID mice. For each injection, 1 × 106 cells were suspended in 100 μl of a serum-free medium and Matrigel mixture (1:1 ratio). Tumor growth was monitored and recorded weekly for up to 7 weeks, after which the animals were euthanized for tumor collection and analysis. For UV-induced skin tumors, SKH-1 hairless mice were subjected to a standard UV irradiation protocol where mice were irradiated twice weekly with 180 mJ/cm2 of UVB, as we previously reported.15 For DMBA-induced skin tumors, SKH-1 mice (6 weeks of age) were treated with DMBA (1 mg/mouse in 0.1 ml of corn oil) by oral gavage once a week for 5 weeks in total. Tumor formation was monitored weekly. Mice were euthanized when tumors reached 1 cm in diameter or 6 months after the first UV or DMBA treatment. Upon euthanization, skin tumors and nontumor-bearing skin were collected for gene expression analysis.
RNA extraction, qRT-PCR, and RNA-seq analysis
Total RNA was isolated from cultured shCELF2 and shScr SCC cells using the RNeasy kit (Qiagen, Hilden, Germany). For qRT-PCR, 2 μg of RNA was reverse-transcribed into cDNA. qRT-PCR was performed with RT2 SYBR Green qPCR Mastermix (Qiagen), and the results were analyzed using the Bio-Rad CFX 96 Touch system (Bio-Rad, Hercules, CA, USA). RNA-seq analysis was performed as previously described.16, 20 To enrich mRNAs for library preparation, the total RNA (500 ng) from each sample was subjected to poly-A pulldown with the Illumina TruSeq RNA prep kit (Illumina, San Diego, CA, USA). The resulting libraries were sequenced on an Illumina HiSeq4000 at the University of Minnesota Genomics Center. Sequencing reads were mapped to the human reference genome (NCBI/build37.2) using Tophat (version 2.0.4). Genes differentially expressed between CELF2-KD (shCELF2) and control (shScr) cells were determined using the DESeq2 software package with a fold-change cutoff set at >2 or <0.5.
Western blotting
Primary antibodies used for western blotting (WB) and immunostaining were CELF2 (Aviva Systems Biology), β-actin (St. Louis, MO, USA), and H3K9me1/2 (Woburn, MA, USA). For WB, a cell pellet was collected after trypsinizing the cells. The cell pellet was lysed in RIPA with protease inhibitor for 30 minutes, followed by centrifugation at 10,000 rpm for 20 min. The supernatant was collected, and protein was measured using the Bicinchoninic acid kit (Thermo Fisher). As we previously reported, approximately 25 μg of total protein from each sample was run on 4–20% SDS gel for WB.22
Immunofluorescence staining
The human SCC tumors with matched adjacent normal skin tissues were collected in the Department of Dermatology, Columbia University Medical Center (IRB protocol #AAAB2667, approved by the Institutional Review Board of Columbia University).23 As previously described, immunofluorescence staining of cultured SCC cells on glass coverslips or frozen tissue sections (6 μm in thickness) was performed.20 Briefly, slides were fixed in cold ethanol for 20 min and 4% paraformaldehyde for 10 min and then washed thrice with PBS. After incubation in blocking buffer (0.1% Triton X-100 and 10% normal serum in PBS) for 1 hour, slides were incubated with a primary antibody (1:100 dilution in blocking buffer) overnight at 4 °C in a humidified chamber. After three consecutive 5-min washes with PBST, the slides were incubated with a secondary antibody (Alexa Fluor 488 goat anti-mouse IgG or Alexa Fluor 568 goat anti-rabbit IgG; Thermo Fisher; 1:100 dilution) at room temperature for 1 hour. Negative controls were stained with secondary antibodies alone. Sections were mounted with Gelvatol mounting medium containing 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories, Newark, CA, USA). Images were acquired using a fluorescence confocal microscope (Zeiss, Thornwood, NY, USA). All IF evaluation experiments were performed in at least three independent experiments.
Statistical analyses
Data were analyzed by one-way anova or Dunnett's t-test using the GraphPad Prism 9 software (GraphPad Prism 9, GraphPad Software). All experiments were done in triplicate. The data were expressed as mean ± SD. A value of P < 0.05 was set as statistically significant. All pertinent data are available within the manuscript or upon request.
Results
CELF2 expression is lost in human SCCs and during UV- and DMBA-induced skin tumorigenesis
To assess the role of CELF2 in skin tumorigenesis, we first analyzed CELF2 mutation rates in three nonmelanoma skin cancer genomic datasets available through the cBioPortal database.17-19 In these datasets, the CELF2 mutation rate was as high as 14% in human skin SCCs (Figure 1a), suggesting a potential genomic instability affecting CELF2 function in SCC tumors. Based on an analysis of differential gene expression between human SCCs and adjacent normal skin using previously published datasets,23 we found that CELF2, but not CELF1, mRNA expression was reduced by 50% or more in SCCs compared to matched normal skin (Figure 1b, n = 5 per group). Immunofluorescence staining in primary human SCCs showed a more pronounced loss of CELF2 expression in invasive human SCCs than in situ (Figure 1c). Using tumors collected from UV-induced and DMBA-induced skin tumorigenesis mouse models, we found reduced CELF2 expression in tumors compared to nontumor-bearing skin by both IF and western blotting (Figure 1d,e). These studies revealed the loss of CELF2 expression during skin tumorigenesis in both clinical samples and mouse models.
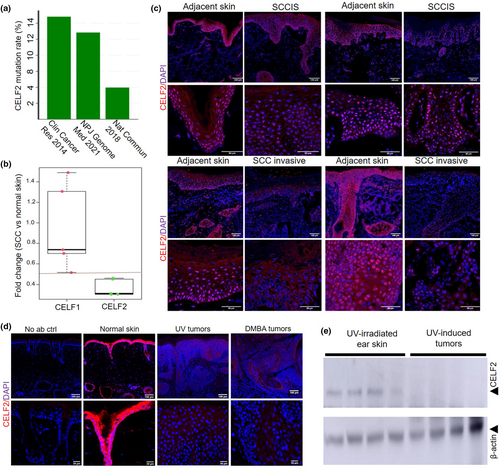
CELF2 knockdown increases SCC13 cell proliferation, colony formation, and wound healing process
To further define the role of CELF2 in SCC tumorigenesis, we performed shRNA-mediated CELF2 KD in human SCC13 cells. SCC13 cells were infected with lentiviruses expressing scrambled shRNA (shScr) as a control and CELF2-targeting shRNAs (shCELF2) to abrogate CELF2 expression. Western blotting and IF staining verified the efficiency of CELF2 KD (Figure 2a,b). Following successful CELF2 KD, we performed cell proliferation assays, which showed that CELF2 KD significantly increased SCC cell proliferation compared with the shScr control (Figure 2c). Next, we conducted a soft-agar colony formation assay to assess the impact of CELF2 KD on the anchorage-independent clonogenicity of SCC cells. The colony number and size were significantly higher for the CELF2 KD SCC cells than for the shScr control group (Figure 2d). Furthermore, a wound-healing assay demonstrated that CELF2 KD significantly enhanced wound closure compared with shScr control cells (Figure 2e). Together, these results indicate that loss of CELF2 augmented SCC cell proliferation, colony formation, and wound closure in vitro.
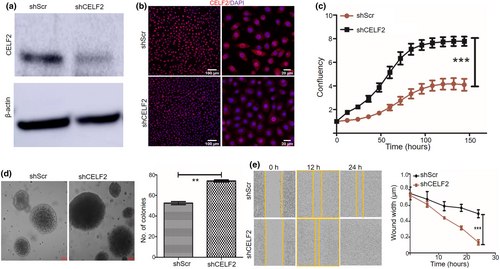
CELF2 KD increases SCC xenograft tumor growth in mice
To further assess the impact of CELF2 KD on SCC tumor growth in vivo, CELF2 KD cells and shScr control SCC13 cells were injected subcutaneously into NOD SCID mice. Tumor growth was measured twice weekly. The tumor growth rate showed significant differences between the shScr and shCELF2 groups from around 37 days after tumor cell injection (Figure 3a). At the end of the experiment, tumors were collected and weighed, and the results revealed an approximately 49.21% increase in the tumor weight of the shCELF2 tumor group compared to the shScr control group (Figure 3b,c). Immunofluorescence staining confirmed lower CELF2 expression in shCELF2 tumors than in shScr control tumors, as illustrated in the representative images of CELF2 expression in Figure 3d. These results show that CELF2 KD promoted SCC tumor growth in xenograft mouse models.
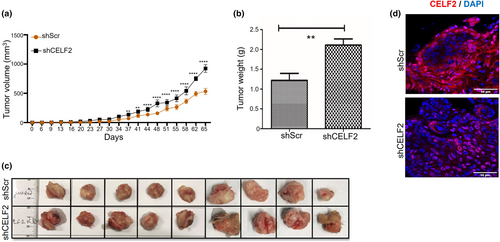
CELF2 knockdown increases drug resistance of SCC13 cells
UNC0642 is a selective small molecule inhibitor of the G9a histone methyltransferase that can effectively reduce genomic H3K9 methylation.24, 25 Previous studies have established that UNC0642 possesses potent anticancer activity partly mediated by inhibiting global histone H3K9 methylation.25, 26 To test if UNC0642 exerts an anti-proliferative effect on SCC cells, we treated SCC13 cells with various concentrations of UNC0642. We found UNC0642 significantly inhibited their proliferation (Figure 4a), which led to a robust reduction in the levels of mono- (H3K9me1) and di-methylation (H3K9me2) of histone H3K9 (Figure 4b), consistent with its ability to inhibit G9a histone methyltransferase activity. While both CELF2-KD and shScr control SCC13 cells appeared sensitive to UNC0642, we noticed that CELF2 KD rendered the cells more resistant to UNC0642 than the control transfection (Figure 4c). Consistent with these findings, we also showed that UNC0642 significantly inhibited control SCC tumor growth in NOD SCID mice but had no significant impact on CELF2-KD tumor growth (Figure 4d,e), validating the results from the in vitro cell culture studies. To test whether CELF2 loss could induce resistance to other chemotherapeutic drugs, we treated control and CELF2 KD SCC13 cells with different concentrations of doxorubicin, an FDA-approved chemotherapeutic drug widely used in cancer treatment. While 5 nm doxorubicin significantly inhibited the growth of shScr control cells, it had little effect on CELF2-KD cells (Figure S2), suggesting CELF2 loss could induce drug resistance in SCC cells.
Loss of CELF2 increases KRT80 expression in SCC cells
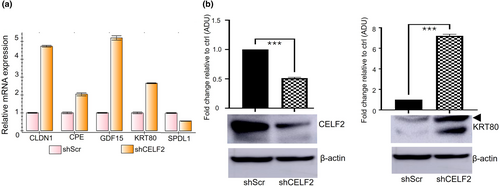
To identify downstream target genes that might mediate the tumor suppressor activity of CELF2 in SCC cells, we performed RNA-seq studies to identify genes differentially expressed between CELF2-KD and shScr control SCC13 cells. Among the top-ranked differentially expressed genes (Table S1), we found several candidate targets showing consistent changes following CELF2 KD in SCC13 cells and two breast cancer cell lines (Figure S3). We selected CLDN1, CPE, GDF15, SPDL1, and KRT80 for further validation using qRT-PCR and western blotting based on the change in their expression following CELF2 KD is consistent with their known or implicated function as either an oncogene or tumor suppressor gene (Table S1). The qRT-PCR results showed that the changes in mRNA expression of the selected targets were consistent with the RNA-seq results (Figure 5a). Western blotting validated the upregulation of KRT80 following CELF2 KD (Figure 5b), but the results for the rest of the selected targets were inconclusive due to the lack of good-quality antibodies. KRT80 encodes a type II epithelial keratin protein that plays an important role in cell differentiation and tumor progression. Our findings indicate that suppressing KRT80 expression might partly mediate CELF2's antitumor activity.
Discussion
As an RNA-binding protein, CELF2 has been shown to function as a tumor suppressor in various cancer models. Based on comprehensive analyses of TCGA RNA-seq data, Wang et al.14 reported that CELF2 expression is significantly downregulated in multiple human cancers, including bladder, brain, breast, lung, lymphoma, ovarian, and prostate cancers, relative to their respective adjacent normal tissues. However, the function of CELF2 in skin cancer and its effect on skin cancer behavior has not been reported. This study found decreased CELF2 expression in human SCC tumors and during UV- and chemical-induced skin tumorigenesis, although the mechanism underlying CELF2 loss during skin tumorigenesis is unknown. We also showed that depletion of CELF2 in SCC cells significantly increases cell proliferation and migration in vitro and tumor growth in vivo. Furthermore, CELF2 KD rendered SCC cells more resistant to the anticancer drugs UNC0642 and doxorubicin. This is consistent with previous studies suggesting that CELF2 overexpression could sensitize ovarian cells to cisplatin.13
Mechanistically, CELF2 modulates the expression of its target genes post-transcriptionally via pre-mRNA alternative splicing, which influences mRNA translation and stability. Yeung et al.11 reported that CELF2 exerts its antitumor activity by interacting with PREX2 protein to reduce the association of PREX2 with PTEN. Guo et al.13 showed that CELF2 expression reduced ovarian cancer cell growth and survival by increasing the stability and function of FAM198B, a potent tumor suppressor. The RNA-seq results described here identified several dysregulated genes following CELF2 KD in SCC cells, including activating the KRT80 tumor promoter. Abnormal KRT80 activation has been found in colorectal, gastric, and breast cancers and is linked with increased tumor growth, invasion, and drug resistance.27-30 Epigenetic activation of KRT80 driven by SREBP1-mediated enhancer activation was shown to promote resistance to endocrine therapy for breast cancer and cell invasion through KRT80-mediated actin cytoskeleton rearrangements.27 Thus, KRT80 may have broad application to cancer diagnosis, prognosis, and treatment, although the CELF2-KRT80 axis in SCC tumorigenesis and its potential therapeutic implications remain to be determined. Future studies are warranted to define the mechanism by which CELF2 regulates KRT80 expression and to exploit its therapeutic potential in the treatment of CELF2-deficient tumors. Besides KRT80, we have identified other CELF2 targets (CLND1, GDF15, SPDL1, etc.) that might have important functional implications in skin cancer. However, we could not validate their protein expression following CELF2 KD in this study due to the lack of quality antibodies. Nevertheless, understanding the function of these novel CELF2 targets in skin cancer might lead to new molecular targets in future skin cancer prevention and treatment.
CELF2 plays a vital role in regulating the expression of various genes involved in tumorigenesis; control of CELF2 expression has also been investigated in several studies. Pique et al.10 reported that CELF2 is epigenetically silenced by promoter DNA methylation in human breast cancer. Fan et al.31 reported that CELF2 is a direct target of miR-95-3p, and expression levels of CELF2 and miR-95-3p are negatively correlated in glioma tissues and cell lines. Brucein-D, a drug that targets miR-95, can reactivate CELF2 and impair the proliferation of hepatocellular carcinoma cells.32 More recently, CELF2 was shown to be targeted by ALKBH5 for m6A modification, leading to its degradation by YTHDF2 in pancreatic cancer cells.33 Curcumin, a polyphenol derived from the rhizome of the turmeric plant (Curcuma longa), has been demonstrated to have therapeutic efficacy for many cancer types in vivo and in vitro.34-36 Curcumin can directly activate CELF2, leading to cell cycle arrest, inhibited cell growth, and improved drug sensitivity in pancreatic cancer cells.37 Overexpression of CELF2 significantly inhibits lung cancer growth in vitro or in vivo by inhibiting the PI3K/AKT signaling pathway phosphorylation.38 Our studies demonstrate that UNC0642 can effectively inhibit SCC cell proliferation in vitro and tumor growth in vivo. Whether UNC0642 activates CELF2 expression remains to be determined in future studies. Notably, the antitumor impact of UNC0642 diminishes in CELF2-KD cells, underscoring the significance of CELF2 expression in the therapeutic effectiveness of certain anticancer medications.
In summary, our findings demonstrate that CELF2 expression consistently decreases in human SCCs and during UV- and chemical-induced skin tumorigenesis in mice, suggesting a potential inhibitory role for CELF2 in skin tumorigenesis and progression. CELF2 loss renders SCC cells more resistant to experimental and chemotherapeutic drugs, underscoring the prognostic biomarker potential of CELF2 in cancer diagnosis and therapy. A limitation of the current study is its focus on one SCC cell line. Utilizing additional cell lines will help determine if the control of KRT80 expression by CELF2 is a conserved mechanism in other SCC cell lines. A deeper understanding of how CELF2 KD increases KRT80 expression is needed in future investigations. Moreover, the specific molecular targets and pathways through which CELF2 exerts its tumor-suppressive activity in skin cancer cells require further exploration. The lack of quality antibodies against other CEFL2 target genes (CLDN1, GDF15, CPE, SPDL1) restricts our ability further to characterize their function in SCC cells and tumor samples.
Acknowledgments
We thank Dr. Juan Abrahante Lloréns for expert bioinformatics support and Drs. Yibin Deng and Rebecca Morris for their insightful comments. This work was supported by the Hormel Foundation, NIH/NCI R01CA196639, and the Prevent Cancer Foundation research award. Bindeshwar Sah and Jasvinder Singh were supported by the Eagle's Cancer Telethon Postdoctoral Fellowship awards. The materials presented here solely represent the authors' views and do not represent the views of the US Department of Veterans Affairs or the United States Government.
Open Research
Data availability statement
The data generated during the current study are available from the corresponding author upon reasonable request. RNA-seq datasets were deposited into the Gene Expression Omnibus (GEO) database repository (GSE244862).




