Efficacy and tolerability of tirbanibulin 1% ointment in the treatment of cancerization field: a real-life Italian multicenter observational study of 250 patients
Conflict of interest: None.
Funding source: None.
Abstract
Background
Tirbanibulin 1% ointment is approved for the field treatment of Olsen grade I actinic keratoses (AKs) of the face and scalp.
Methods
We performed a multicenter retrospective study involving 15 dermatologic units in Italy to investigate the efficacy and tolerability of tirbanibulin in a real-life setting. 250 patients were enrolled. Tirbanibulin, 1% ointment, was applied daily for five consecutive days. The efficacy of treatment was measured with modifications of the Actinic Keratosis Area and Severity Index (AKASI). A satisfactory response was defined by complete (100% reduction in the number of lesions) or partial clearance (75–99%) of treated AKs.
Results
Overall, the AKASI score was significantly reduced in the studied population (mean, from 4.1 ± 2.7 to 1.4 ± 1.5; P < 0.001). A satisfactory response was observed in 222 (88.8%) cases. The proportion of satisfactory responses was higher when follow-up was performed after 8 weeks (34/35, 97.1%). The reduction in AKASI was significant in patients with Olsen grade II or III lesions (from 5.3 ± 2.8 to 1.6 ± 1.6; P < 0.001). A satisfactory response was observed in 91/104 (87.5%) cases. AKASI reduction was also significant in patients with trunk or limb AKs (from 7.0 ± 1.3 to 2.0 ± 1.6; P = 0.018) since a satisfactory response was observed in 7/8 (87.5%) cases. Tirbanibulin was well tolerated; all adverse events (AEs) included transient local reactions at the site of treatment. Overall, 231 patients had at least one AE. Only 7 (2.8%) grade 4 AEs were recorded.
Conclusion
Our retrospective study confirmed that tirbanibulin 1% ointment is effective and well tolerated in a real-life setting and is also promising for Olsen grade II and grade III AKs and AKs localized on difficult-to-treat areas.
Introduction
Actinic keratosis (AK) is a cutaneous lesion that results from the intraepidermal proliferation of atypical keratinocytes and presents as a scaly or keratotic patch or thin plaque on an erythematous base. AKs develop more frequently in males, lighter phototypes (Fitzpatrick I-II), the elderly, and patients with a history of longstanding sun exposure. Typical areas include the face, neck, dorsum of the forearm and hand, and legs.
It is considered a premalignant lesion that can progress to cutaneous squamous cell carcinoma (SCC).1 The progression rate to SCC is reported in a wide range of cases, from 0.025 to 16%, and is estimated to be around 0.06–0.5% per single lesion per year. Of note, AKs can also occasionally regress without the need for treatment.2 However, since progression and regression of single lesions are essentially unpredictable, treatment of AKs is always recommended.3-5
Cryotherapy and other physical or pharmacological treatments are usually prescribed for single lesions.6 Multiple AKs, however, require a so-called “field therapy,” which targets AKs and the sun-damaged skin area surrounding them. Field therapies can be useful in both treating existing AKs and preventing new lesions from developing, since they also address subclinical damage.7 Currently, available field treatments primarily include topical agents such as 5-fluorouracil ointment/cream, imiquimod cream, diclofenac gel, and photodynamic therapy. Compared with the “spot treatment” of single lesions, field therapy carries a higher risk of local skin reactions and might require a prolonged application to be effective.8 Regarding the chemoprophylaxis of actinic keratosis and skin cancers, the efficacy of oral nicotinamide 500 mg twice daily has been recently confirmed both in immunocompetent and immunocompromised patients.9, 10
Recently, tirbanibulin 1% ointment was developed and approved by the European Medicines Agency (EMA) and the United States Food and Drug Administration for the treatment of cancerization field on Olsen grade I AKs (i.e., patches or thin plaques that are more readily palpated than visualized thanks to their surface roughness) of the face and scalp.11 Tirbanibulin inhibits tubulin polymerization and Src kinase signaling, resulting in antiproliferative and pro-apoptotic activity.12, 13 Its effectiveness and safety on lesions of the face and scalp area were demonstrated by two phase III clinical trials.14 More recently, tirbanibulin was also proven to be effective on AKs of the upper limbs.15, 16 In a recent economic evaluation in Scotland, tirbanibulin appeared to be cost-saving compared with diclofenac sodium 3%, imiquimod 5%, and 5-fluorouracil 5%. Moreover, although clearance rates were similar, the shorter treatment course and fewer local adverse events (AEs) may favorably impact patients' adherence to therapy.17
Considering the evidence supporting the use of tirbanibulin, its administration should be optimized, contemplating data derived from clinical practice. Therefore, this multicenter retrospective study was designed to analyze the efficacy, safety, and tolerability of tirbanibulin 1% ointment on AKs in a real-life setting and to describe its effects on lesions with different grades and localization.
Materials and methods
This multicenter retrospective study involved 15 dermatologic centers in Italy. Consecutive adult patients with multiple actinic keratoses were enrolled between March 1, 2023, and July 31, 2023. All patients signed an informed consent form for the publication of anonymous clinical data.
The Olsen grade of actinic keratoses was assessed at baseline. Treatment with tirbanibulin was performed as recommended by European regulation, according to current clinical practice,18 applying the 1% ointment daily for five consecutive days. Follow-up visits were scheduled according to the local clinical practice. The efficacy of treatment was measured once at follow-up visits. The Actinic Keratosis Area and Severity Index (AKASI)19 was recorded before and after treatment to evaluate the overall lesion burden and its modifications with therapy. Clearance of treated lesions was also recorded after treatment. A satisfactory response was defined by the detection of either complete (100% reduction in the number of lesions) or partial clearance (75–99%) of treated AKs. In comparison, an incomplete response was defined as a <75% reduction in the number of lesions.
The study was conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), with the Helsinki Declaration of 1975, as revised in 2000, and with the Taipei Declaration. All patients provided written informed consent for the publication of the material. Because of the retrospective nature of the study, only a notification to the Ethics Committee was requested.
Statistical analysis
Data were summarized by descriptive analysis. Means, median, and standard deviations (SDs) were calculated for continuous variables, while absolute values and frequency (percentage) were calculated for categorical variables. A t-test was performed to compare mean values. The Wilcoxon-signed rank test was used to compare repeated measurements in each group. ANOVA test was used to analyze variable correlations. A P < 0.05 significance level was considered for all the statistical tests. All analyses were performed with IBM SPSS Statistics for Windows, Version 26.0.
Results
Patients' characteristics and history
Overall, 250 patients were enrolled and treated with tirbanibulin 1% ointment; 178 (71.2%) were males, and 72 (28.8%) were females, with a mean age of 74.7 ± 9.0 years. Twenty-one (8.4%) presented Fitzpatrick phototype I, 159 (63.9%) phototype II, and 70 (28.0%) phototype III. Comorbidities were reported in 171 (68.4%) patients. The most common were hypertension and chronic ischemic heart disease (94 cases, 37.6%) and diabetes mellitus (27, 10.8%). As far as risk factors for AKs were concerned, 170 (68%) patients reported sunburn episodes in childhood, and oncological history was positive for basal cell carcinoma (BCC) in 98 (39.2%) patients, for SCC in 60 (24.0%), and for cutaneous melanoma in 20 (8.0%). Forty-seven (18.8%) BCCs and 48 (19.2%) SCCs, but no melanoma, had developed in the head and neck area (Table 1).
| Sex | |
| Male | 178 (71.2%) |
| Female | 72 (28.8%) |
| Mean age ± SD, years | 74.7 ± 9.0 |
| Fitzpatrick phototype | |
| 1 | 21 (8.4%) |
| 2 | 159 (63.6%) |
| 3 | 70 (28.0%) |
| Childhood sunburns | 170 (68%) |
| Previous melanoma | 20 (8.0%) |
| Head and neck | – |
| Chest | 8 (3.2%) |
| Back | 10 (4.0%) |
| Upper limbs | 2 (0.8%) |
| Lower limbs | 1 (0.4%) |
| Previous basal cell carcinoma | 98 (39.2%) |
| Head and neck | 47 (18.8%) |
| Chest | 32 (12.8%) |
| Back | 37 (14.8%) |
| Upper limbs | 10 (4%) |
| Lower limbs | 6 (2.4%) |
| Previous squamous cell carcinoma | 60 (24.0%) |
| Head and neck | 48 (19.2%) |
| Chest | 4 (1.6%) |
| Back | 2 (0.8%) |
| Upper limbs | 3 (1.2%) |
| Lower limbs | 3 (1.2%) |
| Previous AKs | 195 (78.0%) |
| Face and scalp | 156 (62.4%) |
| Rest of the body | 9 (3.6%) |
| Face and scalp and rest of the body | 30 (12.0%) |
| Olsen grade of previous AKs | |
| I | 32 (12.8%) |
| I and II | 42 (16.8%) |
| I and III | 1 (0.4%) |
| I, II, and III | 7 (2.8%) |
| II | 81 (32.4%) |
| II and III | 10 (4.0%) |
| III | 22 (8.8%) |
| Mean number of previous therapies for AKs ± SD | 1.9 ± 1.4 |
| Number of previous therapies for AKs | |
| None | 59 (23.6%) |
| 1 | 39 (15.6%) |
| 2 | 64 (25.6%) |
| 3 | 58 (23.2%) |
| 4 | 25 (10.0%) |
| 5 | 4 (1.6%) |
| 6 | 1 (0.4%) |
- AKs, actinic keratoses; SD, standard deviation.
Most patients (195, 78.0%) had previously received a diagnosis of AK. AKs had arisen more frequently on the face and scalp area (156, 62.4%); 9 patients (3.6%) had developed AKs on other body areas and 30 (12.0%) on both sites. Previous lesions were classified as Olsen grade I in 32 (12.8%) cases, grade II in 81 (32.4%), and grade III in 22 (8.8%), while 60 (24.0%) patients presented lesions with different Olsen grades at the same time. Fifty-nine (23.6%) patients had not undergone any therapy, while 39 (15.6%) had received one treatment, and 152 (60.8%) had received two or more treatments (Table 1). When data regarding previous therapies were available, these included pharmacological therapy (201, 87.4%), cryotherapy (143, 59.8%), photodynamic therapy (93, 39.7%), and surgery (30, 13.2%).
At baseline, 236 (94.4%) patients presented AKs in the face and scalp area, 8 (3.2%) in the rest of the body, and 6 (2.4%) in both sites. AKs were classified as Olsen grade I in 114 (45.6%) cases, grade II in 83 (33.2%), and grade III in 18 (7.2%), while 35 (14.0%) patients showed lesions with different Olsen grades.
Tirbanibulin outcomes
All patients (250, 100%) completed the 5-day treatment course with tirbanibulin 1% ointment. Follow-up visits after treatment application were performed at different time intervals, depending on availability in clinical practice: after 1 week for 5 (2.0%) patients, after 2 weeks for 16 (6.4%), after 3 weeks for 19 (7.6%), after 4 weeks for 29 (11.6%), and after more than 4 weeks for 181 (72.4%).
After treatment, the AKASI score was significantly reduced in the overall population (n = 250; mean, from 4.1 ± 2.7 to 1.4 ± 1.5; median, from 3.2 [range: 0.6–12.5] to 1.2 [range: 0–8.2]; P < 0.001) (Figure 1, Table 2). The percent reduction of the AKASI score was significantly lower in patients with a history of childhood sunburns than in the overall population (Estimated marginal means: 67.3, 95% CI: 53.6–81.0, vs. 78.5, 95% CI: 63.4–93.6). Conversely, the percentage of reduction in the AKASI score increased with the growing age of patients.
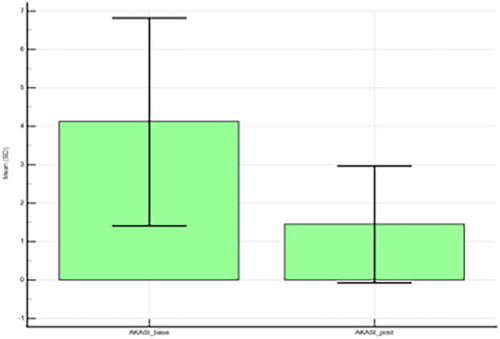
| Overall population | |||
| AKASI | Pre-treatment | Post-treatment | Paired test P-value |
| Mean ± SD | 4.1 ± 2.7 | 1.4 ± 1.5 | <0.001 |
| Median (range) | 3.2 (0.6–12.5) | 1.2 (0–8.2) | |
| Olsen grade I AKs of the face and scalp | |||
| AKASI | Pre-treatment | Post-treatment | Wilcoxon test P-value |
| Mean ± SD | 3.1 ± 2.4 | 1.2 ± 1.4 | <0.001 |
| Median (range) | 2.4 (0.6–10.2) | 1.2 (0–5.4) | |
| AKs of the trunk and limbs | |||
| AKASI | Pre-treatment | Post-treatment | Wilcoxon test P-value |
| Mean ± SD | 7.0 ± 1.3 | 2.0 ± 1.6 | 0.018 |
| Median (range) | 7.0 (6.1–8.2) | 1.8 (1.0–2.8) | |
| Olsen grade II and grade III AKs of the face and scalp | |||
| AKASI | Pre-treatment | Post-treatment | Wilcoxon test P-value |
| Mean ± SD | 5.3 ± 2.8 | 1.6 ± 1.6 | <0.001 |
| Median (range) | 4.8 (2.8–7.4) | 1.2 (0–2.4) | |
- AKs, actinic keratoses; AKASI, Actinic Keratosis Area and Severity Index; SD, standard deviation.
Overall, a satisfactory response (≥75% clearance) was observed in 222 (88.8%) patients, while 28 (11.2%) subjects had incomplete clearance (<75% clearance) (Table 3). In the overall population, among the five patients controlled after only 1 week, 4 (80%) already had partial clearance at the time of the visit. In those controlled after 2 weeks, a satisfactory response was present in 11/16 (68.7%). Among those controlled after 3 weeks, a satisfactory response was observed in 18/19 (94.7%) cases. Among those controlled after 4 weeks, a satisfactory response was found in 22/29 (75.9%) cases (Table 3).
| Complete clearance (100%) | Satisfactory clinical response (≥75% clearance) | |
|---|---|---|
| Overall population | 89/250 (35.6%) | 222/250 (88.8%) |
| Follow-up visit after 1 week | – | 4/5 (80%) |
| Follow-up visit after 2 weeks | 2/16 (12.5%) | 9/16 (56.3%) |
| Follow-up visit after 3 weeks | 7/19 (36.8%) | 11/19 (57.9%) |
| Follow-up visit after 4 weeks | 10/29 (34.5%) | 12/29 (41.1%) |
| Olsen grade I AKs of the face and scalp | 44/111 (39.6%) | 99/111 (89.2%) |
| Follow-up visit after 4 weeks | 36/68 (52.9%) | 62/68 (91.0%) |
| Follow-up visit after 8 weeks | 19/35 (54.3%) | 34/35 (97.1%) |
| AKs of the trunk and limbs | 4/8 (50.0%) | 7/8 (87.5%) |
| Olsen grade II and grade III AKs of the face and scalp | 37/104 (35.6%) | 91/104 (87.5%) |
- AKs, actinic keratoses.
A subgroup of 111 patients adhered strictly to the approved indication for tirbanibulin, as they had actinic keratoses with Olsen grade I, localized in the face and scalp area. In this group, the AKASI was significantly reduced after treatment (mean, from 3.1 ± 2.4 to 1.2 ± 1.4; median, from 2.4 [range: 0.6–10.2] to 1.2 [range: 0–5.4]; P < 0.001) (Table 2). The clearance was complete in 44/111 (39.6%) cases, and the response was satisfactory in 99/111 (89.2%) (Figure 2).
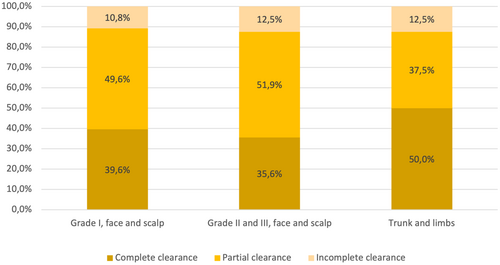
In this subgroup, 68 patients were controlled more than 4 weeks after the treatment, and a satisfactory response was detected in 62/68 (91.2%) patients, while complete clearance was observed in 36/68 (52.9%). A satisfactory response was present in 28/33 (84.8%), and complete clearance in 17/33 (51.5%) patients was observed between 4 and 8 weeks after treatment. Finally, a satisfactory response was observed in 34/35 (97.1%) and complete clearance in 19/35 (54.3%) patients with Olsen grade I lesions of the face and scalp area controlled more than 8 weeks after treatment (Table 3).
The reduction in mean AKASI was also significant in the subgroup of patients with lesions Olsen grade II or III (from 5.3 ± 2.8 to 1.6 ± 1.6; P < 0.001) (Table 2). A satisfactory response was observed in 91/104 (87.5%) cases, while complete clearance was observed in 37/104 (35.6%) cases (Figure 2, Table 3).
The reduction in mean AKASI after tirbanibulin treatment was also significant in the subgroup of patients with actinic keratoses of the trunk or limbs (from 7.0 ± 1.3 to 2.0 ± 1.6; P = 0.018) (Table 2). Among these patients, complete clearance at the follow-up visit was obtained in 4/8 (50%) cases and satisfactory response in 7/8 (87.5%) cases (Figure 2, Table 3).
Tirbanibulin tolerability
Topical tirbanibulin 1% ointment application was well tolerated, and no unexpected AE was reported. All AEs included transient local reactions at the site of treatment (Table 4). Reported AEs included erythema (230 patients, 92.4%), scaling (169, 67.6%), swelling (112, 44.8%), crusting (98, 39.2%), vesicles or pustules (37, 14.8%), and ulceration (36, 14.4%). 231 patients had at least one AE, and only 7 (2.8%) grade 4 AEs were recorded. In addition, 79 (31.6%) patients reported grade 1 AEs, 109 (43.6%) grade 2 AEs, and 36 (14.4%) grade 3 AEs (Figures 3 and 4).
| Patients with at least 1 AE | 231 (92.4%) |
| Highest AE grade | |
| Grade 1 | 79 (31.6%) |
| Grade 2 | 109 (43.6%) |
| Grade 3 | 36 (14.4%) |
| Grade 4 | 7 (2.8%) |
| Erythema | 230 (92.0%) |
| Grade 1 | 103 (41.2%) |
| Grade 2 | 93 (37.2%) |
| Grade 3 | 30 (12.0%) |
| Grade 4 | 4 (1.6%) |
| Swelling | 112 (44.8%) |
| Grade 1 | 66 (26.4%) |
| Grade 2 | 41 (16.4%) |
| Grade 3 | 5 (2.0%) |
| Grade 4 | – |
| Scaling | 169 (67.6%) |
| Grade 1 | 106 (42.4%) |
| Grade 2 | 53 (21.2%) |
| Grade 3 | 9 (3.6%) |
| Grade 4 | 1 (0.4%) |
| Crusting | 98 (39.2%) |
| Grade 1 | 59 (23.6%) |
| Grade 2 | 34 (13.6%) |
| Grade 3 | 4 (1.6%) |
| Grade 4 | 1 (0.4%) |
| Vesiculation and/or pustulation | 37 (14.8%) |
| Grade 1 | 23 (9.2%) |
| Grade 2 | 13 (5.2%) |
| Grade 3 | – |
| Grade 4 | 1 (0.4%) |
| Erosion and/or ulceration | 36 (14.4%) |
| Grade 1 | 17 (6.8%) |
| Grade 2 | 15 (6.0%) |
| Grade 3 | 4 (1.6%) |
| Grade 4 | – |
- AE, adverse event.

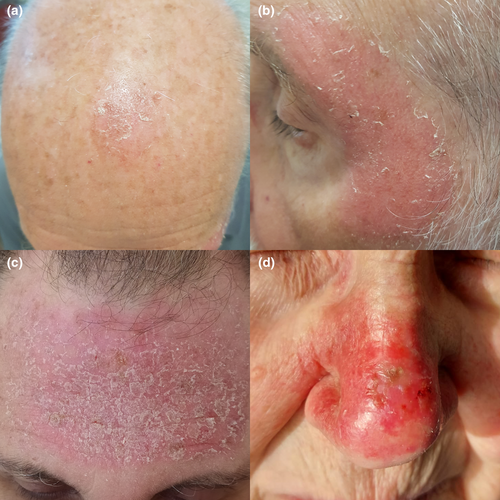
Tolerability was similar in the overall population and the subgroup of 111 patients with Olsen grade I actinic keratoses localized in the face and scalp area. In this subgroup, the incidence of at least one AE was 100/111 (90.1%); 100 (90.1%) patients had erythema, 39 (35.1%) swelling, 61 (55.0%) scaling, 29 (26.1%) crusting, 9 (8.1%) vesicles or pustules, and 5 (4.5%) ulceration.
Discussion
This observational retrospective multicenter study investigated the efficacy and tolerability of tirbanibulin 1% ointment for treating AKs in a real-life setting.
Tirbanibulin is a novel synthetic molecule that has shown antiproliferative and antitumoral effects both in vitro and in vivo. It reversibly binds the colchicine-binding site of β-tubulin and guarantees a dose-dependent inhibition of tubulin polymerization, which is essential during cell division. Moreover, it causes the cell cycle to arrest in the G2/M phase, thus activating both intrinsic and extrinsic pro-apoptotic signaling pathways and promoting expression of the p53 onco-suppressor gene.13 Interestingly, tirbanibulin seems to promote the release of fewer pro-inflammatory cytokines than other medications used in treating AKs, such as 5-fluorouracil, and selectively target highly proliferating cells. Therefore, tirbanibulin might be associated with a milder inflammatory local reaction than other drugs.13 Tirbanibulin also inhibits the Src kinase signaling pathway.20 Src expression is enhanced in AK and SCC when compared to healthy surrounding skin, and its downstream targets are likely implicated in tumor progression and invasive potential.13
Our study prescribed tirbanibulin according to current clinical practice and local regulations.11 However, this real-life setting also included a subgroup of patients with Olsen grade II or III AKs and patients with lesions located elsewhere.
In Blauvelt et al.'s two identical phase III studies, tirbanibulin 1% ointment was applied once daily for five consecutive days on a 25-cm2 contiguous area containing 4 to 8 lesions. On Day 57, complete clearance was obtained in 49% of patients, which was significantly higher than in the placebo group. Partial clearance (≥75% reduction in the number of lesions) occurred in 72% of the treated patients. 47% of patients showed AK recurrence after 1 year, and 27% were estimated to maintain complete clearance.14 A direct comparison with these results is not feasible, primarily due to the different study designs and the variable time intervals at which follow-up visits were performed in our case. Still, a ≥75% reduction in the number of lesions was achieved in most of our patients as well.
In addition, our study showed that tirbanibulin 1% ointment could also be effective and tolerated for Olsen II-III lesions and AK of the trunk and limbs. Indeed, we could evaluate patients with lesions of different Olsen grades, and we had similar clinical results. In such cases, clinicians report having patients use topical keratolytic agents (e.g., salicylic acid ointment or urea cream) for a few days before using tirbanibulin ointment. Additionally, the outcomes we observed on AK localized outside the face and scalp area were in agreement with the retrospective case review by Iglesia-Puzas et al.,15 which showed good activity of tirbanibulin 1% ointment in subjects with AK of the upper limbs. So, it can be suggested that tirbanibulin 1% ointment is effective and tolerated on lesions that are more challenging to treat (with a higher Olsen grade and localization on the trunk and limbs) than those recommended.
Due to the real-life setting, follow-up visits were performed at a variable time lapse from the treatment, while the two clinical trials reported by Blauvelt et al.14 evaluated all patients after 2 months. We could observe patients in a very early phase after treatment, and we found that 4/5 patients had already had a partial response in a week. In the trials, complete clearance of AKs of any localization was reported in 44 and 54% of cases, and at least partial clearance in 68 and 76%. Our observation showed that the best results were observed at least 8 weeks from treatment in patients with grade I AK of the scalp or face area. Nevertheless, we report that partial clearance may be obtained within a week, and complete clearance frequency is similar starting from 3 weeks controls. This may suggest that tirbanibulin 1% ointment effects are quicker than indicated by the trials, and this result may be relevant for clinical practice as it suggests that follow-up visits could not necessarily be scheduled at 8 weeks.
Overall, tirbanibulin 1% ointment is well tolerated. The most common AEs were local reactions at the application site, particularly erythema and flaking/scaling, which were self-limiting and healed without sequelae, consistent with literature data.14, 21 Real-life data revealed that the median onset of AEs occurs on the seventh day after treatment, and the meantime for resolution is 5 days. In our cohort, since AEs were mostly self-reported by patients, the exact time of occurrence and resolution were difficult to assess. Interestingly, a more intense inflammatory local reaction does not correlate with a better clinical outcome.21, 22 A recent meta-analysis demonstrated a lower incidence of severe local AEs with tirbanibulin 1% ointment when compared to some other medications of AKs (namely, a combination of 5-fluorouracil 0.5% and salicylic acid; diclofenac 3%; imiquimod 3.75 and 5%). However, no data were available for comparison with cryotherapy, photodynamic therapy, and 5-fluorouracil 4 and 5%.23 Furthermore, an expert opinion among dermatologists in charge of evaluating a tailored approach for AKs considered tirbanibulin 1% ointment among the topical treatments with the highest tolerability.24
Conclusions
Our study confirms the already published data regarding the efficacy and safety of tirbanibulin 1% ointment for Olsen grade I AKs of the face and scalp. In particular, the best results in this subgroup of patients were observed at least 8 weeks after treatment. Moreover, tirbanibulin shows promising outcomes when prescribed for higher grade lesions (Olsen grade II or III) as well as lesions located on the trunk and limbs, supporting its potential use regardless of grade and location of AKs. However, further studies are needed to broaden its indication criteria and directly compare tirbanibulin with other treatment options for AKs.
Given the real-life nature of this study, we acknowledge the limitations deriving from the absence of a pre-determined schedule for follow-up visits, as well as the fact that AEs were self-reported mainly by patients and not assessed by a trained clinician.
Open Research
Data availability statement
The data supporting this study's findings are available on request from the corresponding author.




