Atopic dermatitis in adults: a population-based study in Finland
Abstract
Background
The prevalence of atopic dermatitis (AD) has increased, but studies in adult or elderly populations are sparse.
Methods
We investigated 12-month and lifetime prevalences of AD in the Finnish adult population ≥30 years of age and analyzed living environment factors, socioeconomic factors, lifestyle-related factors, and serum vitamin D levels for their associations with AD in a national health examination survey.
Results
The lifetime prevalence was 21.9% and 12-month prevalence 10.1%. The highest prevalence (lifetime 28.6%, 12-month 15.4%) was seen in subjects 30-39 years of age. Prevalence decreased with age. Subjects with highly educated parents were more likely to have active AD, though there was no effect of higher education in subjects themselves. Younger age and being an ex-smoker were associated with active AD. Female sex and daily smoking increased the risk in subjects 30-49 years of age. There was no dose–response relationship to serum vitamin D levels and no association with the living environment.
Conclusions
Our data show that the number of adult patients with atopic dermatitis has grown and prevalence numbers of AD in Finnish adults are among the highest reported. Together with the aging of the society, the burden of AD is not limited to childhood.
Introduction
The prevalence of atopic dermatitis (AD, atopic eczema) has increased since the mid-20th century but without a uniform global pattern.1, 2 This increase suggests a likelihood of environmental risk factors and gene–environment interactions. The onset of AD usually occurs in childhood, and it has generally been accepted that the majority of childhood AD will clear up by early adolescence.3 However, the majority of patients who have symptoms in their adolescent years still have symptomatic eczema later in life.4 Even if AD is mostly a childhood disease, it has been estimated that up to one-sixth of adult patients have an adult-onset type of AD.5 Additionally, AD among the elderly seems to be increasing.6 However, epidemiological studies on the prevalence of AD in adults, especially in senior populations, are sparse. Earlier studies in adults have reported 12-month prevalence numbers up to 17.1%, challenging the view of AD as a disease that clears up with age.7
In some countries, childhood AD seems to be associated with high socioeconomic status (SES) and a higher number and order of siblings, but a few studies have also shown this correlation in adults.8 In studies on the association of rural living environments with the risk of AD, results vary.9 In some populations, high SES has been shown to have an impact on the risk of AD, its persistence into adulthood, and onset after puberty.10 Nevertheless, the effects of SES and living environment on the risk of AD have primarily been studied in pediatric and adolescent populations, and little is known of the long-term effects of these factors.
Most of the recent epidemiological research on AD has focused on childhood, adolescence, and young adulthood. Studies on lifestyle determinants and AD are few. AD patients have had a higher prevalence of smoking and alcohol abuse in some studies and a recent meta-analysis, while studies on the relationship of obesity and physical activity with AD have shown conflicting results.11-13 There has been an increasing interest in the relationship of vitamin D and AD, and a recent meta-analysis showed a possible ameliorating effect of vitamin D supplementation on AD symptoms.14
This study aimed to investigate 12-month and lifetime prevalences of AD in the Finnish adult population based on data of the Health 2000 survey, which has not been previously published. In addition, we aimed to analyze living environment factors, socioeconomic factors, lifestyle-related factors, and serum vitamin D levels for their associations with AD.
Materials and methods
Study population
This cross-sectional population-based study used data collected through the Health 2000 Survey, which was a national health examination survey carried out between autumn 2000 and spring 2001 to obtain comprehensive data on general public health issues and the functional and working capacities of the population.15, 16 The survey was carried out in several phases and included questionnaires, extensive in-home interviews, laboratory and functional capacity testing, and a comprehensive clinical examination. A two-stage stratified cluster sampling procedure was used to ensure the representativeness of the study sample, which consisted of 8,028 subjects (54.7% women), with a mean age of 54 years (range 30–99). Written informed consent was obtained from all participants. The local ethics committees approved the study.
Data collection
Medical history and socioeconomic data on income level, number of siblings, childhood in a farming environment, current living environment (rural, semi-urban, urban; official classification by Statistics Finland), and the highest education level of parents were collected as part of an extensive in-home interview. Detailed data on exercise habits, alcohol use, and smoking were also obtained (Table 1). Exercise level was defined as “none,” “on average, less than 3 hours weekly,” “on average, ≥3 hours weekly,” or “doing competitive sports.” Alcohol use was assessed by both the absolute amount of ethanol (grams per week converted from detailed questionnaires) and the frequency of use. Smoking history was defined as “never,” “ex-smoker,” or “daily or occasionally” (Table 1). Serum cotinine, a widely used biomarker of cigarette smoke exposure, was also measured to assess a possible dose-dependent effect of smoking.
| n (all) | nAD (%) | Before MLRM | After MLRM | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Age, years | ||||||
| 30–39 | 1,211 | 187 (33.81) | 1 | 1 | ||
| 40–49 | 1,316 | 122 (22.06) | 0.56 | 0.48–0.71 | 0.58 | 0.45–0.76 |
| 50–59 | 1,206 | 129 (23.32) | 0.66 | 0.52–0.84 | 0.72 | 0.55–0.96 |
| 60–69 | 848 | 72 (13.01) | 0.51 | 0.38–0.68 | 0.58 | 0.41–0.82 |
| ≥70 | 900 | 43 (7.78) | 0.28 | 0.20–0.39 | 0.33 | 0.21–0.50 |
| Sex | ||||||
| Male | 2,536 | 260 (46.93) | 1 | 1 | ||
| Female | 2,946 | 294 (53.07) | 1.03 | 0.86–1.22 | 1.19 | 0.96–1.48 |
| Living environment | ||||||
| Rural | 3,336 | 352 (63.53) | 1 | 1 | ||
| Semi-urban | 798 | 65 (11.73) | 0.79 | 0.60–1.04 | 0.84 | 0.62–1.13 |
| Urban | 1,348 | 137 (24.73) | 1.01 | 0.82–1.25 | 1.07 | 0.85–1.36 |
| Education level | ||||||
| Low | 2,203 | 178 (32.36) | 1 | 1 | ||
| Intermediate | 1,753 | 210 (38.18) | 1.14 | 0.91–1.44 | 1.19 | 0.93–1.53 |
| High | 1,507 | 162 (29.45) | 0.96 | 0.75–1.23 | 0.99 | 0.75–1.32 |
| Childhood on a farm | ||||||
| Yes | 1,770 | 159 (29.33) | 0.94 | 0.77–1.15 | 1.01 | 0.80–1.27 |
| No | 3,530 | 383 (70.66) | 1 | 1 | ||
| Highest parental education | ||||||
| Low | 4,555 | 442 (85.99) | 1 | 1 | ||
| Vocational education | 325 | 37 (7.20) | 1.00 | 0.70–1.44 | 0.98 | 0.66–1.44 |
| University degree | 217 | 35 (6.80) | 1.53 | 1.05–2.23 | 1.58 | 1.05–2.38 |
| Number of siblings | ||||||
| 0 | 273 | 31 (5.66) | 1 | 1 | ||
| 1 | 893 | 99 (18.06) | 0.92 | 0.60–1.41 | 0.78 | 0.51–1.25 |
| 2 | 1,030 | 116 (21.17) | 0.97 | 0.64–1.48 | 0.87 | 0.56–1.34 |
| 3 | 866 | 90 (16.42) | 0.94 | 0.61–1.45 | 0.78 | 0.49–1.22 |
| 4 | 602 | 53 (9.67) | 0.83 | 0.52–1.32 | 0.69 | 0.42–1.14 |
| ≥5 | 1,782 | 159 (29.01) | 0.94 | 0.63–1.43 | 0.81 | 0.52–1.25 |
| Smoking | ||||||
| Never | 2,804 | 237 (43.01) | 1 | 1 | ||
| Ex-smoker | 1,221 | 142 (25.77) | 1.52 | 1.21–1.90 | 1.45 | 1.13–1.85 |
| Daily/occasionally | 1,436 | 172 (31.21) | 1.27 | 1.02–1.58 | 1.15 | 0.93–1.53 |
| Alcohol consumption, g/week | ||||||
| 0 | 1,713 | 140 (25.58) | 1 | 1 | ||
| 0.1–25 | 1,148 | 116 (21.17) | 1.09 | 0.84–1.42 | 1.05 | 0.79–1.40 |
| 25.1–100 | 1,321 | 148 (27.00) | 1.18 | 0.91–1.52 | 1.17 | 0.89–1.55 |
| >100 | 1,173 | 144 (26.28) | 1.31 | 1.00–1.72 | 1.26 | 0.93–1.70 |
| mean/median | 72/19 | |||||
| Body mass index, kg/m2 | ||||||
| <20 | 176 | 26 (4.70) | 1 | 1 | ||
| 20–25 | 1,811 | 193 (34.90) | 0.72 | 0.46–1.12 | 0.73 | 0.45–1.19 |
| 25–30 | 2,250 | 220 (39.78) | 0.72 | 0.46–1.12 | 0.69 | 0.42–1.14 |
| ≥30 | 1,236 | 114 (20.61) | 0.71 | 0.45–1.13 | 0.68 | 0.41–1.14 |
| Exercise at leisure | ||||||
| Never | 1,494 | 161 (29.49) | 1 | 1 | ||
| <3 hours a week | 2,940 | 295 (54.03) | 0.90 | 0.73–1.10 | 0.89 | 0.71–1.11 |
| ≥3 hours a week | 845 | 80 (14.65) | 0.77 | 0.58–1.99 | 0.80 | 0.59–1.09 |
| Competitive sports | 74 | 10 (1.83) | 0.98 | 0.49–1.99 | 1.00 | 0.44–2.15 |
| Vitamin D quintile, mmol/l | ||||||
| 0–30 | 1,039 | 106 (19.63) | 1 | 1 | ||
| 31–38 | 1,041 | 96 (17.78) | 0.86 | 0.65–1.16 | 0.85 | 0.62–1.17 |
| 39–46 | 1,062 | 135 (0.25) | 1.28 | 0.97–1.67 | 1.28 | 0.96–1.72 |
| 47–57 | 1,078 | 104 (19.26) | 0.96 | 0.72–1.27 | 0.91 | 0.67–1.25 |
| ≥58 | 1,089 | 99 (18.33) | 0.94 | 0.70–1.25 | 0.89 | 0.65–1.23 |
| Mean/median | 44.9/43 | |||||
| Asthma | ||||||
| No | 4,994 | 475 (86.05) | 1 | |||
| Yes | 475 | 77 (13.95) | 2.16 | 1.62–2.87 | ||
- Results with bold font represent statistically significant values (P < 0.05); MLRM, multivariate logistic regression model.
Participants were defined as having a positive history of AD if they had signs of AD upon clinical examination (criteria of Williams et al.),17 a confirmed diagnosis of AD in medical records, or a positive response for the validated diagnostic question “Have you ever had itchy dermatitis called atopic eczema, atopic dermatitis, or flexural dermatitis?”.18, 19 We did not analyze the variable of memory recall vs. EHR data because of the heterogeneity of the EHR data and because most of the patients had been clinically examined or a positive response to the validated diagnostic question. The symptomatic age was defined further by the question “When did you have this type of eczema?” (at the age of <2 years, 2-7 years, 7-18 years, or ≥ 18 years, within the past 12 months, or currently). Because of the fluctuating nature of AD, symptomatic disease in the past 12 months was considered “active AD.” “Adulthood” was defined as the age of ≥18 years. Data on lifetime, adulthood, and 12-month prevalences of AD were available for 76%, 72%, and 68% of the subjects, respectively.
Serum vitamin D (25-hydroxycholecalciferol) levels were determined by radioimmunoassay (DiaSorin, Stillwater, Minnesota, USA) from frozen fasting serum samples taken during months with lower exposure to sunlight (from September to March). Because of the high amount of participants and the timing of the study for a long period, we could not synchronize the serum vitamin D investigations for specific months. A quintile classification was used for the values (Table 1). All medical professionals received training for the study protocol. Physicians performed clinical examinations, and nurses interviewed the participants. Of the study sample, 6,986 subjects (87%) were interviewed at home or an institution, and 6,354 subjects (79%) participated in a comprehensive physical examination. In total, 416 subjects (5%) in poor health were examined at home or an institution without a skin examination.
Statistical analyses
For the analyses on associations of AD with environmental, socioeconomic, and lifestyle-related variables, odds ratios (OR) for AD with 95% confidence intervals (CI) were calculated. First, ORs were calculated adjusting for age and sex with “active AD” as the outcome variable, then a multivariate logistic regression model with all variables and “asthma” as confounding factors was used for the final adjusted ORs. Wald’s test was used to test the statistical significance of the associations. Analysis weights correcting for the oversampling and general population structure were provided with the data by the National Institute for Health and Welfare. All analyses were conducted with IBM SPSS (version 24.0; IBM Corp, Armonk, NY, USA).
Results
Prevalence of atopic dermatitis
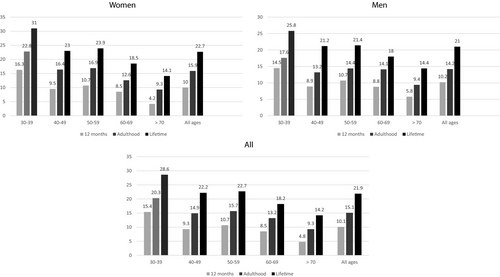
The 12-month prevalence of AD was 10.1%, and it decreased with age from 15.4% (subjects of 30-39 years) to 4.8% (subjects ≥70 years). The prevalence of AD in adulthood was 15.1%. The lifetime prevalence of AD was 21.9%, with the highest lifetime prevalence (28.6%) seen in the youngest age group and the lowest (14.2%) in subjects ≥70 years of age. The lifetime prevalence was highest (31.0%) in 30- to 39-year-old women. In subjects ≥70 years of age, active AD was more common in men, with a 12-month prevalence of 5.8% (vs. 4.2% in women), but the difference was not statistically significant (P = 0.28; Figure 1).
Age of onset and persistence of symptoms
Of all the subjects with a history of AD, 41.1% reported onset before the age of 7, 16.7% between the ages of 7 and 18, and 42.2% in adulthood. Of the subjects reporting the onset of AD symptoms before the age of 7, 37.4% continued having symptoms into adult life, and 25.6% had active disease. Subjects reporting the onset of AD before the age of 7 were significantly more often women (P < 0.001), while men reported adult-onset AD more frequently (P = 0.013). Subjects reporting onset before the age of 7 were also significantly younger than subjects reporting later onset (mean age 48 vs. 56 years, P < 0.001).
Demographics, socioeconomic status, and living environment
Subjects with university-educated parents were more likely to have active AD (OR 1.58, 95% CI 1.05-2.38; Table 1), but there was no association of AD with the education level or the level of income (data not shown) of the subjects. An inverse relationship between age and AD was found in the whole study population (OR per year 0.98, 95% CI 0.97-0.99). Sex was not a determinant of AD when assessed in the entire cohort, but in a subgroup analysis of subjects <50 years of age, female sex was associated with active AD (OR 1.33, 95% CI 1.01-1.75). AD was not associated with the childhood or adulthood living environment or the number of siblings (Table 1).
Vitamin D
Regarding serum vitamin D levels, there was a borderline significance of the variation in AD risk (p-value for heterogeneity, 0.057). This was mainly because of the higher prevalence in the third quintile than in the second, fourth, and fifth ones. There was no dose–response effect of serum vitamin D levels (OR per unit 0.99, 95% CI 0.97-1.00).
Lifestyle determinants
Being an ex-smoker was associated with an increased risk of AD (OR 1.45, 95% CI 1.13-1.85; Table 1). Daily smoking was not significantly associated with AD when assessed in the entire cohort, but there was an association with AD in the subgroup analysis of subjects <50 years of age (OR 1.41, 95% CI 1.05-1.93). Serum cotinine did not associate with AD, and there was no dose–response effect among smokers either. Alcohol consumption, body mass index, and leisure-time physical exercise showed no significant associations with the prevalence of AD.
Asthma and allergic rhinoconjunctivitis
Lifetime prevalence was 9.1% for asthma and 35.2% for allergic rhinoconjunctivitis. Both asthma and allergic rhinoconjunctivitis were associated with an increased prevalence of active AD, with respective ORs of 2.16 (95% CI 1.62-2.87) and 2.72 (95% CI 2.24-3.30). Of the subjects with a history of AD, 14% had asthma and 51% allergic rhinoconjunctivitis.
Adult-onset atopic dermatitis
In the subgroup analysis of subjects reporting onset of symptoms in adult life, age and parental education level showed no association with active AD. There was also no association with sex or any other socioeconomic or environmental factor. Of lifestyle factors, only a history of smoking (but not current smoking) showed an association with active AD in this group, with an OR of 1.49 (95% CI 1.08-2.05). There was no association with asthma, but active adult-onset AD was associated with allergic rhinoconjunctivitis, with an OR of 2.03 (95% CI 1.57-2.63).
Discussion
This study presents the first population-based data on AD in the Finnish general adult population, including the elderly. Both the 12-month prevalence of 10.1% and the lifetime prevalence of 21.9% are high in comparison to many reports from other industrialized countries. A study from Italy showed a lifetime prevalence of 8.1% in 20- to 44-year-old subjects, while in the German population-based ESTHER study, the lifetime prevalence was only 4.3% among subjects aged 50-75 years.20, 21 Our results are nevertheless in concordance with earlier self-reported lifetime prevalence numbers from Finland (28.1% among subjects 25-54 years of age in the North Karelia district) and Denmark (34.1% among young Danish adults of 28-30 years).4, 22 The same applies to previously reported 12-month prevalence rates of 6.9–11.6% among American, Japanese, and Swedish adults.1, 7, 23
The female sex has been shown to be a risk factor for AD in younger adults in many populations, and our data support this view.24 However, in our study, the opposite trend was seen in subjects ≥70 years, among whom the 12-month prevalence was somewhat but not significantly higher in men than in women (5.8% vs. 4.2%). Similar results have been previously reported in smaller studies and by Muto et al. among Japanese medical center health check-up visitors.25, 26 In the elderly, however, asteatotic eczema and, especially among men, seborrheic dermatitis and infrequent skin care can be confounding factors in self-reported data; more studies are needed on this matter.
In a meta-analysis by Kim et al., only 20% of children with AD were symptomatic after a follow-up of 8 years and 5% after 20 years.27 The mean follow-up time of the studies included in the analysis, however, was only 3.9 years (range 0.25-23.0), which is short for conclusions regarding the long-term course of AD. In a Swedish study, 40–60% of the patients were still symptomatic after the 24-year follow-up.28 In a Finnish study, 55–91% (depending on the severity of AD at diagnosis) of adolescent patients from a tertiary referral center setting still had symptomatic disease after the follow-up of 10–18 years.29 In a Danish study, half of the children diagnosed with AD during their school years continued to be symptomatic at the age of 28-30.4 In this study, 37.4% of subjects reporting the onset of symptoms ≤ 7 years old were still symptomatic in adulthood, and 25.6% had active disease.
In former studies, the proportion of patients with adult-onset symptoms has typically been less than 20%.5 In this study, 42.2% of subjects reported the onset of symptoms in adulthood, and this was significantly more common in men. Women reported childhood onset of AD more often, which is in line with earlier reports showing female sex as a risk factor for more persistent AD.30 Asthma did not seem to associate with adult-onset AD, and the association with allergic rhinoconjunctivitis was weaker compared to subjects with the onset of symptoms in childhood. This is in concordance with studies on other populations, underlining the significance of AD in atopic march and the development of atopic comorbidities.31 Since juvenile AD can clear up, temporarily or permanently, early childhood cases may have been missed in this study, especially in older age groups, because of recall bias leading to false positives in adult-onset cases. Subjects reporting childhood onset AD were significantly younger than the ones reporting onset later in life, which may reflect this bias. Because of this, the lifetime prevalence can also be underestimated. Our results are, however, parallel to those by Pesce et al. from Italy and Silverberg et al. from the United States, where about 40% of study subjects reported the onset of symptoms in adult life.19, 31
In Finland, the frequency of filaggrin loss-of-function (null) mutations in the population is notably low (5.6%), making environmental factors a feasible explanation for the high prevalence of AD.32 Higher parental education level was associated with a higher prevalence of active AD. This presumably represents an actual risk increase and not only bias on the information level, possibly connected to the social class and hygienic status of the family during childhood. This is further supported by the finding that the adult-onset form showed no association with parental education. Additionally, we could not demonstrate any increased risk linked to the income or education level of subjects themselves. There was no association between childhood or adulthood living environment and AD. Our results differ from those of the German, Italian, and US adult populations1, 20, 21 but are in concordance with previous results from Finland and neighboring Sweden.10, 33 Many earlier studies have been conducted in children and adolescents, and it is possible that the effects of gene–environment interactions caused by exposures in early life are not life-long.33
Active and passive smoking seem to be associated with increased AD prevalence.12 In our Finnish study population, being an ex-smoker associated with active AD and the association remained when only patients reporting the onset of AD symptoms in adulthood were analyzed. A recent cessation of smoking (<12 months) was even more clearly associated with active AD, with an OR of 3.03 (95% CI 1.85-4.98), which may imply that the mediator of this effect is increased stress caused by the recent quitting of smoking. AD flares have been linked to stress mediated by the hypothalamic–pituitary–adrenal (HPA) axis, and this could be one possible explanation.34 In earlier studies and a recent meta-analysis, daily smoking was associated with AD, but in this study, this was limited to subjects aged <50 years.12, 30 The association could be partly explained by the psychiatric comorbidities of AD that increase the tendency to smoke as AD has been associated with significantly increased anxiety and depression.11, 35 There was no association between serum cotinine concentration and AD, which makes a direct dose–response effect of smoking on the inflammation in AD unlikely.
Body mass index did not associate with AD in our Finnish data, which further supports results from a recent meta-analysis by Zhang et al. where obesity was associated with AD only in North America but not in Europe.36 We also did not find any association between exercising habits and AD. Low vitamin D levels have been shown to associate with an increased likelihood of AD in adult populations in a few previous studies, but the results have been inconsistent.37 A recent Spanish study from Esenboga et al. found that vitamin D levels correlate inversely with AD severity in children.37 Imoto et al. found the patients with sufficient vitamin D levels had lower SCORAD values, and vitamin D supplementation could be used to decrease the severity of AD as an adjuvant to topical therapies.38, 39 In our adult study population, we did not observe similar effects and considering the lack of a dose–response relationship, the borderline significance observed in this study was likely because of chance alone.
The Health 2000 survey provided a representative sample of the Finnish general adult population ≥30 years of age. This allowed us to study possible associations of different variables in adulthood AD with minimal sampling bias. The criteria used to define adult AD were based on self-reported data, confirmed diagnoses from medical records, and a clinical assessment of symptoms according to an established protocol. This makes results less prone to reporting bias. However, examination of the skin was not included in the health examinations conducted at home for those in poor overall health, and the severity of AD was not assessed. Data on environmental and lifestyle factors were mostly based on questionnaires, which may lead to reporting bias in habits considered unfavorable (i.e., smoking, excessive alcohol consumption, absence of exercise). Serum cotinine provided more objective data on smoking. Regarding vitamin D, we did not have data on supplements or sunny holidays during the study period.
Epidemiological data are essential in estimating the burden and costs caused by a disease. Our data show that the number of adult patients with atopic dermatitis has grown, and prevalence numbers of AD in Finnish adults are among the highest reported. Together with the aging of the society, the burden of AD is not limited to childhood.




