Emerging trends of injectable hydrogels for vital pulp therapy: A comprehensive review
[Corrections added on 18 July 2025, after first online publication: few texts have been modified in this version.]
Rafiqul Islam and Hany Mohamed Aly Ahmed contributed equally to this study.
Abstract
Background
Injectable hydrogels are widely used in biomedicine and tissue engineering. Recently, they have been introduced as promising capping materials for vital pulp therapy (VPT) owing to their biocompatibility, in situ adaptability, minimal immunogenicity, and ability to modify the cellular activities of dental pulp exposed to caries or accidental injuries. Although injectable hydrogel-based biomaterials facilitate pulp healing and reparative dentine formation, their clinical utility has not been fully explored.
Objectives
This review highlights the gaps in current research, including the lack of studies on the long-term in vivo effects of injectable hydrogels and comprehensive interactions between injectable hydrogel and the dentine–pulp complex, which impede clinical translation, especially for VPT.
Methods
A comprehensive literature search was conducted in PubMed and Scopus in April 2024 using relevant keywords related to injectable hydrogels and VPT. Eligible articles published between 2014 and 2024 included laboratory, animal and clinical studies, addressing the biological function, design, or clinical applicability of injectable hydrogels for VPT.
Results
This review covers various biomaterial-based injectable hydrogels developed using hyaluronic acid, gelatine methacryloyl, chitosan, collagen, decellularized extracellular matrix, self-assembling peptides, and nanoparticles for VPT, highlighting possibilities for future clinical translation and innovation. Laboratory studies on injectable hydrogels provide promising results, including enhanced pulp healing, reduced inflammation, homogeneous reparative dentine formation, and also key innovations to enhance the functionality, adaptability, and characteristics of novel injectable hydrogels developed for VPT.
Conclusion
Hydrogels functionalized with advanced biomaterials and bioengineering approaches can overcome the existing shortcomings, enabling their smooth transition into clinical practice. Nevertheless, further research is required to elucidate their long-term effects and optimal application methods.
INTRODUCTION
Almost 2 billion adults and 514 million children suffer from dental caries each year (WHO, 2022). Dental caries is a multifactorial disease mainly caused by the release of acidic by-products from the bacterial breakdown of dietary carbohydrates initiated by the localized demineralization of dental hard tissues (Selwitz et al., 2007). If demineralization advances, then bacterial components readily penetrate into the dentine–pulp complex, leading to inflammation and infection (Bjørndal et al., 2018; 2019). These clinical conditions are treated with vital pulp therapy (VPT) if pulpal inflammation is limited to the pulp chamber or root canal therapy if pulpal inflammation advances apically to the root canals (Ahmed et al., 2023; Moussa & Aparicio, 2019).
VPT has been widely adopted as one of the most conservative endodontic treatments (AAE, 2021; ESE, 2019), which is minimally invasive in nature, maintaining the vitality and integrity of dental pulp injured by caries, trauma, and defective restorative procedures (AAE, 2021; ESE, 2019). VPT of permanent teeth includes indirect pulp capping, direct pulp capping (DPC), partial pulpotomy, and full pulpotomy, aiming to facilitate the formation of a reparative dentine bridge (RDB) (AAE, 2021; ESE, 2019), which is mainly regulated by the differentiation of odontoblastic cells (Ishimatsu et al., 2009).
Several dental biomaterials have been developed for the VPT, including calcium hydroxide (CH), calcium silicate-based materials such as mineral trioxide aggregate (MTA), Biodentine®, and other premixed bioceramic materials. Despite having advantageous properties, these biomaterials require a long setting time, induce tooth discolouration, and are difficult to handle for clinicians (Islam et al., 2021, 2024; Ozturk et al., 2023; Pelepenko et al., 2024; Schwendicke et al., 2015; Toida et al., 2022). These VPT materials also fail to reach optimized consistency, and importantly, they fail to induce biological signalling, which is necessary for progenitor cellular differentiation (Islam et al., 2023; Moussa & Aparicio, 2019). Moreover, these materials significantly increase the intracellular level of reactive oxygen species (ROS), which adversely affects biocompatibility (Bossu et al., 2021). Therefore, alternative biomaterials, such as nanocomposite scaffolds, epigenetic modulating agents, and injectable hydrogels, have been developed to induce dentine–pulp complex regeneration for VPT (Atila & Kumaravel, 2023; Duncan et al., 2023; Han, Dal-Fabbro, et al., 2024; Kearney et al., 2018; Quigley et al., 2024).
Injectable hydrogels have emerged as promising alternatives owing to their advantageous properties such as in situ adaptability, formability, targeted drug delivery, and the ability to uniformly incorporate therapeutic molecules and cells (Gao et al., 2021; Ghandforoushan et al., 2023; Liu et al., 2019). Moreover, injectable hydrogels in the sol state are characterized by low viscosity, allowing their deformation and conformation to the required shape of the confined space of the tooth cavity (Hamidi et al., 2008; Ladet et al., 2008). Injectable hydrogels have been fabricated from various natural polysaccharides and proteins, especially hyaluronic acid (HA), chitosan, gelatine, alginate, and collagen, which exhibit superior biocompatibility compared with synthetic polymers (Chen et al., 2024; Liu et al., 2020). Additionally, injectable biomaterials developed for VPT demonstrated favourable physical characteristics, biological properties, and antimicrobial effects (Guo et al., 2024; Zhang, Bi, et al., 2023). Recent studies have shown that several injectable hydrogels contribute to antimicrobial activity against Streptococcus mutans, Lactobacillus casei, and Actinomyces naeslundii (Qiu et al., 2023; Xie et al., 2024). Additionally, they contribute to the physiological mechanisms of pulpal wound healing by initiating haemostasis, further promoting inflammation, proliferation, and remodelling (Fang et al., 2023; Siddiqui et al., 2021; Sun et al., 2018; Yi et al., 2024). Although injectable hydrogel-based biomaterials show favourable properties for VPT, their clinical applicability has not been assessed extensively. Additionally, to the best of knowledge, the information on the characteristics of injectable hydrogels for VPT purposes is limited.
Therefore, this review aims to highlight the significant gaps in current research and knowledge of interactions between injectable hydrogels and the dentine–pulp complex. Additionally, this review covers the characteristics and potential applications of various types of injectable hydrogels developed for VPT. It also discusses challenges and future strategies for the clinical translation of injectable hydrogels in the endodontic field.
LITERATURE SEARCH METHODOLOGY
An electronic search was conducted in the PubMed and Scopus databases by two authors (MRRI and RI) in April 2024. The search included a combination of keywords and MeSH terms relevant to the topic. Search terms included ‘injectable hydrogel’, ‘vital pulp therapy’, ‘reparative dentine bridge formation’, ‘pulpal inflammation’, ‘odontogenic differentiation’, ‘angiogenesis in pulp’, ‘biomaterials in endodontics’, and ‘nanoparticles in endodontics’. Boolean operators and truncation symbols were used to refine the search. No restriction on publication year was initially applied. However, to ensure the standardization, most of the included studies were published between 2014 and 2024, and only articles in English were considered. Titles and abstracts were first screened for relevance, and studies were included if they addressed the biological function, design, or clinical applicability of injectable hydrogels for VPT. Reviews and articles of laboratory, animal and clinical studies were all eligible for inclusion. In addition, a manual search was conducted in several journals, including Bioactive Materials, Advanced Healthcare Materials, Acta Biomaterialia, International Endodontic Journal, and Journal of Endodontics, to identify studies that may have been missed during the database search.
INJECTABLE HYDROGELS: BACKGROUND AND CLASSIFICATION
Since their inception and initial development, covalently crosslinked hydrogels (Danno, 1958; Van Bemmelen, 1894) have been extensively studied by researchers owing to their favourable physical, chemical, and biological properties, along with their suitability for diverse biomedical applications (Li, Lv, et al., 2019; Li, Yang, et al., 2021; Li & Mooney, 2016; Tang et al., 2019; Yeom et al., 2020). Amongst them, injectable hydrogels have attracted attention for regenerative medicine owing to their ability to transform from a flowable sol state into a non-flowable gel state in response to native body conditions at the injection site, making them highly convenient for minimally invasive procedures (Basu et al., 2020; Chen et al., 2019; Rizzo & Kehr, 2021; Yu & Ding, 2008).
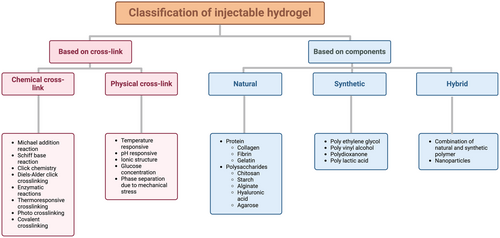
Depending on their cross-linking abilities and the components, injectable hydrogels can be divided into several categories. First, injectable hydrogels can be classified into chemically crosslinked and physically crosslinked hydrogels depending on their gelation mechanism (Lee, 2018; Nguyen et al., 2015). Chemical crosslinking methods include Michael-addition reactions, Schiff-base reactions, click chemistry, Diels–Alder click crosslinking, enzymatic reactions, thermo-responsive crosslinking, ionic crosslinking, photo crosslinking, and covalent crosslinking (Nguyen et al., 2015). In contrast, physical crosslinking occurs in response to changes in physicochemical parameters, such as temperature, pH, ionic strength, glucose concentration, and mechanical stress, which trigger polymer conformational changes and phase separation, leading to the formation of networks through polymer chain aggregation (Figure 1) (Jagur-Grodzinski, 2010; Ulijn et al., 2007; Ullah et al., 2015). Secondly, injectable hydrogels can be classified according to their various polymer components, such as natural, synthetic, and hybrid polymers (Gyles et al., 2017). Natural polymers are frequently used to fabricate injectable hydrogel scaffolds for tissue engineering and biomedical applications owing to their biodegradability, safety, and low cytotoxicity (Almawash et al., 2022; Gyles et al., 2017). Moreover, natural polymers derived from native-like proteins (such as collagen, fibrin, and gelatine) and polysaccharides (such as chitosan, starch, alginate, HA, and agarose) are similar to native tissues in bioactivity and function (Kumar et al., 2023). In contrast, synthetic polymers, such as polyethylene glycol, polyvinyl alcohol, polydioxanone, and polylactic acid, offer precise regulation of cell interactions (Bolívar-Monsalve et al., 2021; Van Vlierberghe et al., 2011). Hybrid hydrogels are novel hydrogel systems with complex structures that integrate chemically and functionally diverse molecules or nanoparticles (NPs) from synthetic and natural polymers (Figure 1) (Hashimoto et al., 2018; Mahinroosta et al., 2018).
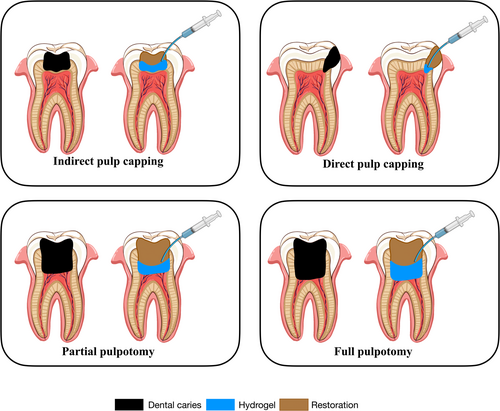
Owing to their biocompatibility, biodegradability, antimicrobial activity, and scaffold porosity, injectable hydrogels facilitate favourable healing of localized inflammation of pulp tissues, achieving successful dentine–pulp complex regeneration for VPT (Medina-Fernandez & Celiz, 2019). In addition, injectable hydrogels are often loaded with cells or bioactive molecules and morphologically modified to enhance pulp tissue regeneration and restore pulp functions (Ahmadian et al., 2019; Atila, Chen, et al., 2022; Atila, Hasirci, et al., 2022; Siddiqui et al., 2022). In the preclinical setting, these hydrogels can be injected through a needle for DPC and partial or full pulpotomy, fitting into irregular cavities to provide microenvironments similar to the natural extracellular matrix (ECM) (Figure 2). Recent randomized clinical trials have shown that injectable hydrogels applied for VPT exhibit no signs of clinical failure, such as spontaneous pain, swelling, or the development of abscesses (Holiel, Mahmoud, & Abdel-Fattah, 2021; Holiel, Mahmoud, Abdel-Fattah, & Kawana, 2021; Holiel et al., 2023). Compared with traditional bulk hydrogels, injectable hydrogels improve the conditions for oxygen and nutrient transfer, enhancing cell viability and functions when compared with traditional bulk hydrogels (Daly et al., 2020). In addition, they protect cells, improve cell survival rates, reduce cell damage, decelerate cell dislocation, and prevent rapid cell death (Khurshid et al., 2022). These properties are essential for the success of VPT.
The following section discusses the characteristics of injectable hydrogels based on HA, gelatine methacryloyl (GelMA), chitosan, collagen, and treated dentine matrix (TDM), which are suitable for VPT due to their biocompatibility, bioactivity, and in situ bioadaptability. Table 1 lists all the injectable hydrogels employed for VPT to date, along with their main effects.
| Ref. | Hydrogel | Additives (biomolecules, fibrin, bioceramics, bioactive glass, stem cells, peptides etc.) | Additives (nanoparticles) | Crosslinking method | Cell type | Evaluation methods | Antimicrobial efficacy | Results |
|---|---|---|---|---|---|---|---|---|
| Silva et al. (2018) | HA |
|
CNC | Chemical crosslink |
hDPSC HUVEC |
|
– |
|
| Almeida et al. (2018) | HA |
|
– | Photo crosslink | hDPSC |
|
– |
|
| Atila, Chen, et al. (2022) | HA |
|
Chitosan | Chemical crosslink |
hDPSC HUVEC |
|
– |
|
| Wang, Fu, et al. (2021) | GelMA |
|
– | In situ ultra violate crosslink | mDPC |
|
– |
|
| Sadeghian et al. (2023) | GelMA |
|
– | Chemical crosslink under LED light | SHED |
|
– |
|
| Atila et al. (2023) | GelMA combined with PecTH |
|
– | Ultra violate crosslink | hDPSC |
|
– |
|
| Qiu et al. (2023) | GelMA |
|
– | Ultra violate crosslink |
rDPSC HUVEC |
|
|
|
| Xie et al. (2024) | GelMA |
|
– | Ultra violate crosslink | hDPSC |
|
|
|
| Zhang, Huang, et al. (2024) | GelMA | – | C-NZ | Ultra violate crosslink | hDPSC |
|
– |
|
| Zhu, Chatzistavrou, et al. (2019) | Chitosan |
|
Ag-doped BG | Chemical crosslink | hDPSC |
|
– |
|
| Osmond and Krebs (2021) | Chitosan |
|
CaP | Chemical crosslink | hDPSC |
|
– |
|
| Hoveizi et al. (2023) | Chitosan |
|
TiO2 | Chemical crosslink | EnSC |
|
– |
|
| Xu et al. (2023) | Collagen-I |
|
– | Chemical crosslink | hDPSC |
|
|
|
| Li et al. (2020) | hDDPM | – | – | Physical crosslink | hDPSC |
|
– |
|
| Yi et al. (2024) | DPM | Pepsin | – | Chemical crosslink | hDPSC |
|
– |
|
| Holiel, Mahmoud, and Abdel-Fattah (2021)a | TDM combined with SA | – | – | Chemical crosslink | – |
|
– |
|
| Holiel, Mahmoud, Abdel-Fattah, and Kawana (2021)a | TDM combined with SA | – | – | Chemical crosslink | – |
|
– |
|
| Holiel et al. (2023)a | TDM combined with SA | – | – | Chemical crosslink | – |
|
– |
|
| Wen et al. (2023) | PAA combined with TDM |
|
ACP | Chemical crosslink | hDPSC |
|
– |
|
| Xia et al. (2020) | SAP |
|
– | Chemical crosslink | HUVEC |
|
– |
|
| Zhao et al. (2024) | Fmoc-triphenylalanine hydrogel |
|
CNP | Chemical crosslink | hDPSC |
|
|
|
| Li, Tian, et al. (2024) | DFSCs-derived small EVs combined with SA |
|
– | Chemical crosslink | rDPSC |
|
– |
|
- Abbreviations: ACP, amorphous calcium phosphatase; AFM, atomic force microscopy; Ag, silver; ARS, alizarin red S; BG, bioactive glass; CaP, calcium phosphate; CBCT, cone beamed computed tomography; CMC, carboxymethyl chitosan; CNC, cellulose nanocrystals; CNP, cerium oxide nanoparticles; C-NZ, carbon dot nanozymes; Cryo-EM, cryogenic electron microscopy; CS, chondroitin sulfate; DDM, demineralized dentine matrix; DECM, decellularized extracellular matrix; DFSC, dental follicle stem cell; DMP-1, dentine matrix protein-1; DPC, direct pulp capping; DPSC, dental pulp stem cell; DPM, decellularized pulp matrix; DSC, differential scanning calorimetry; ELISA, enzyme-linked immunosorbent assay; EnSC, human endometrial stem cell; EV, extracellular vesicles; FTIR, Fourier transform infrared; GelMA, gelatin methacryloyl; GF, growth factor; HA, hyaluronic acid; hDDPM, human decellularized dental pulp matrix; hDPSC, human dental pulp stem cell; 1H NMR, proton nuclear magnetic resonance; HRTEM, high resolution transmission electron microscopy; HUVEC, human umbilical vein endothelial cell; MDA, malondialdehyde; mDPC, mouse dental papilla cell; MEL, melatonin; MTA, mineral trioxide aggregate; Micro-CT, micro computed tomography; NgR1, notoginsenoside R1; PAA, polyacrylic acid; PecTH, thiolated pectin; p-DPM, decellularized matrix hydrogel derived from porcine dental pulp digested by pepsin; PL, platelet lysate; PMMA, polymethylmethacrylate; PPAR𝛾, peroxisome proliferator-activated receptor gamma; rDPSC, rat dental pulp stem cell; RGD, arginylglycylaspartic acid; RhB, rhodamine B; ROS, reactive oxidative stress; RONS, reactive oxygen and nitrogen species; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; SA, sodium alginate; SAP, self-assembling peptide; SEM, scanning electron microscopy; SEM–EDX, SEM with energy dispersive X-ray; SHED, stem cells from human exfoliated deciduous teeth; SOD, superoxide dismutase; Td, Tideglusib®; TDM, treated dentine matrix; TEM, transmission electron microscopy; TiO2, titanium oxide; VEGF, vascular endothelial growth factor; XPS, X-ray photoelectron spectroscopy; XRD, X-ray diffraction.
- a Clinical studies on humans.
HA-based injectable hydrogels
HA has gained significant attention owing to its favourable physical and chemical properties (Singh et al., 2023). Recent studies have focused extensively on developing HA-based materials for bone regeneration, tissue engineering, and cancer therapy (Della Sala et al., 2022; Saravanakumar et al., 2022). HA-based injectable hydrogels developed for biomedical applications can be sub-classified into two main categories depending on their injection mechanism: in situ-forming injectable hydrogels and shear-thinning injectable hydrogels (Mashaqbeh et al., 2024). HA-based injectable hydrogels are distinguished by their adjustable rheological and mechanical characteristics, biocompatibility, biodegradability, and efficient mass transfer capabilities (Tiwari & Bahadur, 2019). Owing to their customizable composition, structure, and size, HA-based injectable hydrogels are promising candidates for biomedical applications, such as retinal cell therapy, bone tissue engineering, anti-tumour therapy, fertility restoration, wound healing, wound dressing, and drug delivery (Singh et al., 2023). Because they allow cell infiltration and induce odontoblastic differentiation, HA-based injectable hydrogels are considered ideal biomaterials for pulp repair and regeneration (Ahmadian et al., 2019). Additionally, HA-based injectable hydrogels emulate the ECM of dental pulp because they comprise glycosaminoglycans, which initiate the development of dental pulp (Goldberg & Hirata, 2017; Inuyama et al., 2010).
HA conjugated with platelet lysate
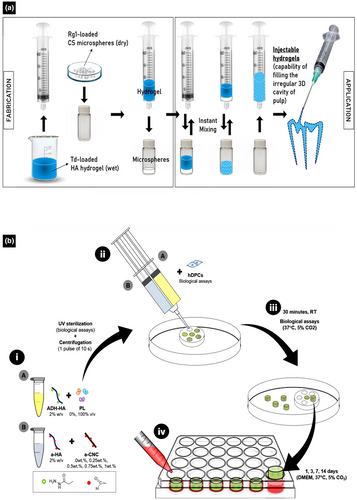
HA-based injectable hydrogels have been modified with platelet lysate (PL) to promote the repair and regeneration of dentine–pulp complexes (Almeida et al., 2018; Astudillo-Ortiz et al., 2021). PL acts as a composite of growth factors, such as fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and insulin-like growth factor (IGF), in addition to cytokines and clot-forming proteins, which are mainly obtained by rupturing the membranes of platelets during the freeze-drying of platelet concentrates. These components are widely known for promoting wound healing and their anti-bacterial activities (Crespo-Diaz et al., 2011; Fekete et al., 2012; Zimmermann et al., 2001). HA-based injectable hydrogels conjugated with PL (HA–PL hydrogel) have been reinforced with cellulose nanocrystals (CNCs) as nanofillers to promote cell migration and angiogenesis for pulp tissue regeneration (Figure 3b) (Silva et al., 2018). Reinforcement can be achieved by incorporating CNCs through hydrazone cross-linking between hydrazide/amine and aldehyde groups, which enhance the mechanical properties of the HA–PL hydrogels and enable the sustained release of chemotactic agents, promoting angiogenesis in an ex vivo model (Silva et al., 2018). In vitro, photo-crosslinked HA-PL hydrogels increased cellular metabolism and stimulated the mineralized matrix deposition by human dental pulp stem cells (hDPSCs), providing clear evidence of the potential of the proposed system for their repair and regeneration of damaged pulp/dentine tissues and regeneration (Almeida et al., 2018). In addition to the sustained release of growth factors, which play a crucial role in angiogenesis and RDB formation, HA–PL injectable hydrogels can fit into defects of any size, demonstrating their applicability for VPT (Astudillo-Ortiz et al., 2021).
HA combined with biomolecules
Recently, an innovative hydrogel was prepared from an anionic HA hydrogel incorporating cationic chitosan microspheres, Tideglusib® (Td), and Rg1 (Figure 3a) and its applicability for VPT was evaluated (Atila, Chen, et al., 2022). In vitro, this combination has the potential to improve strategies for vital pulp regeneration (Atila, Chen, et al., 2022). Td, which is popularly known as a potential candidate for treating Alzheimer's disease, is a glycogen synthase kinase-3 (GSK-3) inhibitor that enhances the regeneration of hard tissues (Alpan et al., 2020; Atila, Chen, et al., 2022; Lovestone et al., 2015). In contrast, Rg1, a biomolecule derived from the root of Panax ginseng, upregulates the phosphorylation of glucocorticoid receptor and activates p13K/Akt signalling pathways important for the vascularization of human umbilical vein endothelial cells (HUVECs) (Zheng et al., 2013). Moreover, immunocytofluorescence assays have shown that Rg1 significantly increases VEGF production, indicating the activation of angiogenesis (Zheng et al., 2013). This hydrogel was developed as an alternative, well-integrated HA-based injectable hydrogel for the controlled release of cationic chitosan microspheres along with Td and Rg1 to induce the regeneration of vital pulp (Atila, Chen, et al., 2022). Notably, the responses of cells to this injectable hydrogel are promising, with the sustained release of multifunctional chitosan microspheres, Td, and Rg1 promoting the odontogenic differentiation of dental pulp stem cells (DPSCs) and vascularization, demonstrating the biocompatibility and applicability of this injectable hydrogel as a prospective candidate for VPT (Atila, Chen, et al., 2022). However, there is still a lack of in vivo evidence to confirm the suitability of the HA-based hydrogels incorporating Td and Rg1 for VPT.
GelMA-based injectable hydrogels
In recent decades, gelatin biomaterials have been used to synthesize hydrogels and scaffolds for biomedical applications (Li, He, et al., 2023). Gelatine, a natural protein-based polymer approved by the FDA, is derived from the controlled hydrolysis or thermal denaturation of collagen (Kang et al., 1999; Tan et al., 2018; Zhao et al., 2016), which can be sourced from the bones, skin, tendons, ligaments, and connective tissues of terrestrial animals, predominantly mammals such as pigs, cattle, fish, and poultry (Ferraro et al., 2016; Liu et al., 2015; Nikoo et al., 2014; Wang et al., 2009; Young et al., 2020). GelMA is a gelatin-based biomaterial that has drawn significant attention since its development by Van Den Bulcke et al. (2000). GelMA-based hydrogels exhibiting diverse structures, including 3D scaffolds, injectable hydrogels, bioprinted scaffolds, and electrospun fibrous membranes, have been fabricated using advanced methods, such as light-induced crosslinking, extrusion 3D bioprinting, and electrospinning, for a wide range of biomedical applications (Pramanik et al., 2024; Wang et al., 2024; Yue et al., 2015). In dentistry, GelMA-based injectable hydrogels have been investigated for dental pulp regeneration and RDB formation (Huang et al., 2024). Recently, GelMA-based hydrogels incorporating DPSCs and HUVECs have been fabricated for pulp regeneration (Khayat et al., 2017).
GelMA loaded with notoginsenoside R1
In 2021, a novel injectable colloidal gel composed of GelMA loaded with notoginsenoside R1 was fabricated using an ultraviolet method to induce reparative dentine formation under biological conditions (Wang, Fu, et al., 2021). Notoginsenoside R1, a bioactive and biocompatible monomer isolated from Panax notoginseng, exhibits anti-inflammatory effects for the treatment of cardiovascular disease and osteoporosis (Fang et al., 2018; Wang, Zhang, et al., 2015; Yu et al., 2016; Zhang et al., 2018). Additionally, notoginsenoside R1 promotes osteocalcin expression and ECM mineralization, which are known as late and final dentinogenic differentiation markers of odontoblast-like cells and DPSCs, respectively (Liu et al., 2016). Novel injectable colloidal gels composed of GelMA-based hydrogels loaded with notoginsenoside R1 are promising candidates for VPT because they induce dentinogenic differentiation in vitro and RDB formation in vivo with minimal inflammation (Wang, Fu, et al., 2021). In vitro, these gels significantly enhance the odontogenic differentiation of mouse dental papilla cells by elevating the expressions of alkaline phosphatase and osteocalcin and promoting ECM mineralization. Similarly, they promote the formation of a favourable RDB in a Sprague Dawley rat model of injured pulp, illustrating the potential of the gels to promote dentinogenesis. Moreover, the novel notoginsenoside R1-loaded GelMA-based injectable hydrogel shows no significant shrinkage, indicating its ability to withstand mechanical distortion during VPT (Wang, Fu, et al., 2021).
GelMA loaded with bioactive glass
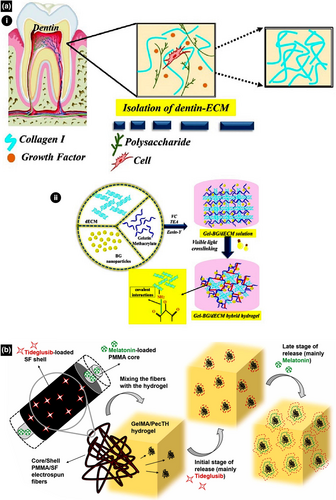
Despite the proven biocompatibility of GelMA, it neither induces the remineralization of dentine–pulp complexes nor mimics the complexity of ECMs, which are crucial for dentine–pulp complex regeneration. Therefore, Sadeghian et al. (2023) developed an injectable hydrogel from GelMA and bioactive glass (BG) to load novel dentine ECM for dentine–pulp complex regeneration. Evaluation of the physical and biological responses of human exfoliated deciduous teeth stem cells (SHED) to this hydrogel in vitro (Figure 4a) revealed that this novel injectable hydrogel has favourable mechanical properties, along with excellent biocompatibility, alkaline phosphatase (ALP) activity, and osteoconduction, indicating that its bioactivity is promising for VPT (Sadeghian et al., 2023).
GelMA combined with thiolated pectin
An injectable hydrogel has been developed by integrating GelMA and thiolated pectin (PecTH) with electrospun core/shell fibres of melatonin (MEL), polymethylmethacrylate (PMMA), and Td–silk fibroin for the proliferation and differentiation of odontoblastic DPSCs, thereby regenerating vital pulp (Figure 4b) (Atila et al., 2023). In this hydrogel, PMMA and silk fibroin fibres ensure the sustained and controlled release of bioactive agents Td and MEL (Atila et al., 2023). PecTH is known to undergo gelation and muco-adhesive polymerization owing to its rheological properties (Bernkop-Schnurch, 2005; Daas et al., 2000; Sharma & Ahuja, 2011). Furthermore, Td is an agent for treating degenerative neuro-disorders and promoting hard tissue regeneration, whilst melatonin is a tryptophan-derived neuro-hormone that promotes sleep, immune system activation, anti-inflammation, anti-oxidation, odontogenic differentiation, and DPSC proliferation (Alpan et al., 2020; Dubocovich et al., 2003; Konieczny et al., 2017; Liu et al., 2013; Liu, Fan, et al., 2017; Lovestone et al., 2015; Tolosa et al., 2014). PecTH improves the injectability and adhesion of the hydrogel to the dentine wall of the cavity (Atila et al., 2023). The sustained and controlled release of bioactive agents, such as Td and MEL, ensures long-term DPSC proliferation and odontogenic differentiation (Chan et al., 2022; Kornsuthisopon et al., 2023). High cell viability and ALP activity indicate the biocompatibility and bioactivity of the bioengineered GelMA–PecTH injectable hydrogel, which incorporates electrospun core/shell fibres of MEL–PMMA and Td–silk fibroin (Atila et al., 2023). Gene expression assays revealed an upregulation of dentine matrix protein-1 (DMP-1), dentine sialophosphoprotein (DSPP), and axin-2. In particular, axin-2 upregulation indicates that the bioengineered hydrogel induces RDB formation for VPT through the Wnt/β-catenin signalling pathway (Neves et al., 2017).
GelMA loaded with strontium copper tetrasilicate
An innovative GelMA-based injectable hydrogel loaded with strontium copper tetrasilicate (SrCuSi4O10) was introduced to treat infected dental pulp and improve VPT efficacy (Figure 5a) (Qiu et al., 2023). SrCuSi4O10 is a microscale bioceramic composite that promotes osteogenic and odontogenic differentiation and angiogenesis, in addition to suppressing bone tumours (Huang et al., 2016; Lu et al., 2022; Wu et al., 2013; Yang et al., 2020). Applied with near-infrared (NIR) light, the SrCuSi4O10/GelMA-based injectable hydrogel exhibits excellent mechanical properties, photothermal performance, and favourable biocompatibility, regardless of the experimental approach. Owing to its photothermal properties, the SrCuSi4O10/GelMA-based injectable hydrogel exhibits greater antimicrobial activity against Streptococcus mutans and Lactobacillus casei than other VPT materials, such as MTA and bioceramic putty (Qiu et al., 2023). Under NIR radiation, the SrCuSi4O10/GelMA injectable hydrogel promotes vascularization of HUVECs in vitro and odontogenesis of DPSCs in an infected pulp tissue model in vivo (Qiu et al., 2023). The presence of well-organized odontoblast-like cell layers, collagen structures, newly formed blood vessels, and intact and thick RDB was observed above the pulp chamber, which further confirms the favourable efficacy of the SrCuSi4O10/GelMA-based injectable hydrogel as a VPT biomaterial (Qiu et al., 2023).
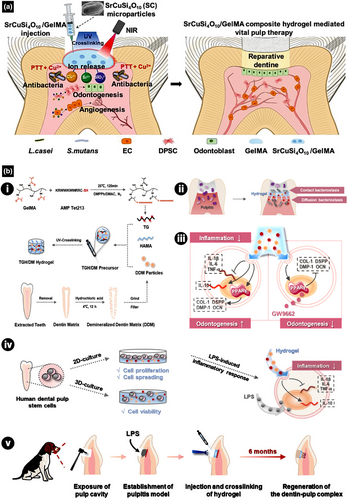
GelMA conjugated with antimicrobial peptide
In another innovative study, a GelMA-based injectable hydrogel (TGH/DM) incorporating novel antimicrobial peptide Tet213 and demineralized dentine matrix (DDM) was developed for VPT in vitro and in vivo, including biofilm interaction (Figure 5b) (Xie et al., 2024). In vitro, TGH/DM exhibits potent antibacterial activity against Lactobacillus casei and Actinomyces naeslundii. Additionally, TGH/DM promotes the polarization of macrophages towards the M2 phenotype, indicating that TGH/DM promotes tissue regeneration by disrupting the membranes of microorganisms, reducing inflammation, and converting immunocompetent cells to anti-inflammatory phenotypes. TGH/DM also triggers the synthesis of peroxisome proliferator-activated receptor gamma (PPAR-gamma), a prominent regulator of inflammation and homeostasis, to promote odontogenic differentiation in the late stage of mineralization (Xie et al., 2024). Additionally, an in vivo study was conducted using a beagle dog model of injured pulp to investigate the response of the injured pulp. Cone-beam computed tomography results revealed a thick mineralized dentine layer around the exposed pulp. The histological observation confirmed the presence of newly formed RDB with polarized odontoblast-like cells and well-observable dentine tubules (Xie et al., 2024). Overall, TGH/DM is considered a promising material for VPT, which prolongs the lifetime of teeth with pulp injuries (Xie et al., 2024).
GelMA incorporating carbon dot nanozymes
Nanozymes have recently gained attention due to their ability to catalyse reactions under various conditions, offering advantages such as high catalytic efficiency, favourable stability, and cost-effectiveness (Huang et al., 2019). Nanozymes are nanomaterials (NMs) with excellent catalytic activity and selectivity, serving as promising platforms for biomedical applications (Ren et al., 2022), including the treatment of pulpal pathosis (Xie et al., 2023). Carbon dot nanozymes (C-NZ) resemble nitrogen-doped graphene, exhibiting ultrafast electron transfer and superlative catalytic activity, along with excellent neutralization of reactive oxygen and nitrogen species (RONS). This results in significant cytoprotection and reduced apoptosis under oxidative stress, as well as favourable biocompatibility (Forman & Zhang, 2021; Mu et al., 2021). Considering the favourable properties of C-NZ, Zhang, Huang, et al. (2024) introduced an innovative GelMA-based injectable hydrogel incorporating C-NZ (C-NZ/GelMA) to provide antioxidation effects in pulp microenvironments. Assessment of the novel material (Zhang, Huang, et al., 2024) revealed that C-NZ/GelMA exhibits suitable mechanical properties, degradation rates, and swelling kinetics, in addition to inducing M1 to M2 phenotype polarization and hDPSC proliferation whilst efficiently scavenging intracellular RONS in vitro. Moreover, C-NZ/GelMA effectively maintains the oxidative stress microenvironment during pulpal inflammation, reduces pulp tissue damage, regulates the immune environment to control inflammation, and promotes pulp regeneration and RDB formation, thereby achieving tissue repair in vivo. Consequently, C-NZ/GelMA represents a significant advancement in the development of bioactive materials, offering a new strategy for VPT (Zhang, Huang, et al., 2024).
Chitosan-based injectable hydrogel
Amongst the materials explored for biomedical applications, natural products are non-toxic and thus in high demand. Chitosan, a natural co-polymer consisting of aminoglucose and N-acetylglucosamine, has gained popularity owing to its biodegradability, biocompatibility, non-toxicity, bioadhesion, and antimicrobial activity (Jiménez-Gómez & Cecilia, 2020). Chitosan is derived from chitin, a natural biopolymer commonly found in the exoskeletons of crabs, shrimps, and insects, as well as in the cell walls of algae and bacteria (Hisham et al., 2024). Currently, chitin is in the limelight as a candidate for developing biomaterials owing to its natural abundance and low cost (Crini, 2019). However, the water solubility of chitin is significantly lower than that of chitosan (Qian et al., 2023). Therefore, chitosan and its derivatives have found extensive applications as pharmaceuticals, cosmetics, wound dressings, and tissue scaffolds (Azmana et al., 2021; Tang et al., 2020; Wang, Xie, et al., 2020; Wang, Zheng, et al., 2020; Wang & Zhuang, 2022; Xiong et al., 2024; Zhang, Liu, et al., 2024). In particular, chitosan-based hydrogels have gained attention as effective dressings to accelerate wound healing (Zhang, Liu, et al., 2024). Hydrogels incorporating chitosan exhibit exceptional biocompatibility, biodegradability, non-toxicity, and antimicrobial activity, making them highly effective as wound healing accelerators (Azmana et al., 2021; Wang, Xie, et al., 2020; Wang, Zheng, et al., 2020; Wang & Zhuang, 2022; Xiong et al., 2024). Considering their favourable properties, chitosan-based hydrogels have been evaluated as potential biomaterials for VPT (Samiei et al., 2022).
Chitosan combined with BG-NPs
The biological effect of a chitosan-based silver-doped BG-loaded (Ag-BG) injectable hydrogel as a pulp capping agent has been evaluated (Zhu, Chatzistavrou, et al., 2019). Compared with MTA pulp capping materials, the Ag-BG injectable hydrogel induces the odontogenesis of DPSCs in vitro and downregulates the infiltration of inflammatory cells in vivo, demonstrating its bioactivity and biocompatibility (Zhu, Chatzistavrou, et al., 2019). Favourable inflammatory response and optimum RDB formation are attributed to the activation of p38 mitogen-activated protein kinase (MAPK) signalling (Zhu, Chatzistavrou, et al., 2019). MAPK signalling is activated when calcium in both MTA and the Ag-BG injectable hydrogel is converted into Ca2+/calmodulin-dependent kinase II, which is responsible for osteogenic and odontogenic differentiation (Rong et al., 2019; Zhu, Chatzistavrou, et al., 2019). In vivo results showed that the newly formed dentine-like barrier has regular fibrous structures. Furthermore, immunohistochemistry revealed that the expression of DSPP, Nestin, and vWF is high near the newly formed dentine-like structures (Zhu, Chatzistavrou, et al., 2019). In addition to its bactericidal activity, silver-doped BG promotes osteogenic and odontogenic differentiation in vitro and tissue calcification in vivo (Gholipourmalekabadi et al., 2015; Hench, 2006; Meretoja et al., 2014; Nommeots-Nomm et al., 2017; Shih et al., 2015; Wang, Chatzistavrou, et al., 2015; Wren et al., 2015).
Chitosan combined with calcium phosphate NPs
A tuneable injectable hydrogel based on chitosan and calcium phosphate NPs has been evaluated in vitro as a potential candidate for DPC (Osmond & Krebs, 2021). Characterized by a high compressive strength and biocompatibility, the chitosan/calcium phosphate NP-based injectable hydrogel promotes the regeneration of dental pulp and dentine. Incorporating growth factors and chemokines increase the migration and proliferation of stem cells for the regeneration of dental hard tissues (Osmond & Krebs, 2021).
Chitosan loaded with titanium oxide NPs
A chitosan/titanium oxide (TiO2) NP-based injectable hydrogel encapsulating human endometrial stem cells (EnSCs) has been evaluated in vivo using a rat model for dental pulp regeneration (Hoveizi et al., 2023). The results indicated that the formation of RDB was complete, and the RDB was uniformly thick. Additionally, Masson's trichrome staining confirmed that the collagen formation at the newly formed RDB was high (Hoveizi et al., 2023). TiO2 NPs in this hydrogel promote the proliferation and differentiation of odontoblastic cells (Patel et al., 2020). The antimicrobial activity of TiO2 NPs is greater than that of Ti, and combining TiO2 NPs with chitosan and glass ionomer powders not only provides antimicrobial effects but also improves compressive and tensile strengths of the resulting hydrogel (Bakhtiar et al., 2022; Ghilini et al., 2021). Additionally, combining EnSCs with chitosan and TiO2-NPs provides synergistic effects, promoting the differentiation of odontoblastic cells in vitro and the formation of dentine bridges in vivo (Hoveizi et al., 2023).
Collagen-based injectable hydrogels
Collagen plays a crucial role in modulating cellular activities to stimulate tissue repair and regeneration (Chen et al., 2022; Ferreira et al., 2012; Sorushanova et al., 2019; Zhu et al., 2022). Collagen-based biomaterials developed for tissue repair and regeneration modulate various cell–material interaction pathways (Haywood et al., 2016; Joyce et al., 2021; Lebbink et al., 2009; Zhou et al., 2016), including the extracellular signal-regulated kinase (ERK) 1/2 mitogen-activated protein kinase (MAPK) signal transduction pathway, which promotes cell adhesion, survival, and migration (Joyce et al., 2021). In diverse biomedical applications, optimized collagen biomaterials such as collagen scaffolds (mineralized or structured) are used to induce or guide bone regeneration (Li, Du, et al., 2021; Zhu et al., 2022). Additionally, crosslinked collagen, collagen complexes, and lyophilized hydrogels promote the regeneration of cartilage defects (Kwon et al., 2019; Parmar et al., 2015), whilst collagen-based hydrogels developed as artificial vessels promote the regeneration of cardiovascular defects (Kumar et al., 2013; Li, Ruan, et al., 2023). Owing to their versatility and bioactivity, collagen-based bioactive materials have promising applications in gynaecology, reproductive medicine, plastic repair, cornea regeneration, and dental tissue regeneration (Atieh et al., 2016; Chen et al., 2022; Lei et al., 2022; Liu, Dai, et al., 2022; Majumdar et al., 2018; McTiernan et al., 2020; Wang, Xu, et al., 2021; Xu, Wang, et al., 2019). Notably, collagen-based injectable hydrogels for dentine–pulp complex regeneration have advanced significantly with innovative approaches (Han, Xu, et al., 2024; Pankajakshan et al., 2020; Xu et al., 2023).
Collagen combined with chondroitin sulfate
A crosslinked type I collagen (COL-I)-based hydrogel incorporating chondroitin sulphate (CS) was first introduced in 2018 (Zhou et al., 2018). CS, the most common glycosaminoglycan, is predominantly found in the ECM and performs crucial functions in various cells (Wang et al., 2017). For instance, CS regulates the proliferation of DPSCs and promotes vascularization during pulpal healing (Ida-Yonemochi et al., 2022). CS plays a crucial role in the development of tooth germs (Liu, Chen, et al., 2017). CS has been integrated into genipin/COL-I hydrogels to create a desirable environment for DPSCs, with the aim of evaluating the effect of immobilized CS on the odontogenic differentiation of DPSCs. The results showed that COL-I-based hydrogels incorporating CS significantly enhance the proliferation of DPSCs in vitro. Furthermore, immobilization of CS within the COL-I hydrogel induces the formation of calcified tissues with uniform dentine tubule-like structures in a rat model, which indicates the superior biocompatibility and bioactivity of CS for VPT (Xu et al., 2023). However, whether the hydrogels can induce the formation of sufficient RDB to completely cover the larger area of exposed pulp remains questionable. Further studies should be conducted to investigate the incorporation of bioactive glass and nanohydroxyapatite, which might help to improve the sealing ability and mechanical properties of the CS-COL-I hydrogel (Xu et al., 2023). Nevertheless, the interaction between CS and collagen needs to be evaluated in the future to elucidate the mechanism underlying favourable tissue repair.
Other collagen-based injectable hydrogels
Recently, Pankajakshan et al. developed a novel tuneable collagen-based injectable hydrogel by incorporating DPSCs into low and high stiffness oligomeric collagen-based injectable hydrogels loaded with bone morphogenic protein-2 (BMP-2) and VEGF to induce dentine and pulp regeneration, respectively (Pankajakshan et al., 2020). Oligomeric collagen was precisely tuned to navigate cells of a specific stem cell lineage and to enhance the differentiation of DPSCs by morphogenetic signals. Incorporating BMP-2 and VEGF into low- and high-stiffness oligomeric collagen-based injectable hydrogels, respectively, facilitates slow, sustained release, which induces endothelial and odontogenic differentiation. This process is regulated by the VEGF/MEK1/ERK signalling pathway, along with the Wnt/β-catenin and p38 MAPK-activated canonical Wnt pathways (Bento et al., 2013; Pankajakshan et al., 2020; Yang et al., 2015; Zhang et al., 2016). Moreover, low- and high-stiffness methacrylate type I collagen promotes endothelial and odontogenic/osteogenic differentiation along with dentine–pulp complex regeneration in vivo (Han, Xu, et al., 2024). The novel approaches used in both studies hold promise for translation from basic research to clinical applications (Han, Xu, et al., 2024; Pankajakshan et al., 2020), including VPT.
Decellularized dental pulp matrix-based injectable hydrogel
The decellularized extracellular matrix (DECM) is a naturally derived biomaterial that has been used for tissue engineering in the last few decades (Yao et al., 2019). Biomaterials based on the muscle cellular matrix were first introduced in 1948 (Poel, 1948). In 2008, whole organ decellularization was developed to extract cells along with their components, especially DNA and RNA, from the ECM to produce a natural matrix with mechanical integrity. Thus, technological advancements have provided DECM-based biomaterials for regenerative medicine and tissue engineering (Ott et al., 2008). Notably, these advancements provide biomaterials that mimic natural tissues and their biological structures, ensuring minimal or no cytotoxicity and immunogenicity (Amirazad et al., 2022).
Hydrogels based on mammalian DECMs leverage the biological benefits of the ECM (Capella-Monsonís et al., 2024; Hussey et al., 2020; Saldin et al., 2017). Moreover, hydrogels incorporating mammalian DECM provide promising effects in preclinical animal models (Bordbar et al., 2020; Ghuman et al., 2018; Hussein et al., 2020; Lin et al., 2018; Mohiuddin et al., 2019; Xu et al., 2021). However, the exploration of decellularized dental pulp for endodontic regeneration is relatively new. One study revealed that DECM scaffolds based on dental–pulp promote odontoblastic differentiation of human periodontal ligament stem cells and bone marrow stromal cells without added growth or differentiation factors (Ravindran et al., 2014). Moreover, decellularized human dental pulp promotes the proliferation of stem cells from the apical papilla and their differentiation into odontoblast-like cells (Song et al., 2017). To date, there has been limited research on the potential of dental pulp DECM-based injectable hydrogels as tissue-specific materials for dental pulp regeneration (Li et al., 2020; Liang et al., 2024; Yi et al., 2024; Yuan et al., 2023; Zheng et al., 2023).
In 2020, a novel approach was introduced to develop injectable hydrogels using the decellularized matrix derived from human dental pulp (hDDPM) for dental pulp regeneration (Li et al., 2020). Owing to their biocompatibility, hDDPM-based hydrogels are suitable carriers of exogenous stem cells. The results of proteomic analysis confirmed that hDDPM-based hydrogels regulate cell adhesion, proliferation, and migration. ECM proteins contribute to odontogenesis, neurogenesis, and angiogenesis, indicating that hDDPM-based hydrogels can be applied for VPT, mainly pulpotomy. The same research group conducted another extensive study to investigate the gene expression responses of cells in response to various DECM-based hydrogels (Liang et al., 2024), revealing that 1728 differentially expressed genes are enriched from biological processes related to phosphorylation, osteoblastic differentiation, and bone mineralization, highlighting their co-relationship with tissue mineralization. Moreover, TGF-β, Wnt, and particularly, Notch signalling pathways play crucial roles in the odontogenic differentiation of DPSCs.
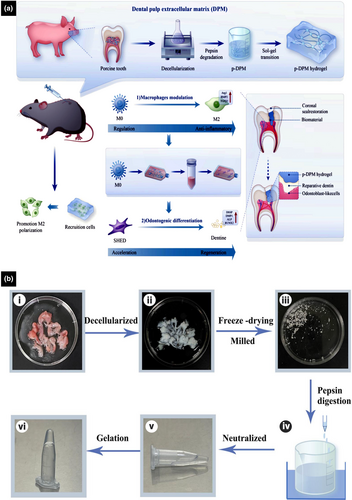
Decellularized dental pulp matrix digested by pepsin
DECM-based injectable hydrogels derived from the matrix of porcine dental pulp, further digested by pepsin, exhibit rheological properties and biocompatibility that make them effective materials for the regeneration of dental pulp (Yuan et al., 2023). Under physiological conditions, DECM-based hydrogels undergo sol–gel conversion without the addition of cross-linking agents. The rheology of DECM-based hydrogels highlights the basis of their excellent injectability and conformability to the irregular shapes of root canals (Yuan et al., 2023). Both in vitro and in vivo experiments showed that DECM-based hydrogels derived from dental pulp tissue retain VEGF to promote angiogenesis. Moreover, DMP-1 and DSPP, known as positive regulators of hard tissue mineralization, are upregulated. Collectively, the findings suggest that DECM-based hydrogels are effective tissue-specific scaffolds for regenerating dentine–pulp complexes (Yuan et al., 2023). Recently, a novel injectable hydrogel based on decellularized matrix derived from porcine dental pulp digested by pepsin (p-DPM) was developed for immunomodulation during RDB formation (Figure 6) (Yi et al., 2024). Evaluation of the immunomodulatory effects of p-DPM (Zeng et al., 2022) revealed that M2 macrophage markers and anti-inflammatory cytokines are upregulated in macrophages, contributing to the significant release of TGF-β1, TGF-β2, and BMP-2, which play crucial roles in the proliferation and differentiation of DPSCs along with the repair and regeneration of dentine (Kadowaki et al., 2022; Li et al., 2020; Park et al., 2017). Apart from that p-DPM modulates the immunogenicity of the pulp microenvironment in vivo, thereby reducing dental pulp inflammation and facilitating RDB formation (Yi et al., 2024). Injectable hydrogels based on the decellularized matrix derived from dental pulp hold immense potential for VPT and clinical translation.
Treated dentine matrix-based injectable hydrogels
Identifying and developing appropriate scaffolding materials for the regeneration of tooth structures, particularly dentine–pulp complexes, is crucial in the field of endodontic tissue engineering (Nakashima & Akamine, 2005; Scheller et al., 2009). Treated dentine matrix (TDM) is a special type of DECM derived from dentine (Bi et al., 2022). A major constituent of the tooth, dentine is composed of 70% hydroxyapatite, with ECM and odontoblasts as minor components, and thus it is less mineralized yet more elastic than enamel (Stern et al., 2009). Scaffolds that replicate the natural structure of dentine or TDM efficiently promote dentine regeneration in vivo (Lluch et al., 2009). Additionally, TDM derived from rats induces the differentiation of dental precursor cells into dentine in a rat model (Guo et al., 2009).
TDM combined with sodium alginate
A special type of TDM-based injectable hydrogel incorporating sodium alginate has been demonstrated to offer convenient application and significant potential for VPT (Figure 7a) (Holiel, Mahmoud, Abdel-Fattah, & Kawana, 2021; Holiel et al., 2023; Holiel, Mahmoud, & Abdel-Fattah, 2021; Holiel & Sedek, 2023). Several TDM-based injectable hydrogels incorporating sodium alginate have been evaluated for the repair and regeneration of injured human dentine using conventional gold standards, such as MTA and Biodentine® (Holiel, Mahmoud, & Abdel-Fattah, 2021; Holiel, Mahmoud, Abdel-Fattah, & Kawana, 2021; Holiel et al., 2023). Applying the TDM-based injectable hydrogel on human teeth induces the complete formation of a thick RDB in the pulp without inflammation (Holiel, Mahmoud, & Abdel-Fattah, 2021; Holiel, Mahmoud, Abdel-Fattah, & Kawana, 2021). Notably, TDM/sodium alginate-based injectable hydrogels promote the regeneration of injured dentine, with the formation of a uniform dentine and no tunnel defects, owing to the sustained release of DMP-1, DSPP, biglycan, COL-1, TGF-β1, and decorin, which play significant roles in the proliferation of DPSCs and differentiation of odontoblastic cells, leading to the formation of an uninterrupted homogenous dentine bridge (Jiao et al., 2014). Nonetheless, TDM is composed of highly mineralized particles, and thus scaffold covalent bonds are susceptible to breakdown during fabrication, and scaffold functional activity in vivo is unstable without adhesive carriers (Du et al., 2015; Wen et al., 2023).
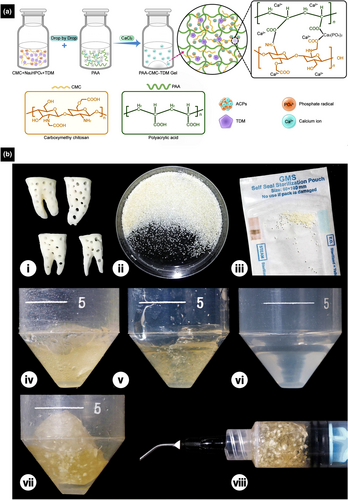
TDM combined with polyacrylic acid
A novel composite injectable hydrogel based on amorphous calcium phosphatase (ACP), polyacrylic acid (PAA), carboxymethyl chitosan (CMC), and TDM was fabricated to improve the formability and extend the applicability of TDM (Figure 7b) (Wen et al., 2023). Incorporating ACP induces the mineralization of collagen fibrils and improves the structural stability of hydrogels (Li et al., 2018; Li, Cui, et al., 2021; Wen et al., 2023; Zhao et al., 2012). Owing to the electrostatic interaction between CMC and PAA, this novel biomaterial shows increased hardness, reduced swelling, and prolonged degradation (Wen et al., 2023). TDM exhibits favourable biocompatibility and bioactivity, inducing the differentiation of mesenchymal stem cells (MSCs) for odontogenesis and osteogenesis (Chen et al., 2017; Tanoue et al., 2018), whilst CMC and PAA facilitate hard tissue regeneration and biomimetic mineralization (Tang et al., 2020; Wen et al., 2023). Because the injectable hydrogel based on ACP, PAA, CMC, and TDM exhibits favourable biocompatibility and minimal immunogenicity, and induces minimal pulp inflammation, it improves the repair of dentine in vivo, making it a promising candidate for VPT and other clinical applications (Li, Cui, et al., 2021; Wen et al., 2023).
Other injectable hydrogels for VPT
Other injectable hydrogels, such as self-assembling peptide (SAP)-based hydrogels, extracellular vesicle (EV)-based hydrogels, and NP-based hydrogels, have been evaluated for VPT (Li, Tian et al., 2024; Moore et al., 2015; Sharma et al., 2017; Siddiqui et al., 2021; Xia et al., 2020; Zhao et al., 2024).
SAP-based hydrogels
Recently, smart biomaterials based on SAPs have attracted the attention of researchers in the field of tissue engineering (Holmes et al., 2000; Pérez et al., 2015; Sargeant et al., 2012). SAPs readily attach to DPSCs to promote angiogenesis during pulp regeneration (Guo et al., 2024; Piva et al., 2014). Additionally, different types of short bioactive peptides, such as arginylglycylaspartic acid (RGD), and various growth factors, such as VEGF and BMP, have been incorporated into hydrogels to promote cellular adhesion, proliferation, and differentiation (Casagrande et al., 2010; Suzuki et al., 2011; Zhu, Dissanayaka, et al., 2019). RAD, produced by functionalizing SAPs with RGD and VFGF, is a novel supramolecule that resembles natural ECM. One laboratory study demonstrated that RAD significantly improves odontogenic differentiation and angiogenesis. The study revealed that RAD also promotes favourable inflammatory infiltration and the formation of an intact RDB with well-aligned odontoblastic cell layers (Xia et al., 2020).
EV-based injectable hydrogels
EVs are predominantly nanoscale materials secreted by cells, and over the past several years, they have been a prominent focus of research on regenerative medicine (Ju et al., 2023). Extensive research has demonstrated that EVs, particularly those secreted by MSCs, facilitate the repair and regeneration of various tissues, highlighting their potential as significant players in regenerative medicine (Li, Hosseini-Beheshti, et al., 2019; Liu et al., 2023; Nagelkerke et al., 2021; Safari et al., 2022; Zhang, Wang, et al., 2023). Indicators of oxidative stress, such as ROS, myeloperoxidase and 8-isoprostane, play vital roles in regulating the microenvironment of injured dental pulp (Dogan Buzoglu et al., 2023; Vengerfeldt et al., 2017). Therefore, a smart injectable hydrogel based on EVs derived from dental follicle stem cells was loaded with sodium alginate and an ROS scavenger (SA-RhB) for VPT (Li, Tian, et al., 2024). The smart hydrogel provides a balanced oxidative and antioxidative microenvironment through the sustained release of the ROS scavenger to promote cell proliferation and odontogenic differentiation in vitro, thereby increasing the wound healing rate (Li, Tian, et al., 2024). In vivo, the smart hydrogel promotes the formation of a large RDB, with a relatively favourable pulpal response observed under the RDB (Li, Tian, et al., 2024).
NP-based injectable hydrogels
A triphenylalanine-based injectable hydrogel incorporating cerium oxide NPs (CNPs) and DMP-1 provides anti-inflammatory effects and promotes RDB formation (Figure 8) (Zhao et al., 2024). Owing to its physicochemical properties, this innovative hydrogel readily adapts to irregular dental cavities and facilitates the sustained release of CNPs and DMP-1. The CNP/DMP-1 polypeptide-based injectable hydrogel exhibits favourable biocompatibility in vitro and in vivo to facilitate homogeneous RDB formation and high immunohistochemical expressions of DSPP and DMP-1 in a preclinical VPT rat model of pulp injury. Thus, the CNP/DMP-1 polypeptide-based injectable hydrogel is a promising candidate for next-generation VPT (Zhao et al., 2024).
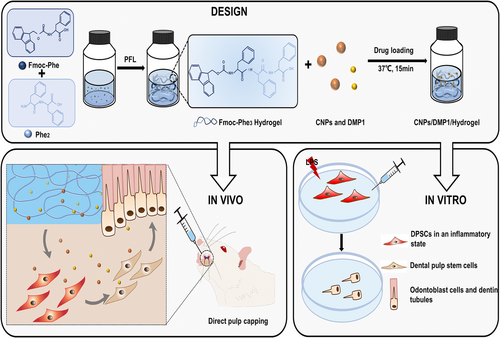
Table 2 outlines the clinical translational feasibility of injectable hydrogels for VPT.
| Ref. | Hydrogel type | Biocompatibility | Ease of delivery | Mechanical stability | Cost | Evidence level | Regulatory status | Clinical translation readinessa |
|---|---|---|---|---|---|---|---|---|
| Silva et al. (2018), Almeida et al. (2018), Atila, Chen, et al. (2022) | HA | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
| Wang, Fu, et al. (2021), Sadeghian et al. (2023), Atila et al. (2023), Qiu et al. (2023), Xie et al. (2024), Zhang, Huang, et al. (2024) | GelMA | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
| Zhu, Chatzistavrou, et al. (2019), Osmond and Krebs (2021) | Chitosan | Favourable | Clinically reasonable | Unknown | Unknown | Preclinical | Experimental | Moderate to high |
| Hoveizi et al. (2023) | Chitosan with NPs | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
| Xu et al. (2023) | Collagen-I | Favourable | Clinically reasonable | Unknown | Unknown | Preclinical | Experimental | Moderate to high |
| Li et al. (2020) | hDDPM | Favourable | Clinically reasonable | Unknown | Unknown | Preclinical | Experimental | Moderate to high |
| Yi et al. (2024) | DPM | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
|
Holiel, Mahmoud, and Abdel-Fattah (2021) Holiel, Mahmoud, Abdel-Fattah, and Kawana (2021) Holiel et al. (2023) |
TDM combined with SA | Favourable | Clinically reasonable | Favourable | Unknown | Clinical | Experimental | Moderate to high |
| Wen et al. (2023) | TDM combined with PAA | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
| Xia et al. (2020) | SAP | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
| Zhao et al. (2024) | Fmoc-triphenylalanine hydrogel | Favourable | Clinically reasonable | Favourable | Unknown | Preclinical | Experimental | Moderate to high |
| Li, Tian, et al. (2024) | SA combined with DFSCs-derived small EVs | Favourable | Clinically reasonable | Unknown | Unknown | Preclinical | Experimental | Moderate to high |
- Abbreviations: DPM, decellularized pulp matrix; EV, extracellular vesicles; GelMA, gelatin methacryloyl; HA, hyaluronic acid; hDDPM, human decellularized dental pulp matrix; NPs, nanoparticles; PAA, polyacrylic acid; SA, sodium alginate; SAP, self-assembling peptide; TDM, treated dentine matrix.
- a Based on qualitative assessment.
CHALLENGES AND FUTURE PERSPECTIVES
- Although injectable hydrogels offer many advantages as potential biomaterials, there are still several challenges that need to be addressed. Antimicrobial effect after VPT is a fundamental requirement for successful treatment (Duncan, 2022). Using antimicrobial agents, such as chlorhexidine, sodium hypochlorite, tetracycline, metronidazole, quaternary ammonium compounds, peptides, and enzymes, can prolong the antimicrobial effects (Montoya et al., 2023; Ribeiro et al., 2022; Xie et al., 2024). However, potential interactions between the antimicrobial agent and injectable hydrogel formulations require further investigations.
- NMs exhibit favourable biocompatibility and antimicrobial activity and are capable of controlled drug delivery; thus, applying NMs is expected to improve the efficacy of VPT (Hu et al., 2014; Kunzmann et al., 2011; Makvandi et al., 2020; Park, 2014). Antimicrobial agents, such as drugs or metallic NPs, provide greater antimicrobial effects than bio-derived materials, although high doses of these agents are required for broad-spectrum action, which may be toxic to pulp cells (Ahmad et al., 2024; Ahmadian et al., 2022). However, some NMs and NPs have adverse effects, such as coronal discolouration of the tooth, inflammatory osteolysis, oxidative stress, carcinogenicity, and genotoxicity (Heo et al., 2020; Moazami et al., 2018; Shakeel et al., 2016). Indeed, NMs can enhance the physical and mechanical properties, injectability, and biodegradability of hydrogels, which are crucial for clinical translation. Therefore, incorporating NPs or NMs into injectable hydrogels at an optimum dose might hold immense promise for achieving high VPT efficacy. Future research should focus on exploring the potential of NP-loaded injectable hydrogels for VPT, aiming to stimulate RDB formation and pulp regeneration.
- Placement of the biomaterials during VPT stimulates the pulp healing by initiating inflammatory reactions within the pulp, which is the most important factor in determining the success of VPT (Arora et al., 2021). Similar to the release of drug from traditional biomaterials for VPT, achieving on-demand release of active molecules from injectable hydrogels to modulate inflammation remains challenging. However, applying the hydrogels can trigger unexpected body reactions, which lead to the rapid degradation of the hydrogels and the formation of undesired by-products that elevate the level of inflammatory mediators (Dal-Fabbro et al., 2023). Therefore, it is crucial to avoid and/or control inflammatory responses following the application of injectable hydrogel (Poustchi et al., 2021). However, a certain level of inflammation is necessary for RDB formation (Cooper et al., 2014). To overcome these challenges, incorporating biological molecules, such as growth factors, anti- and pro-inflammatory cytokines, and epigenetic regulators, into injectable hydrogels might enhance the biomimetic complexity of the hydrogel, thereby improving the outcome of VPT.
- For a successful VPT, bleeding must be controlled by using haemostatic agents before the placement of an appropriate biomaterial, which can allow clinical assessment of the inflammatory level and identification of necrotic tissue (Islam et al., 2023). Although several injectable hydrogels promote favourable haemostasis under various conditions, not all the injectable hydrogels are suitable to be used as haemostatic agents (Fang et al., 2023; Sanyal et al., 2024). Therefore, haemostatic agents, such as sodium hypochlorite, chlorhexidine, hydrogen peroxide, and ferric sulphate (Ballal et al., 2020), can be incorporated into the injectable hydrogels to provide promising effects during VPT. This might promote haemostasis, disinfect the dentine–pulp interface, eliminate biofilms, remove blood clots and fibrin chemically, and clear dentinal chips and damaged cells from the mechanically exposed site (AAE, 2021). Nevertheless, a significant gap remains in understanding of the haemostatic effects during VPT, highlighting the need for future research.
- Improving aspects of injectable hydrogel systems, such as the degree of hydrophobicity/hydrophilicity, is required for cell viability and proliferation within the hydrogel network during and after injection, protection of molecules from degradation, and sustained release of molecules (Rizzo & Kehr, 2021). Injectable hydrogels should be biodegradable, generating non-cytotoxic degradation products or unreactive crosslinkers (Bakaic et al., 2015; Li et al., 2014; Weng et al., 2008). Therefore, it is important to encourage the design and development of multifunctional hydrogels using multiple units with different functionalities (Hendrickson et al., 2010; Xue et al., 2021). Precise molecular design can be a significant drawback because optimization is time-consuming and expensive (Almawash et al., 2022; Sonker et al., 2021). Consequently, the molecular structure of injectable hydrogels represents an advantage and a limitation or a tug of war, that affects their competitiveness with other drug delivery systems (Rizzo & Kehr, 2021). Therefore, incorporating oligosaccharide-based micelles, thermo-responsive elements, and miniaturized implantable micropumps into injectable hydrogel facilitates the controlled and sustained release of bioactive molecules (Chin et al., 2017; Li, Cui, et al., 2024). This approach might facilitate the creation of smart injectable hydrogels capable of precisely delivering drugs within the dental pulp microenvironment for personalized and optimized therapeutic interventions.
- Beyond the chemical properties, the mechanical and physical properties of injectable hydrogels are also vital for convenient application and biocompatibility within the tissue's microenvironment (Bashir et al., 2020). For clinical applicability in VPT, injectable hydrogels must withstand high stress, offer a balance of mechanical stability and elasticity, and adhere to tissues, particularly at the tissue interfaces. However, developing formulations that exhibit these attributes is challenging. Advanced three-dimensional and four-dimensional bioprinting enables the fabrication of injectable hydrogels exhibiting inherent in situ shape recovery and stabilization (Camacho et al., 2021; Liao et al., 2024; Liu, Zhang, et al., 2022; Wan et al., 2020; Wang et al., 2022; Zhu et al., 2021). This may provide precise control over physical and chemical properties, including temperature, pH, and humidity, at specific locations (Mahmood et al., 2023). Moreover, artificial intelligence (AI) allows for possible in silico testing by simulating different injectable scaffold designs, materials, and cell types suitable for VPT, leading to breakthroughs in VPT. AI algorithms can become powerful tools for revealing and predicting how biomaterials promote reparative dentine formation and for identifying suitable growth factors, cytokines, and epigenetic molecules for tissue regeneration (Nosrati & Nosrati, 2023).
- Currently, the primary concern is the lack of in vivo or preclinical data. Therefore, it is important to evaluate in vivo models for extended periods, alongside in vitro and other molecular models. However, standardized application of injectable hydrogels in rodent teeth is challenging owing to the small teeth and cavities of rodents. Although some studies include in vivo rodent models, the use of larger animals, such as dogs and monkeys, might provide more reliable preclinical data (Wen et al., 2023). In addition, randomized controlled trials need to be conducted to confirm the clinical effectiveness of injectable hydrogels.
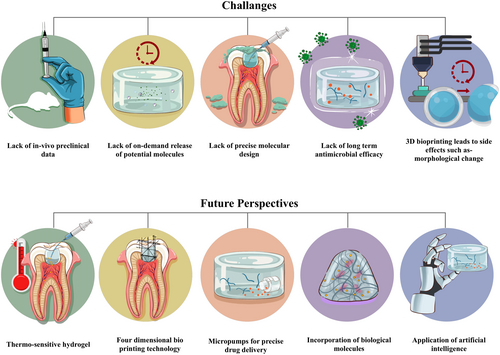
In conclusion, despite the potential applications of injectable hydrogels, challenges remain. A significant limitation is the scarcity of in vivo studies, which restricts the ability to draw definitive conclusions about their clinical efficacy. However, existing studies demonstrate the effectiveness of injectable hydrogels in promoting RDB formation, reducing inflammation, and ultimately preserving pulp vitality. Future studies should focus on optimizing injectable hydrogel components, investigating the long-term VPT outcomes, and translating preclinical successes into clinical applications.
AUTHOR CONTRIBUTIONS
Md Refat Readul Islam: Conceptualization; writing – original draft preparation; writing – review and editing; funding acquisition. Rafiqul Islam: Writing – original draft preparation; writing – review and editing. Hidehiko Sano: Writing – review and editing; resources. Yu Toida: Writing – review and editing; resources. Shuhei Hoshika: Writing – review and editing; resources. Hany Mohamed Aly Ahmed: Writing – original draft preparation; writing – review and editing. Atsushi Tomokiyo: Writing – review and editing; supervision; funding acquisition.
FUNDING INFORMATION
This work was financially supported by Grants-in-Aid for Scientific Research (Project No. JP21K19608, JP23K24529, JP24K19870 and JP25K20259) from the Japan Society for the Promotion of Science.
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflict of interest.
ETHICAL APPROVAL STATEMENT
This study does not require any ethical approval.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




