Present status and future directions: Surgical endodontics
Abstract
Endodontic surgery encompasses several procedures for the treatment of teeth with a history of failed root canal treatment, such as root-end surgery, crown and root resections, surgical perforation repair and intentional replantation. Endodontic microsurgery is the evolution of the traditional apicoectomy techniques and incorporates high magnification, ultrasonic root-end preparation and root-end filling with biocompatible filling materials. Modern endodontic surgery uses the dental operating microscope, incorporates cone-beam computed tomography (CBCT) for preoperative diagnosis and treatment planning, and has adopted piezoelectric approaches to osteotomy and root manipulation. Crown and root resection techniques have benefitted from the same technological advances. This review focuses on the current state of root-end surgery by comparing the techniques and materials applied during endodontic microsurgery to the most widely used earlier methods and materials. The most recent additions to the clinical protocol and technical improvements are discussed, and an outlook on future directions is given. Whilst nonsurgical retreatment remains the first choice to address most cases with a history of endodontic failure, modern endodontic microsurgery has become a predictable and minimally invasive alternative for the retention of natural teeth.
INTRODUCTION
Endodontic surgery has a long-standing tradition as a dental procedure. Various clinicians introduced concepts of apical surgery in the late 19th and early 20th centuries. The intention was to remove necrotic portions of the apex (Beal, 1908; Brophy, 1880; Farrar, 1884) and to excise diseased periapical tissues. These approaches, however, did not take into account the presence of intra-radicular infection (Regan et al., 2005). Root-end resection gained further attention after publications by Partsch (1896, 1898, 1899) and Faulhaber and Neumann (1912).
After these early approaches, there have been many changes and iterations to endodontic surgical techniques, with varying acceptance and success. For many years, surgical burs were used for osteotomy and root-end resection, and amalgam as a root-end filling material (Castenfelt, 1939; Dorn & Gartner, 1991; Harty et al., 1970; Oginni & Olusile, 2002). With advances in initial root canal treatment and nonsurgical retreatment, the utilization of endodontic surgery was called into question due to several clinical studies with very low outcome rates (Allen et al., 1989; Frank et al., 1992). These low outcome rates were likely related to the continued presence of intra- and/or extra-radicular infection after surgical intervention.
- contrast the present status of endodontic surgery in the form of endodontic microsurgery to more common earlier applications,
- give an overview of some of the procedures included within the spectrum of endodontic surgery, including root resection or amputation, crown resection (hemisection, trisection and premolarization or bicuspidization) and
- highlight the most recent additions and technical improvements in endodontic surgery.
Indications
Nonsurgical or surgical retreatment is an intervention to treat persistent or recurrent apical periodontitis. Nonsurgical retreatment is considered the option of choice in case of an insufficient primary root canal treatment and unidentified canals, or if a new definitive restoration is planned. The majority of periapical lesions, including abscesses, cysts (Nair et al., 1996) and granulomas (Bergenholtz et al., 1979; Sjögren et al., 1990; Strindberg, 1956), should respond favourably to adequate nonsurgical retreatment. Surgical retreatment is indicated when nonsurgical retreatment attempts have failed or are deemed to be unfeasible. Prior to choosing surgical retreatment, the aetiology of a persistent pathosis must be thoroughly evaluated (Karabucak & Setzer, 2007). Asymptomatic periapical lesions should be monitored at appropriate follow-up appointments to allow sufficient time for healing.
A variety of tooth-related factors may necessitate surgical retreatment, including complicated root canal anatomy, the pathophysiology of the apical pathosis, severe alterations of the root canal anatomy during endodontic treatments, root filling materials, build-ups or posts impossible to retreat or at unreasonably high risks, as well as perforations, resorptions or root fractures.
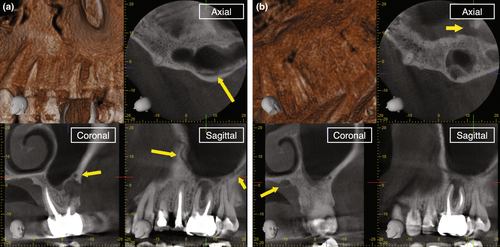
Natural anatomical challenges leading to a decision for surgical retreatment may include immature root development (>1.5 mm in apical diameter), extreme (>30°) or s-shaped root curvatures, root canal bifurcations in the middle or apical third, overly long roots (>25 mm) or severe calcifications (Karabucak & Setzer, 2007). Changes to the original root canal system anatomy that may be difficult to overcome consist of ledges, transportations, perforations, internal and external resorptions, or separated instruments, which may result in residual microorganisms in proximity to the apical constriction (Walton & Ardjmand, 1992) or apical foramen (Siqueira & Lopes, 2001) due to inadequate biomechanical disinfection (Gorni & Gagliani, 2004; Figure 1).
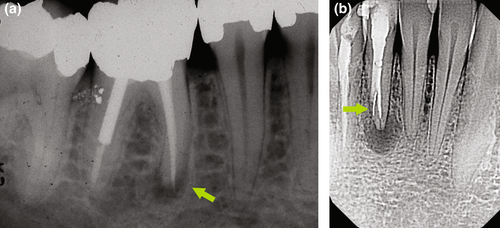
Whilst some of these challenges only become apparent during actual nonsurgical retreatment, some may be predictable during the treatment planning phase. If the original root canal anatomy cannot be successfully explored and submitted to biomechanical instrumentation in cases with the presence of apical periodontitis, the success rate of nonsurgical retreatment dropped as low as 40% (Gorni & Gagliani, 2004). In this context, it must also be stressed that persistent or recurrent apical periodontitis is maintained by a microbiological spectrum posing greater challenges to antimicrobial strategies.
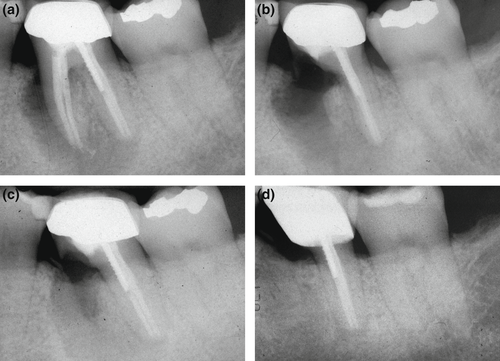
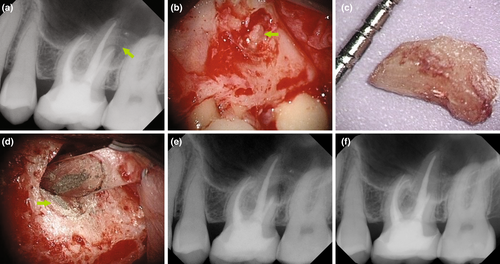
Previous root fillings with hard pastes or silver points may not be completely removable by nonsurgical means. Resorcinol–formaldehyde pastes (‘Russian Red’; Albrecht, 1915) may be impossible to remove from the root canal system, requiring a surgical approach. In addition, the orthograde retrievability of calcium silicate-based sealers or putty materials is subject to debate. The decision to remove an instrument fragment from a root canal by nonsurgical versus surgical means depends on the presence or absence of apical periodontitis, the location of the fragment in the coronal, middle or apical third, and the type of instrument, for example a stainless steel hand versus a nickel–titanium rotary file (Figure 2). The expected loss of hard tissue structure must be justifiable to initiate nonsurgical retreatment (Setzer & Karabucak, 2018). At or beyond a canal curvature, superelastic nickel–titanium instruments may wedge towards the outer canal curvature, which may render removal attempts very difficult or impossible (Parashos & Messer, 2006), favouring surgery as a less invasive option (Karabucak & Setzer, 2007). Cast post and core build-ups, crowns or long metal posts may necessitate the disassembly of an existing coronal restoration, which could come at an elevated risk of hard tissue loss, root fracture or perforation, favouring a surgical approach (Karabucak & Setzer, 2007; Figure 3).
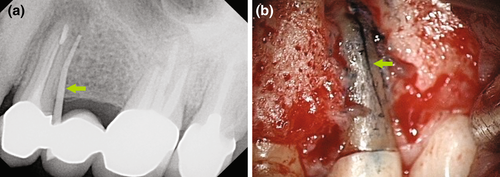
Resorption repairs in the coronal aspect of a tooth likely warrant a nonsurgical approach (Meister et al., 1979; Nicholls, 1962; Pitt Ford et al., 1995a, 1995b; Pitt Ford, Torabinejad, et al., 1995; Setzer & Karabucak, 2018), whereas a more apical position will favour endodontic surgery (Karabucak & Setzer, 2007), in particular, if a resorptive defect did not arrest after nonsurgical treatment (Figure 4). Vertical root fractures can be assessed by exploratory procedures (Figure 5). Root amputation of a fractured root may save a multi-rooted tooth (Figure 6).
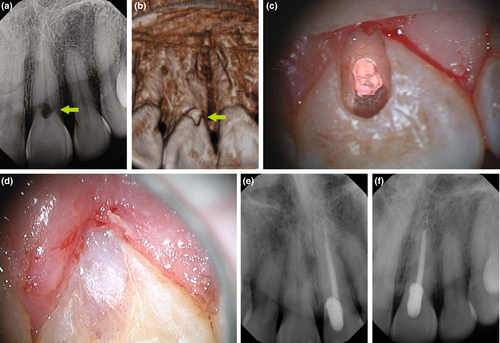
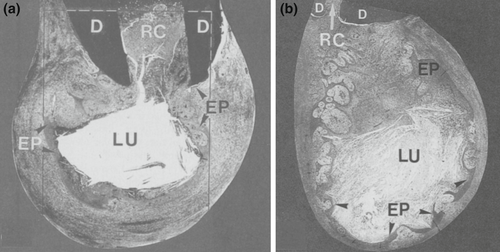
Histologically, periapical lesions are overwhelmingly inflammatory pathoses. According to Nair et al. (1996), 50% of lesions were granulomas, 35% were periapical abscesses, and 15% were pocket or true cysts. Pocket cysts have a direct connection to the infected root canal system, whereas true cysts are independent of the root (Nair et al., 1996, Nair, 2006; Figure 7). Primary endodontic treatment or nonsurgical retreatment may not allow for the healing of true cysts (Nair et al., 1996), necessitating endodontic surgery. Foreign body reactions, by gutta-percha and other filling or sealer materials, paper point remnants and cholesterol clefts were identified within cystic lesions (Nair, 1998, 2006; Nair et al., 1993). Lastly, extra-radicular infections may be found as biofilms on an external root surface (Tronstad et al., 1990) or in the form of Actinomyces and Propionibacterium colonies within asymptomatic lesions (Nair, 1987; Ricucci & Siqueira Jr, 2008; Sjögren et al., 1988; Sunde et al., 2003), and may necessitate endodontic surgery (Figure 8).
The dentist's decision-making has to include a detailed medical and dental history and clinical examination. A patient has to be informed about all appropriate treatment alternatives and their prognoses and risks. Surgical procedures exhibit potential risks of damage to adjacent anatomical structures, postoperative swelling, discomfort or impaired wound healing. A patient must be allowed to choose based on their understanding of the advantages and disadvantages of proposed alternatives, the value their tooth presents to them, costs and their ability to undergo specific dental procedures (Kvist, 2001).
Contraindications
Endodontic surgery can be contraindicated or compromised in situations where the close proximity of sensitive anatomical structures may lead to transient or irreparable damage during a surgical procedure. These structures may include the nasal or sinus cavities (Figure 9), the mental and infra-alveolar nerves (Figure 10) or the palatal neurovascular bundle (Figure 11). Targeted surgical approaches discussed in this review may provide an appropriate solution. Systemic diseases such as congenital bleeding disorders may prohibit a surgical approach. A history of intravenous bisphosphonate therapy may pose a highly elevated risk of bisphosphonate-related osteonecrosis of the jaws (Setzer & Karabucak, 2018). Some cardiovascular diseases contraindicate the use of vasoconstrictors with the local anaesthesia, severely limiting haemostasis during surgery. Lastly, teeth with deficient definitive restorations, a minimal or questionable crown-to-root ratio, advanced periodontal disease or increased mobility may not be suitable for a surgical approach due to a less favourable prognosis.

PRESENT STATUS—ENDODONTIC MICROSURGERY
Treatment planning
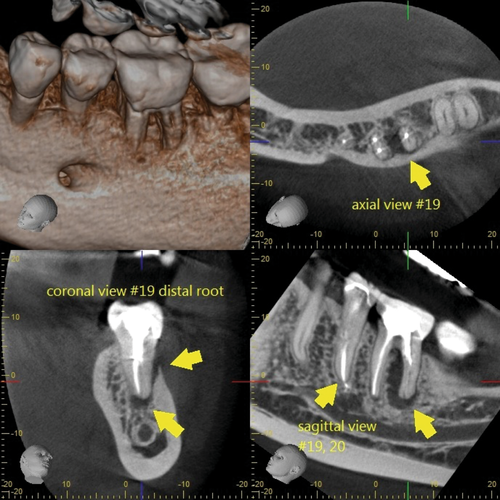
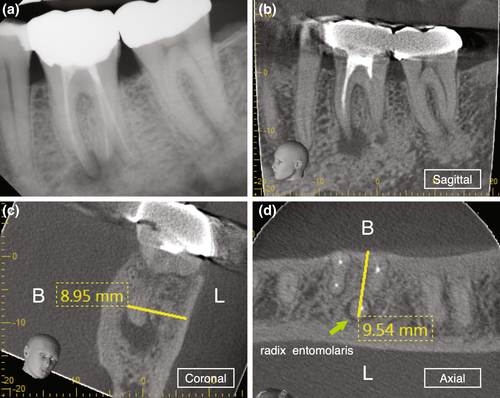
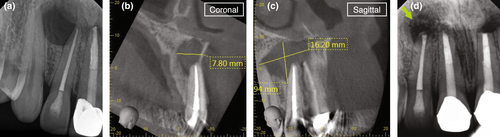
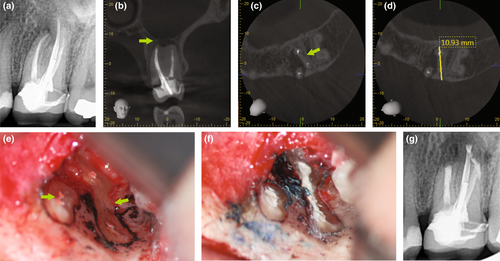
The clinician has to ensure to review a patient's medical and dental history and perform a proper clinical and radiographic examination prior to arriving at a clinical diagnosis. A patient's chief complaint needs to be addressed. If a decision for endodontic surgery has been made, a three-dimensional radiograph should be taken for both diagnostic and medico-legal purposes. For the majority of dental procedures, including endodontic surgery, a limited field-of-view (FOV) cone-beam computed tomogram (CBCT) is sufficient to evaluate not only the tooth or teeth undergoing the surgical operation but also the surrounding tissues to avoid iatrogenic damages during the surgical intervention. However, despite the fact that CBCT has now been widely adopted in Endodontics (Scarfe et al., 2009; Setzer et al., 2017), the relatively high radiation exposure compared to periapical radiographs has to be considered and the ALARA principle followed at all times. CBCT allows for the precise evaluation of the size and location of a periradicular lesion, the bone thickness over the pathosis, and the proximity to adjacent anatomical structures such as the infra-alveolar and mental nerve, sinus and nasal cavities and neighbouring roots (Frank et al., 1996; Setzer et al., 2017). Whilst there are still impediments in the detection of vertical root fractures due to limitations in CBCT resolution and beam hardening effects of root filling materials or other radiographic artefacts, characteristic patterns of bone loss in the form of an abrupt, narrow vertical defect may indirectly indicate their presence (Chang et al., 2016; Menezes et al., 2016; Figure 12). To treatment plan endodontic surgery, CBCT allows accurate measurements of relevant distances, including the buccal bone surface to the root tip or the length of a root (Bornstein et al., 2011; Figure 13). In contrast to two-dimensional radiographs, CBCT also allows for the detection of the posterior superior alveolar nerve and the greater palatine artery canals (Figure 14), making CBCT an important tool for guided microsurgical techniques discussed further below.
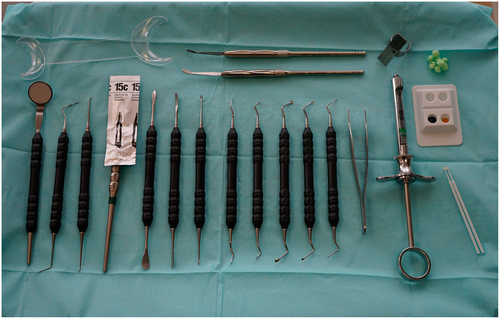
Armamentarium
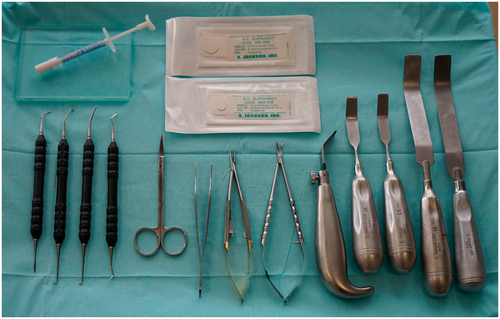
A variety of kits are available for modern endodontic surgery and typically consist of miniaturized versions of standard surgical instruments, often specifically designed to work under high magnification with a dental operating microscope or endoscope. Standard instrument sets should include hand instruments, such as a dental mirror, a periodontal probe, an endodontic explorer and micro-explorer, a surgical blade holder, and tissue elevators for incision and flap elevation; periodontal curettes, surgical curettes, and micro-endodontic curettes for the removal of pathologic tissues; micro-mirrors and handle for inspection; and carriers and pluggers for root-end filling (Figure 15). In addition to hand instruments, the following armamentarium is needed: a 45° surgical handpiece and burs for osteotomy and root resection; tissue retractors; an ultrasonic unit with corresponding tips for root-end preparation; a microsurgical tissue forceps; needle holder and scissors; and miscellaneous instruments such as anaesthesia syringe, college pliers, air–water syringe and a micro-irrigator (Figures 16 and 17). Disposable items include anaesthetic solution, surgical blades, bone-cutting burs, gauze and cotton pellets, haemostatic agents, dyes, saline, root-end filling and perforation repair materials, bone grafting and membrane materials, and sutures. In addition, piezoelectric devices for specific osteotomy techniques, such as the creation of protective grooves or a bone window, are helpful and have recently increased in usage (Figure 18).

Patient positioning
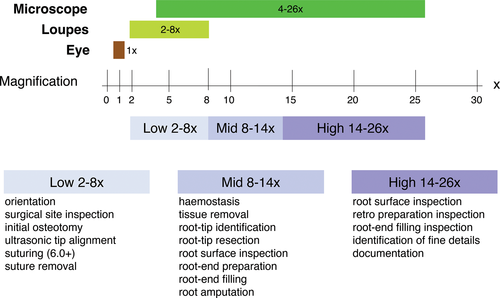
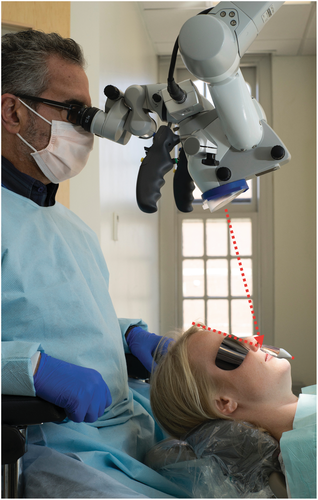
Endodontic microsurgery is applicable to the majority of teeth in both jaws, with the exception of second mandibular molars and wisdom teeth. A key aspect of the procedure is the positioning of the patient, the clinician and the assistant (Jouanny & Safi, 2014; Kratchman & Kim, 2017) for both the comfort of the patient and the ability of the surgical team to execute the procedure to the best of their ability. For endodontic microsurgery, the operating microscope provides high magnification, coaxial illumination and ergonomic seating for the clinician (Kim & Kratchman, 2006; Setzer et al., 2012). The direct vision provided by the microscope allows the operator to examine and treat both pathologic tissues and root structure more precisely and thus minimally invasive. As with increasing magnification, the focal depth becomes smaller, and correct patient positioning becomes important not only for the proper execution but also for the efficiency of the procedure. For each step of the procedure, the appropriate magnification level, low, mid and high, has to be chosen dependent on whether an overview of an area with several teeth, a single tooth, or specific details must be in the field of vision (Figure 19). During soft tissue elevation, osteotomy and root resection, a patient remains in a horizontal position just as the long axis of the tooth undergoing surgery. When the root resection is completed and verified, the patient has to be readied for ultrasonic root-end preparation by ensuring the direct vision of the resected root surface to avoid misalignment of the ultrasonic tip and limit the osteotomy to a reasonable size. This is achieved by uprighting the chair when working on maxillary teeth (Figure 20) and further reclining for working on mandibular teeth (Figure 21). The original position can resume after the root-end filling is completed.

Anaesthesia
Local anaesthesia in endodontic surgery is used for both profound analgesia and haemostasis (Kim & Rethnam, 1997). A resected root surface must be examined under high magnification to identify problems that may have led to the failure of the previous endodontic procedure. Haemostasis provided by a local anaesthetic is achieved by the addition of a vasoconstrictor, ideally 1:50 000 epinephrine (with 1:80 000 epinephrine as an alternative if a higher concentration is unavailable; Buckley et al., 1984; Gutmann, 1993; Kim et al., 2001). A higher concentration of the vasoconstrictor more effectively reduces bleeding if it is injected in the submucosa (Gutmann, 1993; Jastak & Yagiela, 1983; Witherspoon & Gutmann, 1996). Effects of higher epinephrine concentrations on the cardiovascular system were raised as a concern (Jastak & Yagiela, 1983; Troullos et al., 1987), as the plasma level of epinephrine is elevated when a high dose is administered. Epinephrine binds to adrenergic receptors located on the vascular smooth muscles. The predominant receptors in the oral tissues are α-receptors. Thus, epinephrine predominant effect in the oral mucosa, submucosa and periodontium is vasoconstriction (Kim & Kratchman, 2006). Therefore, the application of local anaesthetics containing epinephrine 1:50 000 should be a constraint to injections 1–2 teeth mesial and distal from the tooth receiving surgery into the buccal or palatal submucosa using an aspirating syringe. Higher epinephrine concentrations are unnecessary for an infra-alveolar nerve block since there is no contribution to the surgical haemostasis in the operating field. Unless a direct injection into a blood vessel takes place, potential cardiovascular effects should be short-lived and minimal and well-tolerated by the majority of patients. For patients with a history of cardiovascular disorders or surgery, higher epinephrine concentrations may be contraindicated, and a consultation with the patient's physician will be necessary (Besner, 1972; Knoll-Kohler et al., 1989; Yagiela, 1995).
Flap designs—soft tissue management
With the exception of palatal roots of maxillary molars, surgical access for endodontic microsurgery is generally from the buccal, which presents with three tissue types, alveolar mucosa, the attached gingiva and the marginal gingiva. The alveolar mucosa is a thin, nonkeratinized mucosal layer loosely attached to the underlying bone. The attached gingiva is the portion of gingiva extending from the base of the gingival crevice to the mucogingival junction, firmly connected to the underlying bone and cementum and therefore immovable. The marginal gingiva is the crest of the free gingiva surrounding the tooth, forming the soft tissue portion of the gingival sulcus. Any surgical manipulation must be carefully undertaken to minimize any potential scarring. Proper flap design and tissue elevation must allow for appropriate surgical access to the underlining bone, root structure and pathosis and facilitate uncomplicated and scar-free soft tissue healing (Mitsis, 1970). Many flap designs have been introduced for endodontic surgery over the years (Velvart & Peters, 2005; Velvart et al., 2005a, 2005b; von Arx & Salvi, 2008). The semilunar flap, involving a curved incision entirely situated in the mucosa (Brock, 1961), is now considered obsolete due to its limited access to larger periradicular defects, challenges with reapproximation, delayed secondary healing, increased postoperative swelling and pain, flap shrinkage, and the potential for more scarring compared to other flap designs because of a compromised blood supply to the flap (Kramper et al., 1984). Similarly, a single vertical incision above the root, with lateral retraction to expose bone over the apex (Buckley, 1911; Weaver, 1949), is not considered a viable option nowadays due to reduced access to larger lesions and an increased risk of postoperative infection, as the closed sutures will be directly on top of the osteotomy site. Contemporarily, the two most widely used flap designs are the intra-sulcular and submarginal incisions.
The intra-sulcular incision is a long-established flap design (Hofer, 1935). It is a full-thickness flap that aims at keeping the blood supply intact to allow for primary wound healing (Gutmann & Harrison, 1991; Figure 22). Variations are a triangular flap with a singular, and a rectangular flap with two vertical incisions. For many situations where a single tooth is undergoing surgery, a triangular design allows for sufficient access. A rectangular design may be needed to address a large periapical lesion or in case multiple teeth require intervention. Intra-sulcular flap designs demonstrated excellent reattachment, and minimal postoperative pain and swelling, as postoperative infection of the blood coagulum in the bony crypt is unlikely (Gutmann & Harrison, 1991). Disadvantages may include moderate gingival recession, which may be of concern in an aesthetic area, such as the maxillary anterior region, or when artificial crown margins are present (Kramper et al., 1984). Dental papilla damage may occur (Grung, 1973; Velvart et al., 2004), particularly when the soft tissues are poorly keratinized, the papilla is very thin, or by iatrogenic errors. Preservation of the papilla with this flap design is most predictable, when the vertical incision joins the horizontal incision lateral to the papilla at the cervical margin at a 90° angle, which provides the best blood supply. The intra-sulcular incision may not be well suited for patients with a high smile line or a thin-scalloped periodontal biotype. The papilla base incision is a variation of the intra-sulcular incision that aims at avoiding papilla recession by placing the intra-sulcular component of the incision only in the buccal–cervical aspect of the tooth, but leaving the papilla intact by continuing the incision line through the base of the papilla without detaching it with the flap (Velvart, 2002; Figure 23). Significantly less recession of the papilla may occur if this incision is used (Velvart et al., 2004).
The submarginal incision is a flap design, where, in contrast to the intra-sulcular incision, the free gingiva surrounding the teeth will not be detached (Figure 24). This flap design was introduced over a century ago (Neumann, 1926) and further propagated by Ochsenbein-Luebke (Lubow et al., 1984). The key element of the design is a horizontal, submarginal incision in the middle of the attached gingiva following the contour of the cervical margin. Both rectangular and triangular variations of the flap are available to the operator, with the same indications as outlined above. This flap design is ideally indicated in situations where the attached gingiva is wider, to leave gingival tissues undisturbed, such as in the maxillary anterior region, in the presence of artificial crown margins, or other aesthetically challenging situations (Grung, 1973). Situations where surgical access is limited, or a large periradicular lesion, particularly in the coronal–apical direction, is present, may be contraindications for this flap design. Whilst the submarginal incision provides good preadaptation and wound healing most of the time, postoperative scarring has been noted in circumstances where soft tissues had teared (Kramper et al., 1984) or the horizontal incision did not stay within the attached gingiva, but had been placed directly at the delineation of attached gingiva and mucosa (Figure 25).
Osteotomy
Periradicular lesions may be fully contained inside the bone or may have already penetrated a cortical plate. In the latter situation, simple curettage of the inflammatory tissues may be enough to expose and identify a root tip. If a root tip is still covered by bone, the practitioner will have to use the information gained from pre-surgical treatment planning, including clinical observation and the preoperative measurements derived from periapical radiographs or a CBCT. The operator must be conscious of the proximity of the surgical field to root apices of adjacent teeth, the infra-alveolar nerve, the mental foramen, the nasal cavity or the maxillary sinuses (Jang et al., 2017; Kuzmanovic et al., 2003; Oberli et al., 2007). Endodontic microsurgery is a minimally invasive procedure. An osteotomy site should be kept as small as possible whilst allowing for the removal of inflammatory tissues, adequate root resection, an inspection of the resected root surface, root-end preparation and root-end filling. Boyne et al. (1969) demonstrated differences in the healing of osseous defects in the anterior region depending on the size of the lesion. Lesions 5–8 mm in diameter healed with complete bone regeneration, whilst defects with a 9–12 mm cross section demonstrated herniations with fibrous tissue (Figure 26). Similarly, Hjorting-Hansen (1970) provided evidence that cavity spaces up to 5 mm could result in complete bone regeneration independent of the anatomical site. Radiographic observation also demonstrated a direct relationship between osteotomy size and the speed of healing—the smaller the osteotomy, the faster the healing (Rubinstein & Kim, 1999). An atraumatic minimized hard tissue approach must be supported by careful handling of the surrounding soft tissues and protection of sensitive adjacent structures.
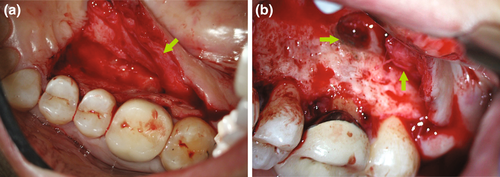
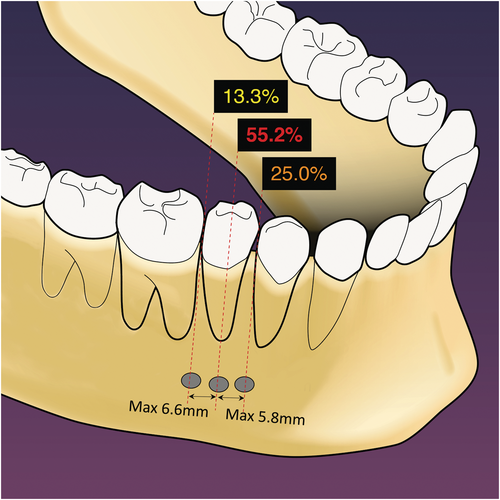
The mental and inferior alveolar neurovascular bundles are frequently encountered in the proximity of a surgical site. Radiographic assessment and CBCT imaging, together with careful treatment planning, are essential for endodontic surgery in the mandibular molar and premolar area (Imada et al., 2014; Moiseiwitsch, 1995; Shibli et al., 2010; von Arx et al., 2013). In a majority of situations, the mental foramen is located near the apex of the second mandibular premolar and less often near the apices of the first molar or the first premolar. The distance of the mental foramen to the closest root ranged from 0.3 to 9.8 mm (Aminoshariae et al., 2014; Figure 27). In more rare instances, its location was shown to be at the same vertical height or coronal to the apices of adjacent teeth or to present with a secondary foramen (Abella et al., 2014; Oberli et al., 2007). The mandibular canal is located inferior and lingual to the root apices of mandibular molars. The canal is usually at a safe distance from the root tips of the first molars. However, due to its close proximity to apices of second molars, together with their posterior location and the thickness of the bone in the area of the ramus, it typically prohibits conventional microsurgical approaches to these teeth.
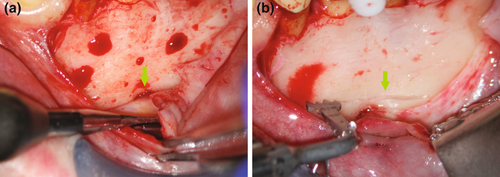
Protection of the mental foramen during mandibular posterior surgery mandates a specific protocol, including an intra-sulcular incision for a better overview, a vertical incision mesial of the first premolar for surgical procedures of both second premolar and first molar, and the placement of a bony groove for safe anchorage of the retractor. This bony groove should not exceed 1.5 mm in width and should only be cut deep enough to provide safe support for a surgical retractor. When correctly applied by either a surgical bur or a piezoelectric device, slippage of a retractor or accidental trauma with a surgical instrument is less likely, and the risks of transient or permanent trauma to the mental nerve are greatly reduced (Abella et al., 2014; Figure 28).
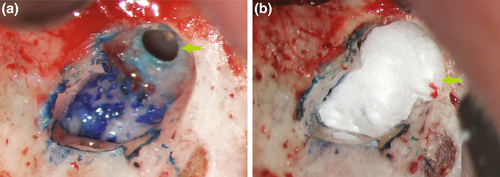
The maxillary sinus may not be only close to the surgical site, but it may also be exposed if a periapical lesion reaches or perforates the membrane. An opening to the oral cavity may occur by excision of the inflammatory tissues (Hauman et al., 2002). Precaution needs to be taken if a sinus perforation is likely to happen or if it has already occurred. No tissue remnants or foreign body materials must be displaced into the sinus cavity. The manner of how to resect the root tip was debated, in particular if it is advisable to carefully reduce a root tip (Lemon et al., 1993) rather than to cut it whilst securing the apex (Guess & Kratchman, 2017). On the one hand, the operator must avoid the displacement of dentinal shavings into the sinus (Figure 29). On the other hand, a root tip might be at risk of displacement as a whole. A sterile cotton pellet secured by a suture can be placed temporarily into the sinus opening to block any shavings or foreign body materials from entering the sinus (Kim & Kratchman, 2006; Figure 30). Watzek et al. (1997) demonstrated that the outcome of endodontic surgery would not be compromised by a sinus opening itself, as the repositioned flap will provide protection after the surgical procedure. Larger sinus openings may require a bone grafting material such as collagen or a membrane covering the buccal defect. Antibiotic coverage may be advised but is not necessary for all patients. However, a decongestant may prevent any pressure issues from within the sinus to disturb wound healing.
The palatine neurovascular bundle may be at risk during palatal surgery, which is largely limited to first molars. Maxillary premolars are treated via a buccal approach. Conventional, nontargeted endodontic microsurgery from palatal is no option for second molars in almost all situations due to the anatomical risks. The greater palatine foramen is located approximately 3–4 mm anterior to the posterior border of the hard palate, with nerves and blood vessels projecting in an anterior direction in the submucosa approximately halfway between the midline of the palate and the gingival margin. Palatal soft tissue elevation to approach the palatal root of a first molar may include the neurovascular bundle within the flap (Figure 11). However, any posterior attempt on a second molar may involve the foramen with danger to the bundle and may greatly increase the risk of severe haemorrhage or nerve injury. The vertical releasing incision for a palatal flap should be placed between the canine and the first premolar (Gutmann & Harrison, 1985, 1991). Transantral approaches to the palatal roots have been described (Altonen, 1975; Walton & Wallace, 1996) but are nowadays limited to root fusions of distobuccal and palatal roots to allow for adequate root-end preparation and root-end filling (Figure 31). For individual palatal roots, a transantral approach may be greatly limited by the distance from the buccal wall to the palatal apex and may pose additional risks of leaving dentinal shavings or foreign body materials in the sinus.
Apical curettage
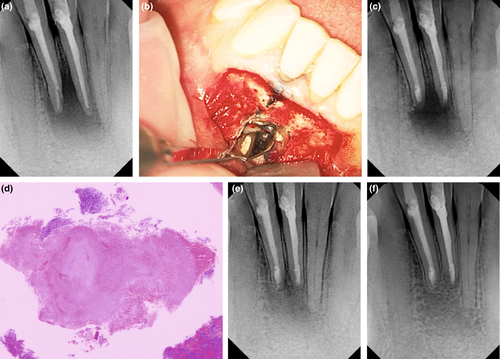
The removal of the inflammatory tissues of the apical periodontitis around the root tip is an integral part of root-end surgery. Limited indications exist for apical curettage as a self-sufficient procedure without root resection or root-end filling, including exploratory procedures for the identification of vertical root fractures or other situations when a tooth is declared nonrestorable. Teeth that undergo a surgical procedure should receive treatment that addresses both potential intra-radicular and extra-radicular causes for failure. Therefore, root-end resection and root-end filling must be part of the procedure to maximize surgical prognosis. Whilst granulomas or abscesses are expected to heal after the aetiology of a failed root canal treatment has been addressed, it cannot be determined during surgery if an extra-radicular infection is present. Thus, the entire periradicular lesion should be removed (Brock, 1961; Lin et al., 2010; Nichols, 1965; Rud & Andreasen, 1972) to include any epithelial remnants that might continue the proliferation of a cystic lesion or any extra-radicular infection. The concept of selective curettage has been recently introduced as a conservative approach to treating large and complicated lesions by removing 50%–70% of the soft tissue lesion to avoid iatrogenic damage to neighbouring structures and successfully demonstrated in a case series (Nesari et al., 2020).
Root-end resection
After removal of the inflammatory tissues from the periradicular area, the root tip should be identifiable. In comparison with the white colour of the bone, which is soft and bleeds upon scraping, a root has a darker, yellowish colour and is hard (Kim et al., 2001). The root-end resection will address intra-radicular infection that may be contained in the anatomical complexities of the apex, including apical ramifications, accessory canals or severe apical curvatures; iatrogenic mishaps that prevented biomechanical disinfection of the root canal system by means of nonsurgical retreatment, such as perforations, ledges, transportations or foreign body materials; apical root fractures or cracks; or apical resorptions that prevented an adequate seal during the conventional procedure. The root-end resection will also aid in removing other aetiological factors from the tissues, such as foreign body materials attached to the tooth or extra-radicular infection in the form of a biofilm on the root surface. The resection of the apex should be performed with a Lindemann bone-cutting bur (Figure 17), a fissure bur or a piezoelectric tip to allow for a smooth resection that permits the inspection of the anatomy on the resected root surface, thus allowing for the identification of potential reasons for the failure of the previous treatment.
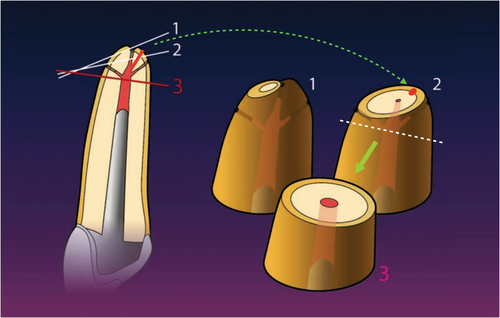
The literature does not present a uniform opinion on how much of the root should be resected. However, there is consensus that resection to the base of the lesion, which had been advocated earlier (Buckley, 1914), is nowadays regarded as unnecessary (Gutmann & Harrison, 1985, 1991). The preservation of as much buccal bone as possible is advised. Gilheany et al. (1994) proposed to resect at least 2 mm to minimize bacterial leakage from the canals. An anatomical study identified that the apical 3 mm of a root tip contained 98% of apical ramifications and 93% of lateral canals, suggesting to use this value as a clinical guideline for resection (Kim & Kratchman, 2006). Nevertheless, the actual amount of resection may vary from case to case. The proximity of anatomical structures such as the mental nerve may necessitate resection of a longer apical portion of the root to minimize the risk of damaging the nerve structure. Cracks or fracture lines must be eliminated completely, if necessary, exceeding the 3-mm guideline. On the contrary, if there is a limited amount of root structure available apical of a post, the tooth presents with significant resorptions, or if there is a history of previous surgical intervention, the amount of resection may be less.
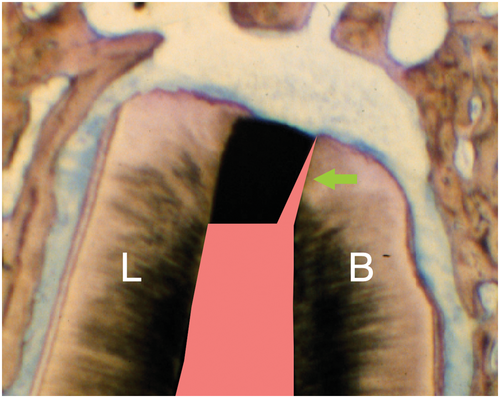
Current recommendations suggest resecting the root at a shallow 0–10° bevel rather than a traditional steep 45 ° bevel (Carr, 1994; Kim & Kratchman, 2006; Rud & Andreasen, 1972; Figure 32). The more perpendicular a resection angle is to the long axis of the root, the more apical root structure may be preserved, the chance of identifying lingual and accessory canals increases, guarantees a complete root resection, exposes fewer dentinal tubules that may harbour intra-radicular infection (Chong et al., 1997; Gilheany et al., 1994; Gutmann et al., 1994; Tidmarsh & Arrowsmith, 1989; Figure 33), and allows for an easier coaxial root-end preparation with ultrasonic tools. Intra-radicular infection may reach the periradicular tissues primarily via the apical foramen and accessory and lateral canals as the major physiological pathways. Whilst bacteria are capable of propagating into dentinal tubules, an intact cementum layer will prevent them from reaching the periodontal tissues. The exposure of microorganisms to the periradicular tissues via dentinal tubules opened by root resection was discussed as a potential source of infection of the surrounding tissues. However, a correlation between the presence of bacteria in dentinal tubules and the degree of periradicular inflammation has not been demonstrated (Rud & Andreasen, 1972). Nevertheless, a shallow resection angle will minimize the number of open dentinal tubules after resection.
Root-end inspection
The inspection of the resected root surface is considered one of the most important steps during endodontic microsurgery (Kim & Kratchman, 2006). Complete haemostasis should be achieved before this procedural step. After rinsing the osteotomy site with saline, areas that still exhibit bleeding may be controlled by using cotton pellets soaked with epinephrine or other haemostatic agents. Ferric sulphate and aluminium chloride are likely the most commonly used today. Bone wax has been described as an effective haemostat in endodontic surgery (Selden, 1970), but it only exhibits a tamponade effect by closing vascular openings in the bone and has no effect on the blood clotting mechanism. In animal studies, bone wax has been shown to produce consistent inflammatory reactions (Ibarrola et al., 1985).
Cotton pellets containing racemic epinephrine hydrochloride (Racellets, Pascal Co.) work both as a mechanical and as a chemical haemostyptic agent. The amount of racemic epinephrine varies with the size of the pellets and ranges between 0.55 and 1.15 mg on average. The topical use of epinephrine causes immediate local vasoconstriction, with very limited absorption into the systemic circulation. The pulse rate of patients did not change with the application of racemic epinephrine in the bony crypt (Besner, 1972). An epinephrine pellet can be placed after all inflammatory tissues are removed. The application of backpressure with additional sterile cotton pellets and the backside of a mirror or college pliers in combination with the epinephrine release has an immediate and profound effect on vasoconstriction (Figure 34). It must be ensured that all cotton pellets are removed before wound closure.
Ferric sulphate (Stasis 21%; Midway Dental Supply Co.) is a chemical agent that achieves haemostasis by agglutination of blood proteins that occlude the capillary orifices after a reaction of the blood with both ferric and sulphate ions under the acidic condition (pH 0.21) of the solution (Evans, 1977). The use of ferric sulphate results in an immediate chemical reaction and does not require the application of backpressure (Figure 35). However, ferric sulphate is cytotoxic and may cause tissue necrosis if left behind in the osteotomy site. It may have significant adverse effects on osseous healing (Lemon et al., 1993) and must be thoroughly flushed out with saline to avoid impaired healing.

Aluminium chloride (Expasyl, Acteon) is a haemostatic agent with a working mechanism similar to ferric sulphate. It has been compared to a variety of other means to establish surgical haemostasis (Menendez-Nieto et al., 2016; Penarrocha-Oltra, Menendez-Nieto, et al., 2019; Penarrocha-Oltra, Soto-Penaloza, et al., 2019; Peñarrocha-Oltra et al., 2022). A systematic review and meta-analysis by Khater et al. (2021) found aluminium chloride to rank first in bleeding control during endodontic surgery, albeit with a low quality of evidence. From a clinical perspective, the paste-like consistency causes it to adhere well to the osteotomy site and may require bony curettage for effective removal in addition to saline irrigation for removal. By the end of the surgical procedure, all haemostatic agents must be removed to clear away any toxic components that might have adverse effects on healing (Haasch et al., 1989; Ibarrola et al., 1985; Jeansonne et al., 1993; Lemon et al., 1993) and allow the osteotomy site to fill in with blood.
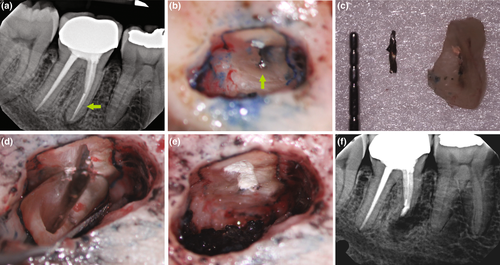
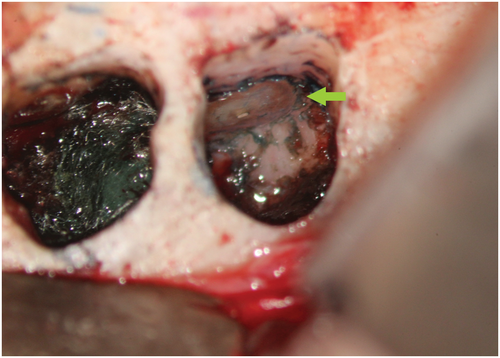
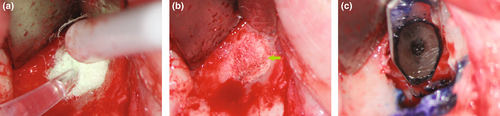
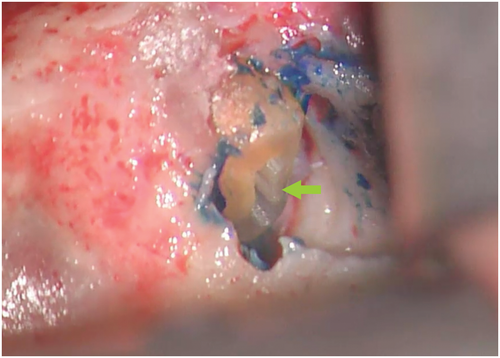
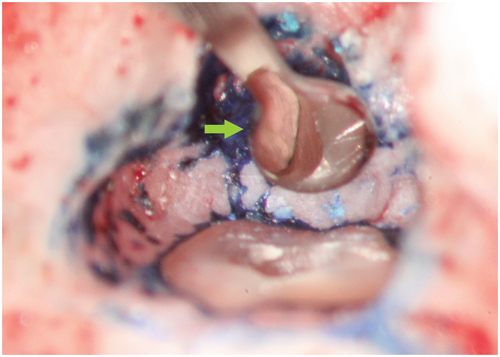
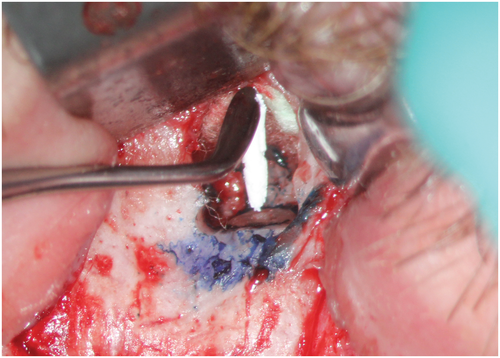
Methylene blue (Vista Apex Dental Products), a thiazine dye, is used to stain the resected root surface (Kim & Kratchman, 2006; Figure 36a) after drying it with a small irrigator, such as the Stropko device (Stropko et al., 2005; Stropko irrigator; Vista Apex Dental Products; Figure 36b). The dye will stain the circumference of the periodontal ligament to verify complete resection and to detect missed canals (Figure 36c), microfractures (Figure 36d), isthmuses (Figure 36e), other anatomical details, aberrations or iatrogenic errors (Figure 36e). The inspection of the resected root surface must be carried out at high magnification (16×–24×; Kim & Kratchman, 2006; Von Arx et al., 2011). Canal isthmuses are narrow, ribbon-shaped communications between two root canals that contain pulpal tissues (Weller et al., 1995). An isthmus is a part of the canal system and not a separate entity. It must be cleaned, shaped and filled as thoroughly as other parts of the root canal system. At a 3-mm resection level from the original apex, isthmuses were found at 90% of the mesiobuccal roots of maxillary first molars, 30% of the maxillary and mandibular premolars, and over 80% of the mesial roots of the mandibular first molars (Hsu & Kim, 1997; Weller et al., 1995). An evaluation of unsuccessful cases identified mismanagement or failure to manage isthmus spaces as the main cause of failure in mesial roots of molars (Hsu & Kim, 1997).
Root-end preparation
Root-end preparation aims at disinfecting portions of a root canal system that had been left untreated by earlier endodontic therapy, including previously non-negotiated canals and cases where the quality of the existing root filling is unsatisfactory, either due to insufficiency of the sealer materials to fill the spaces between the core filling material and the root canal wall or due to anastomoses between canals. Even fine isthmuses connecting main canals should be cleaned during root-end preparation (Mannocci et al., 2005; Figure 37). A root-end preparation will also greatly diminish bacteria inside dentinal tubules, as the majority of bacteria in the apical third of a root are located in the dentine immediately adjacent to the root canal (Jolly & Sullivan, 1956; Shovelton, 1964). A root-end preparation is a Class 1 cavity with walls parallel to and coincident with the anatomy of the root canal space (Carr, 1994).
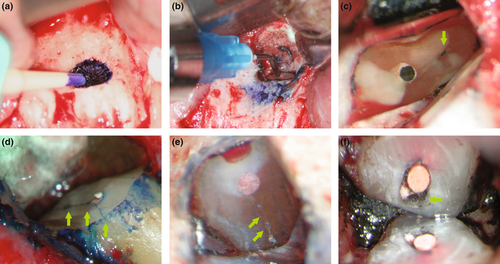
Root-end preparation as part of endodontic microsurgery is carried out using ultrasonic tips (Sultan & Pitt Ford, 1995; Figure 38). It is important to orient the ultrasonic tip in the direction of the long axis of the root at low magnification (4×–8×; Figure 39). Tip variations with different angulations exist for different sections of the jaw. Whilst ultrasonic tools had been under suspicion to create cracks in the dentine after root-end preparation (Frank et al., 1996; Layton et al., 1996), these concerns do not seem to be relevant to the clinical outcome (Beling et al., 1997). A practitioner should choose tip sizes and angulations dependent on the original shape and size of the root canal (Figure 38). Before placing a root-end filling, the retrograde cavity must be dried using the micro-irrigator and inspected at high magnification using a micro-mirror to verify that all filling materials in the cavity were removed completely (Stropko et al., 2005; Figure 40). Filling remnants are often still detected in the buccal aspect of the cavity, as this portion of the root canal wall cannot be directly observed during ultrasonic preparation (Figure 41).

Root-end filling
An ideal root-end filling material has to be biocompatible and demonstrate excellent sealing ability. Bactericidal or bacteriostatic properties would also be preferential, considering the overall purpose of treatment. A root-end filling material should also show good adhesion to the root canal wall, dimensional stability, noncorrosiveness, resistance to dissolution and ease of manipulation with an adequate working time. It should not stain teeth or tissues, exhibit osteo- and cementogenic properties, and be radiopaque. Over time, a wide range of root-end filling materials have been introduced, investigated and reviewed (Chong & Pitt Ford, 2005; Johnson, 1999. Common materials that have been investigated in clinical studies include amalgam (Del Fabbro et al., 2007; Harty et al., 1970), gutta-percha (Frank et al., 1996; Persson, 1964), zinc oxide/calcium sulphate types of cement (Cavit; Finne et al., 1977; Nord, 1970; Persson et al., 1974), polyvinyl resin (Diaket; Tetsch, 1986), glass–ionomer cement (Bronkhorst et al., 2008), composite resin (Geristore, RetroPlast; Al-Sabek et al., 2005), zinc oxide/eugenol cement (IRM, SuperEBA) and calcium silicate types of cement (mineral trioxide aggregate (MTA), biodentine and bioceramic root repair material (BC, RRM)). With the exception of amalgam, which was included due to its historical importance and because it may still be used (Bronkhorst et al., 2008), only the most contemporary root-end filling materials are discussed below.
Amalgam was found to be the most popular and widely used root-end filling material (Block & Bushell, 1982; Friedman, 1991; Von Hippel, 1914). It has been criticized for lack of biocompatibility, corrosion, a risk of crack formation in the root apex, hard tissue staining and soft tissue tattoos due to silver salts (Harrison & Johnson, 1997), and poor prognosis (Allen et al., 1989; Dorn & Gartner, 1990; Finne et al., 1977; Jesslén et al., 1995; Pantschev et al., 1994; Setzer et al., 2010). The leakage of amalgam was found using dye penetration tests (Friedman, 1991; Olson et al., 1990) or the fluid filtration model (Spangberg et al., 1989; Wu et al., 1990). Critics pointed out that these were not in vivo conditions (Peters & Harrison, 1992; Roda & Gutmann, 1995). However, amalgam also demonstrated bacterial leakage (Chong et al., 1995; Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995), consistently allowing for greater leakage than any other material, independent of the methods (Chong et al., 1995). Therefore, amalgam is likely to leak as a root-end filling material as well (Andreasen & Rud, 1972; Chong et al., 1995; Gutmann & Harrison, 1991). Amalgam's lack of biocompatibility stems from the mercury content in the metal alloy mixture. Mercury is an environmental hazard and has great scrutiny attached to its use as a restorative filling material (Eley & Cox, 1993). Histologically, amalgam and its corrosion products repeatedly demonstrated unfavourable inflammatory tissue responses (Baek et al., 2005, Chong et al., 1997), with traces of amalgam still detectable from the root end (Peters & Harrison, 1992). Amongst all materials compared, amalgam showed the most severe and extensive periapical tissue inflammation (Chong et al., 1997; Peters & Harrison, 1992; Pitt Ford et al., 1995a, 1995b; Pitt Ford, Torabinejad, et al., 1995; Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995; Torabinejad et al., 1997).
Composite resins exhibited good sealing abilities as a root-end filling material in vitro (Adamo et al., 1999; McDonald & Dumsha, 1987, 1990). Geristore (Den-Mat), a dual-curing hydrophilic modified composite resin, has been used for conventional root-end filling and the repair of subgingival or subosseous defects and as a barrier material for guided tissue regeneration (GTR). A resin-based technique to seal the resected root apex was first introduced by Rud et al. (1991) and more recently used by von Arx et al. (2010). The protocol requires the preparation of a concave cavity over the entire resected root surface by a round bur, which is then etched with EDTA, and filled over with a bonded resin material in a domelike fashion. The material most frequently used with this technique is RetroPlast (RetroPlast; RetroPlast Trading), a dentine-bonded dual-curing composite resin (Rud et al., 1991; von Arx et al., 2010). Although being incorporated into a microsurgical approach, this technique differs significantly from other approaches, as no conventional root-end cavity is prepared, and no conventional root-end filling is placed.
Whilst this resin-based technique has produced favourable results compared to traditional root-end surgery techniques (Jensen et al., 2002;Rud et al., 1996a, 1996b), it has demonstrated a lower weighted pooled success rate compared to contemporary techniques (Kohli et al., 2018). Most practitioners may limit its indications to situations where a root-end cavity cannot be prepared with ultrasonic tips, for example if a post reaches close to the end of a root. The technique requires moisture control beyond the haemostasis provided with regular bleeding control during surgery to allow for favourable dentine bonding to the resected root surface (Rud et al., 1996a, 1996b), which may be the major disadvantage of the procedure.
Zinc oxide eugenol (ZOE) cement was recommended and used in root-end surgery for many decades (Garcia, 1937; Nichols, 1965). The two most widely accepted materials are intermediate restorative material (IRM; Dentsply Caulk), a ZOE cement reinforced by the addition of polymethacrylate to the powder, and superethoxybenzoic acid (SuperEBA; Harry J Bosworth Company), modified by the partial substitution of eugenol liquid for orthoethoxybenzoic acid with the addition of fused quartz or aluminium oxide (alumina) to the powder. Both materials demonstrated significantly better outcomes than amalgam (Dorn & Gartner, 1990; Rubinstein & Kim, 1999, 2002). Histologically, they are more biocompatible than unmodified ZOE, although some inflammatory cells on the root surface were still observed (Peters & Harrison, 1992; Pitt Ford et al., 1992; Pitt Ford et al., 1995a, 1995b; Pitt Ford, Torabinejad, et al., 1995). Both IRM and SuperEBA display low solubility (Chong et al., 1994), good antibacterial action (Chong et al., 1994; Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995) and little leakage in dye penetration tests (Chong et al., 1994; O'Connor et al., 1995). SuperEBA demonstrated significantly less leakage and better root wall adaptation compared to amalgam. Clinical studies that used either IRM or SuperEBA as root-end filling materials, in combination with ultrasonic root-end preparation and the use of high magnification, qualify for inclusion in a meta-analysis evaluating the outcome of endodontic microsurgery (Kohli et al., 2018; Setzer et al., 2010, 2012).
Calcium silicate cements are dental materials that were originally derived from Portland cement, a silica, alumina and calcium compound construction material. To date, a variety of dental filling and repair materials have been developed, including mineral trioxide aggregate (MTA; Dentsply Sirona), Biodentine (Septodont USA) or Bioceramic root repair material (BC, RRM; Figure 42). All calcium silicate cements are hydrophilic, but there are differences in setting time and methods of preparation. Calcium silicate cements offer significant improvements compared with ZOE cements, including reduced cytotoxicity (Bortoluzzi et al., 2015; Collado-González et al., 2017), increased biocompatibility (Gilheany et al., 1994; Mestieri et al., 2015), increased cell attachment (Collado-González et al., 2017), cemento- and osteoinductive properties (Chen et al., 2016; Gilheany et al., 1994), and increased pH values (Grech et al., 2013). MTA was the first calcium silicate cement to be introduced in Endodontics (Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995) and has been comprehensively investigated. Histologically, MTA demonstrated considerably less inflammation around root-end fillings in in vivo studies (Baek et al., 2005; Regan et al., 2002; Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995; Torabinejad et al., 1997). New cementum and periodontal ligament structure have frequently been shown to form over a resected root surface and the root-end filling itself (Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995; Torabinejad et al., 1997). The formation of a hydroxyapatite (HA) layer on the MTA surface in contact with tissue fluid during the setting of the material, described as ‘biomineralization’, has been observed (Bird et al., 2012). This layer may create a biological seal between MTA and the dentine interface and may enhance its long-term sealing ability. Disadvantages of MTA include handling properties and possible tooth discoloration, for both its grey and white formulations (Kohli et al., 2015). Freshly mixed MTA has the consistency of a moist, granular paste, and it may be difficult to place into a root-end cavity. It may also wash out in the presence of excessive bleeding or other tissue fluids, which may compromise its sealing ability.
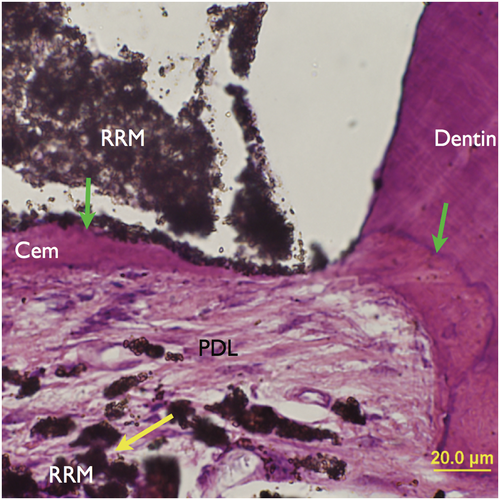
Newer versions of calcium silicate cements have been introduced, including Biodentine, a capsule-based material that has to be mixed in a triturator, and the premixed BC or RRM, which only differ in particle size. These materials alleviated some of the concerns that had arisen with MTA. Both Biodentine and RRM exhibit less tooth discoloration than MTA (Kohli et al., 2015). RRM has been more widely applied as a root-end filling material than Biodentine, which is more often used for perforation repair. RRM is dimensionally stable, demonstrates a high pH and is as short as 2–4 h of setting time. RRM and MTA are not significantly different in their antimicrobial efficacy (Lovato & Sedgley, 2011), biocompatibility (Alanezi et al., 2010) and sealing ability (Nair et al., 2011). An in vivo study comparing RRM and MTA as root-end filling materials (Chen et al., 2015) demonstrated no or only minimal inflammation at the surgical site upon healing, with cementum-like tissues observed adjacent to RRM, comparable to previous findings for MTA (Figure 43). Two randomized controlled trials investigating endodontic microsurgery have shown highly favourable outcomes (Shinbori et al., 2015; Zhou et al., 2017).
Guided tissue regeneration (GTR)
After completing a surgical procedure, the osteotomy site must be inspected and cleaned of any excess materials and haemostatic agents to avoid foreign body reactions and to remove any toxic by-products. Blood should fill back into the osteotomy site so that the formation of a blood clot and subsequent osseous healing can take place. Grafting materials or membranes should be placed at this stage of the procedure. Whilst extensively used in periodontology and implant dentistry, the use of GTR techniques is far less common in endodontics (Tsesis et al., 2011), and the scientific evidence for the benefit of its use was considered low (Corbella et al., 2015). Membranes to separate bone healing from connective tissue healing were introduced for dentoalveolar applications in the 1960s (Boyne, 1969). Membranes for endodontic surgery were investigated in animal studies (Dahlin et al., 1990; Maguire et al., 1998) and in patients (Pecora et al., 1995). Common materials for GTR include polytetrafluoroethylene (ePTFE, Gore-Tex), collagen or polylactide for membranes; and freeze-dried bone allografts, demineralized freeze-dried bone allografts, HA, tricalcium phosphate, bioglass or calcium sulphate for grafts. GTR prevents epithelial ingrowth into lesions and therefore allows bone regeneration in comparison with control lesions without the use of barriers (Baek & Kim, 2001; Villar & Cochran, 2010).
GTR for endodontic surgery was subject to review (Corbella et al., 2015; Deng et al., 2016; Lin et al., 2010; Sumangali et al., 2021; Tsesis et al., 2011; Zubizarreta-Macho et al., 2022). For the application of GTR in endodontic surgery, a distinction can be made between uncomplicated defects, complicated defects and periodontally involved defects. Uncomplicated defects are true endodontic lesions without any periodontal component, such as deep probings on the tooth or a connection between concomitant periodontal and endodontic defects. For uncomplicated defects, the healing rate with or without membrane placement did not demonstrate any differences (Maguire et al., 1998), similarly for the placement of a membrane combined with graft at a 1-year recall (Taschieri et al., 2007). Evaluated by CT imaging, periapical bone healing and bone density after 6 months of follow-up did not exhibit differences (Santamaria et al., 1998). Complicated defects are endodontic lesions with a defect size exceeding 10 mm in diameter, ‘through-and-through’ lesions with a bucco-lingual defect, and/or perforation to the nasal cavity or a large maxillary sinus perforation.
Complicated defects may benefit from GTR. Membrane placement on both the buccal and lingual aspects of ‘through-and-through’ rat calvaria defects showed complete healing versus no healing without membranes in the control group (Bohning et al., 1999). In similar defects, complete healing after endodontic surgery could be seen when a barrier was used versus controls that showed only fibrous connective tissue (Dahlin et al., 1988; Dahlin et al., 1990). Azim et al. (2020) confirmed that the quality of apical bone remodelling after endodontic surgery was improved with GTR techniques. In patients, healing of through-and-through lesions was observed in 88% with GTR placement versus 57% without (Taschieri et al., 2008). Taking these findings together indicates that for through-and-through lesions, both buccal and lingual barriers should be placed for effective healing (Bohning et al., 1999). Lesions exceeding 10 mm in diameter healed faster and with better outcomes when GTR was used versus without (Pecora et al., 1995; Pecora et al., 2001), including radiographic, clinical and histologic results (Pecora et al., 1995; Rankow & Krasner, 1996; Tobon et al., 2002). However, the healing of lesions measuring 10 mm or larger in diameter combined with a loss of both the buccal and lingual cortical plates remained unaffected by the placement of resorbable collagen membranes according to both two- and three-dimensional evaluation (Parmar et al., 2019).
A systematic appraisal of the literature demonstrated some general improvement in surgical endodontic outcomes by GTR techniques. Sumangali et al. (2021) conducted a systematic review and meta-analysis evaluating various bone regenerative materials for periradicular surgery. Their meta-analysis showed higher success rates when bone graft materials were used in conjunction with barrier materials. These findings concurred with the meta-analysis by Deng et al. (2016), who confirmed the increased effectiveness of combinations of membranes and bone replacement analogues. However, the same study also concluded that the singular use of bone replacement materials improved the surgical outcome for endodontic surgery, which was not confirmed by a recent network meta-analysis for bone graft materials alone (Zubizarreta-Macho et al., 2022). Zubizarreta-Macho et al. (2022) appraised the clinical evidence on the efficacy of GTR techniques and included 11 randomized clinical trials comparing six GRT techniques, including bone graft, membrane, membrane plus bone graft, platelet-rich plasma or membrane plus platelet-rich plasma. Both the membrane and the membrane plus bone graft techniques demonstrated statistically significant odds ratios compared to procedures conducted without GTR.
Periodontally involved defects may display apico-marginal or perio-endo communication defects, bone loss in the furcation, or a loss of the buccal plate due to dehiscence or completely denuded root. The use of GTR techniques for chronic periodontic-endodontic lesions was evaluated by Britain et al. (2005). da Silva Pereira (2000) suggested that the combined use of a membrane and graft may significantly increase cementum deposition in situations with complete buccal plate loss. The overall success rate for endodontic surgery is significantly decreased for periodontally involved defects compared to endodontic lesions alone (Kim et al., 2008; Song, Shin, et al., 2011; Song, Jung, et al., 2011).
Wound closure and postoperative care
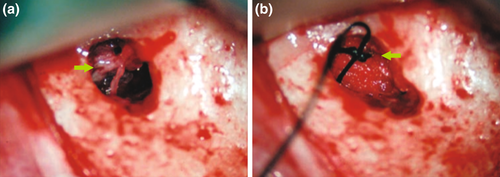
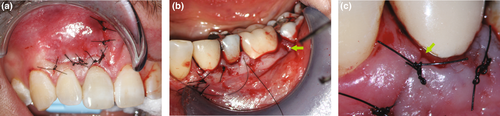
After an osteotomy site has been cleaned and any appropriate grafts or membranes have been placed, the surgical flap can be repositioned and sutured. Wound closure and postoperative care will have a great effect on the biologic and aesthetic healing process. The soft tissue should be moistened with a wet gauze containing saline before the repositioning of the flap to ensure that dehydration during the surgery will not have a negative effect on the natural elasticity of the tissues. Sutures will be necessary to replace the mucoperiosteal flap in its original position. A microscope or loupes should be used for smaller suture diameters. Today, common sutures for endodontic microsurgery are monofilament sutures that range in diameter between 5–0 and 6–0 for standard interventions and between 6–0 and 7–0 for aesthetically demanding areas, such as the anterior maxilla or when a papilla base incision was performed. Traditional silk sutures may promote bacterial growth and impede wound healing. Nylon, polypropylene, or polytetrafluoroethylene (PTFE) monofilament or coated monofilament sutures have become the material of choice for endodontic microsurgery (Velvart et al., 2005a, 2005b). Contact to the underlying bone is needed to establish stable conditions, minimize the thickness of the subperiosteal blood clot and allow for healing by primary intention. Whilst resorbable sutures, such as gut, may reduce the number of office visits, an inflammatory component is added to the wound healing process by resorption of the suture. A suture removal appointment allows the practitioner to check on the patient 48 h (Gutmann & Harrison, 1991) to 4 days (Gutmann & Harrison, 1985; Velvart & Peters, 2005) after the procedure. If sutures remain too long, some flap margins may be compromised by infection (Grung, 1973; Gutmann & Harrison, 1991). Needle size, shape and curvature are important, but its selection may depend on the periodontal biotype, the ability to access the area and the preference of the clinician (Velvart et al., 2005a, 2005b). Single interrupted sutures allow for a better readaptation than continuous sutures (Figure 44a,b). A sling suture is useful for interproximal readaptation in the posterior (Figure 44c).
Instructions for a patient include avoiding exercise for 1–2 days and not pulling up their lips or cheeks to prevent any unnecessary disturbance of the flap, which could result in bleeding or open up flap margins. The underlying blood clot must be protected from any postoperative risk of infection, which may be associated with pain and swelling, or delayed healing. The patient should not smoke for as long as possible and choose food that can easily be cleaned away from the surgical site. Intermittent cooling with an ice pack will prevent excessive swelling (Gutmann, 2005; Gutmann & Harrison, 1991).
Patients need to be informed that besides swelling and pain, temporary discoloration and bruising may occur in a small number of patients. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first choice as analgesics if the patient can tolerate this type of drug. Antibiotic coverage is not advised on a general basis (Gutmann, 2005) but may be needed based on the patient's medical or dental history or, for example, a large opening of the maxillary sinus during the procedure. A chlorhexidine rinse after the first day will allow for better wound healing, as it reduces bacterial content in the oral cavity and therefore reduces the risks of postoperative infection. The patient should receive contact information for after-hour emergencies and instructions on when they should return for follow-up. It is advisable to have the patient return after 4 weeks for a follow-up to check soft tissue healing and the absence of any clinical symptoms. Clinical and radiographic follow-ups should take place after 1, 2 and 4 years to observe the healing process and ensure that long-term success is guaranteed.
Modes of healing
During the healing phase of the periapical tissues, stem and progenitor cells are recruited from the bone marrow, endosteum, periosteum and the periodontal ligament (PDL) to differentiate into osteoblasts fibroblasts and cementoblasts. An excisional wound after surgery will heal faster than a granuloma or cyst after nonsurgical treatment, as the inflamed tissues must first be broken down by phagocytic debridement (Lin et al., 2010). After endodontic surgery, the osteotomy site is filled with blood. The repositioned flap will protect the blood clot from microbes present within the oral cavity. Epithelial cells will proliferate to close surgical incision wound. Healing will occur as two separate processes below the soft tissues, osseous healing and dentoalveolar healing.
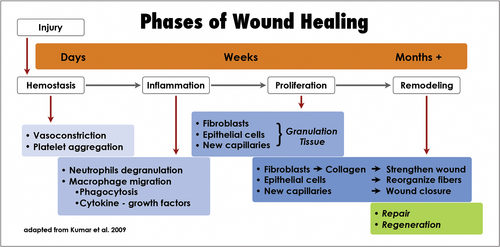
Osseous healing requires haemostasis by vasoconstriction and platelet aggregation (Lin et al., 2010). There are three basic phases of wound healing, which considerably overlap: inflammation, proliferation and remodelling (Kumar et al., 2009; Figure 45). Within these three phases, a complex and coordinated series of events occurs. The inflammatory phase includes chemotaxis and phagocytosis (Kumar et al., 2009). During the proliferation phase, neocollagenesis, epithelialization and angiogenesis result in the formation of a granulation tissue that originates from the PDL and the endosteum (Harrison & Jurosky, 1992; Kumar et al., 2009; Majno & Joris, 2004). In the remodelling phase, active collagen remodelling and tissue maturation take place, which may result either in repair or in regeneration (Kumar et al., 2009). For the osseous wound, this results in a sequence of revascularization of the initial blood clot and the formation of a mineralizing matrix, from which woven bone eventually matures into cancellous bone. New bone formation starts in the centre and progresses externally towards the level of the former cortical plate. When new woven bone approaches the lamina propria, the overlying membrane matures into functional periodontium.
During the dentoalveolar healing process, viable PDL cells from the adjacent root surface will proliferate and cover the denuded resected root (Lin et al., 2010). These PDL stem cells will differentiate into cementoblast-like cells and eventually regenerate cementum (Lin et al., 2010; Lin & Rosenberg, 2011). Root resorption that involves both cementum and dentine can only be repaired by cementoid tissues, as PDL stem cells can not differentiate into odontoblasts. In the absence of infection or severe inflammation, the cementum can grow back to cover the resected dentine surface (Andreasen, 1973, 1981; Harrison & Jurosky, 1992; Rowe, 1967). Both MTA and RRM allow cementum to apposition directly on the surface of the materials (Chen et al., 2015; Torabinejad, Hong, Ford, et al., 1995; Torabinejad, Hong, McDonald, et al., 1995; Torabinejad, Rastegar, et al., 1995; Torabinejad et al., 1997). Moreover, MTA may support the reestablishment of a PDL width comparable to its natural thickness, which was not observed for SuperEBA or amalgam (Baek et al., 2010).
Outcome of surgical endodontics
The most widely used criteria to assess the outcome of endodontic surgery were based on Rud and Molven (Molven et al., 1987; Rud et al., 1972). The authors defined radiographic success as complete healing or incomplete (scar tissue formation) healing and clinical success as the absence of pain, swelling, percussion sensitivity or a sinus tract. Radiographic failure includes uncertain healing (reduced lesion size) and unsatisfactory healing (unchanged or increased lesion size), whilst clinical failure means the presence of any of the symptoms described above. According to Molven et al. (1987), healing-related changes after surgery occur predominantly within the first year. Cases that were considered uncertain healing should be re-evaluated for up to 4 years.
If all historical techniques are included, surgical success may range from 37% to 91%, varying with the operator and the specific techniques (Friedman, 2005). Great variations in treatment protocol and methodology exist between different studies. Therefore, the interpretation of the outcome has to be undertaken cautiously. Variables include study design, sample sizes, inclusion and exclusion criteria, follow-up periods, lack of standardized clinical and radiographic parameters for healing, the periodontal condition of the teeth prior to surgery, variations in the quality of previous endodontic treatment, the coronal restoration, and the surgical materials and techniques itself. Moreover, many historical studies were case series or other studies with a low level of evidence (Mead et al., 2005).
The differences in outcome for endodontic surgery depending on the techniques used in individual studies have been documented by systematic reviews and meta-analyses. Traditional, now obsolete techniques using a straight surgical handpiece, a bevelled root resection and often a retrograde amalgam filling demonstrated a weighted pooled success rate of 59.0% (Setzer et al., 2010). The use of loupes, ultrasonic root-end preparation and more biocompatible filling materials increased cumulative success to 86% (Setzer et al., 2012). Endodontic microsurgery, using the same tools and techniques, only replacing loupes with a dental operating microscope allowing for high magnification, demonstrated even higher success rates ranging from 91.4% to 94.4% for true endodontic lesions (Kohli et al., 2018; Setzer et al., 2010; Torabinejad et al., 2015; Tsesis et al., 2013; Figure 46). Resin-based endodontic surgery, using a concave cavity and a dome-shaped composite filling, demonstrated a weighted pooled success rate of 82.2% (Kohli et al., 2018). BC RRM has been successfully used as an alternative to mineral trioxide aggregate, with success rates ranging between 92.0 and 94.4% (Shinbori et al., 2015; Zhou et al., 2017).
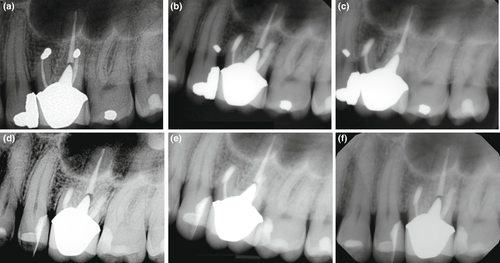
However, a reversal of healing after periradicular surgery has often been debated. Del Fabbro et al. (2007) assessed randomized clinical trials comparing nonsurgical versus surgical retreatment. The authors described faster healing after the surgical procedure after 1 year but a regression of successful cases for endodontic surgery over a follow-up period of 4 years (Del Fabbro et al., 2007). Friedman made similar assertions reviewing the outcome of endodontic surgery (Friedman, 2011). However, the majority of the healing reversals described can be identified as relating to now obsolete techniques, including retrograde gutta-percha or amalgam fillings. There are abundant data today that confirm that surgical procedures with modern techniques and proper case selection do not undergo excessive failure rates. However, as with all dental procedures, there is attrition of cases that were labelled successful at follow-up. According to Song et al. (2012)), teeth that had undergone surgery using microsurgical techniques demonstrated a success rate of 93.3% for more than 6 years. Cases that were considered unsuccessful frequently exhibited failure patterns associated with root fractures or operator mistakes, including missing root-end fillings, incorrect root-end preparation, missed canals, or unaddressed and leaking isthmuses (Song et al., 2012).
The studies described above largely assessed cases of endodontic surgery that are considered true endodontic lesions. Kim and Kratchman (2006) developed a microsurgical classification where true endodontic lesions are characterized as Classes A through C. Briefly, Class A represents a case with unresolved symptoms after nonsurgical approaches have failed but without a lesion present on a periradicular radiograph; Class B, a small periapical lesion together with clinical symptoms; and Class C, a large periapical lesion progressing coronally but without periodontal pocket and mobility. In contrast, periodontally compromised teeth represent Classes D through F, with Class D being similar to those in class C but with deep periodontal pockets; Class E represents a periapical lesion with an endodontic–periodontal communication; and Class F represents an apical lesion and complete denudement of the buccal plate.
Kim et al. (2008) compared teeth undergoing endodontic microsurgery depending on the microsurgical classification described above. The difference in success between teeth in Classes A–C was 95.2% versus a success rate of 77.5% for the periodontally compromised teeth in Classes D–F, which was statistically significant (Kim et al., 2008). The lowest success rates were observed for cases with probing to the apex of the root with or without complete loss of the buccal cortical plate.
Crown and root resection
Root amputation procedures were first introduced for the treatment of multi-rooted teeth with furcation involvement (Faulhaber & Neumann, 1912). Root amputation is considered a ‘root resection’ technique as it only involves the removal of root structure below or at the level of the cementoenamel junction without removal of portions of the crown (Setzer et al., 2019). In contrast, ‘crown resection’ includes hemisection, trisection and premolarization (bicuspidization), respectively, and all procedures where a dissection transverses through the furcation and the crown of a multi-rooted tooth so that a root and the associated portion of the crown may be removed (hemisection, trisection) or all root/crown sections are being retained (premolarization, bicuspidization; Setzer et al., 2019). Indications and contraindications for crown and root resection were introduced and further amended (Minsk & Polson, 2006; Setzer et al., 2019; Staffileno, 1969).
Crown and root resections are indicated for situations with severe bone loss affecting one root, not amenable to other forms of therapy; moderate to advanced furcation involvement with divergent roots; unfavourable root proximity between adjacent teeth; root fracture, perforation, root caries, or external root resorption involving one root or the furcation area; endodontic treatment of a particular root canal cannot be performed, and root-end surgery is contraindicated; a tooth, which is an abutment of a bridge, can be retained after removal of a particular root; or when anatomical situations preclude implant placement.
Crown and root resections are contraindicated if there is insufficient bone support around the remaining roots or in the furcation area; the furcation is too close to the apex, not sufficiently separated, or roots are fused; it is impossible to perform endodontic treatment in the remaining roots, due to unfavourable anatomy of the remaining roots, extensive caries or root resorption in the furcation area and a minimal strategic value of the remaining root structures.
Whenever a crown or root resection is considered, proper endodontic treatment must be carried out prior to the surgical procedure (Minsk & Polson, 2006). A flap should not be raised before metal restorations are dissected or removed or major areas of dentine have been cut so that no metallic fragments or tooth particles will be left behind in the soft tissues. Buccal and lingual full-thickness mucoperiosteal flaps are indicated for proper surgical access and visualization to complete the resection and to achieve proper wound closure. Any inflammatory tissues must be removed. A long fissure bur or a Lindemann bone-cutting bur is recommended to remove the root section from the main trunk of the tooth. The full separation must be verified using a periodontal probe for careful evaluation through the furcation and assessment of the mobility of each root. A resected root must be carefully removed to avoid additional damage to adjacent structures. The remaining crown section must be contoured to ensure that no undercuts remain, which could be attractive to plaque. All granulation tissues must be removed, the root surfaces cleaned, and an osteoplasty performed for the remaining bone to eliminate any irregularities and to provide a sound biologic width for the dentogingival complex. The flap should then be repositioned and sutured. Follow-up instructions follow the same guidelines as for root-end surgery. The definitive restoration, if it remains or be it renewed, should allow good hygiene access.
A number of clinical studies (Bergenholtz, 1972; Bühler, 1988; Carnevale et al., 1991; Carnevale et al., 1998; De Beule et al., 2017; Erpenstein, 1983; Fugazzotto, 2001; Hamp et al., 1989; Hou et al., 1999; Langer et al., 1981; Park et al., 2009; Svärdström & Wennström, 2000; Zafiropoulos et al., 2009) assessed the outcome of crown and root resection therapies. Survival of the tooth has been validated as the positive outcome measure for crown and root resection (Setzer et al., 2019). A systematic review and meta-analysis demonstrated a cumulative survival rate of 85.6% after at least 1 year, with no statistical difference between crown resection and root resection techniques (Setzer et al., 2019).
FUTURE DIRECTIONS—RECENT ADVANCEMENTS
Over decades, endodontic surgery has evolved into endodontic microsurgery. Whilst the most obvious emphasis is on the inclusion of the surgical operating microscope, ultrasonic root-end preparation and, today, calcium silicate materials, there are, in fact, many other technical changes that added to the evolution of endodontic surgery and also Endodontics as a whole. With the realization of long-term problems with dental implants, such as peri-implantitis and peri-implant mucositis, a shift started to take place to refocus on the retention of natural teeth. There is a renewed interest in procedures such as crown and root resections, and other new techniques, such as regenerative endodontics, have emerged. What are the emerging trends for endodontic surgery?
Piezoelectric surgery
The piezotome has long had a place in endodontic surgery. The preparation of a protective groove for safe anchorage of surgical retractors is carried out in the safest way with a piezoelectric tip, which can not harm soft tissues with its cutting action (Degerliyurt et al., 2009). However, in recent years significant progress has been made regarding the development of thinner piezoelectric blades and more powerful devices. These thinner tips allow for the increased application of the bone ‘window technique’ where a bony lid of the cortical plate is resected, temporarily stored in Hanks Balanced Salt Solution (HBSS) and then repositioned at the conclusion of the surgical procedure (Lee et al., 2020). The indications for the bone window technique are an intact cortical plate of at least 1-mm thickness, close proximity to the sinus or large lesions involving multiple roots (Lee et al., 2020). The primary aims of performing a bone window technique are to preserve a more bony structure, to maintain the integrity of the healthy cortical plate (Hirsch et al., 2016), and to have easier access to the target roots without the need for preparation of two separate osteotomies (Figure 47).
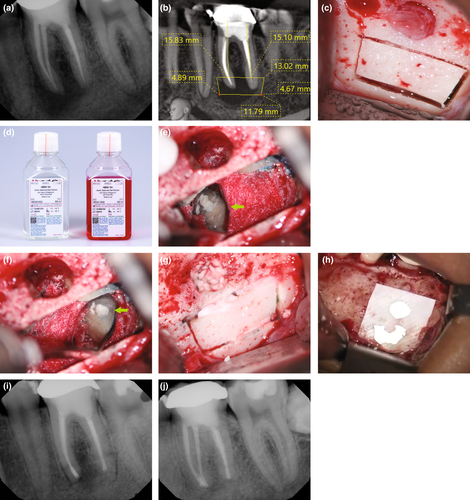
With the improvements in piezoelectric surgery, its future application may not only be with the creation of a protective groove or a bone window but its general use for bone cutting, root resection and root-end preparation. Ultrasonic vibrations were shown to wash away particles from the surgical site resulting in less intraoperative bleeding and better visibility (Goyal et al., 2012; Landes et al., 2008; Pappalardo & Guarnieri, 2014). Horton et al. (1975) discovered that bone healing was faster after using an ultrasonic instrument (piezosurgery) in comparison with a low-speed rotary bur. The original cortical and cancellous structure of the bone was preserved when a piezoelectric electric cutting tip was used compared to a micro-saw or a Lindemann bur (Maurer et al., 2007). The same study also showed that, histologically, the cell viability of the bone at the site of the cut was better maintained with piezoelectric tools. In a patient study, results demonstrated better haemorrhage control during surgery, improved quality of life in the first week post-surgery, less pain and swelling, and a smaller number of analgesics taken (Bharathi et al., 2021). In conclusion, piezosurgery may be used for more applications than to date and make endodontic surgery an even less traumatic experience.
CBCT healing assessment
CBCT technology has become commonplace in endodontic practice with widespread applications. A survey of CBCT use completed by 1083 active members of a professional Endodontic society identified that the most frequent applications were taken either for diagnostic purposes, for example evaluating resorptions or identifying missed canals, or for treatment planning for nonsurgical or surgical retreatment (Setzer et al., 2017). Only a very small percentage of survey participants reported the use of CBCT for healing assessment. One of the traditional problems in endodontic surgery has been the evaluation and classification of lesions that fall into either the ‘incomplete healing’ (scar tissue, success) or ‘uncertain healing’ (failure) categories for healing assessment. CBCT imaging has been confirmed to allow a more precise appraisal of periapical lesions than periapical films. Schloss et al. (2017) demonstrated that cases that were inconclusive in the healing assessment on two-dimensional periapical radiographs could be clearly assigned to a specific healing category using three-dimensional CBCT imaging. Criteria for three-dimensional assessment of periapical healing after endodontic surgery have been introduced (Chen et al., 2015; Schloss et al., 2017; von Arx et al., 2016) and since clinically validated (Safi et al., 2019). The future may also see the traditional healing categories to be replaced by strict volumetric assessment of lesions, which has been already introduced for a number of clinical evaluations (Curtis et al., 2018; Schloss et al., 2017). Volumetric assessment with the aid of semi-automated or fully automated lesion segmentation would then allow the clinician to assess healing progress in absolute measurements for a precise comparison to the preoperative situation. The current progress in CBCT imaging technology, with the availability of limited FOV high-resolution volume scans with minimal distortion, and radiation doses up to 15 times lower than conventional CT scans (Scarfe et al., 2009), will likely continue and increase the ability for practitioners to use CBCT imaging in private practice settings for a more precise healing assessment.
Guided techniques and artificial intelligence
The availability of CBCT technology has driven an entirely new branch of endodontics, with the introduction of guided surgical techniques in endodontic microsurgery. Guided or targeted endodontic microsurgery utilizes three-dimensional printed templates or stents for guided osteotomy and root resection (Ahn et al., 2018; Giacomino et al., 2018; Strbac et al., 2017). The superimposition of preoperative CBCT images with intraoral scans allows for the precise planning of the osteotomy size and location and aids with the three-dimensional printing of the surgical guides. Variations of the technique include access to the osteotomy site with a traditional flap or in the form of a direct flapless approach. The surgical stents were used to guide piezoelectric saws (Strbac et al., 2017), trephine burs (Giacomino et al., 2018) or implant drills (Ahn et al., 2018) for osteotomy and root resection. Targeted endodontic microsurgery has since continued to develop and has since improved with the consolidation of the necessary digital workflows (Ray et al., 2020), the detailed assessment of anatomy specific to guided surgical techniques, such as the location of the greater palatine artery (Smith et al., 2021), and the validation of the techniques by larger case series (Buniag et al., 2021; Figure 48). Another variation of guided surgical techniques is dynamic navigation, a freehand approach to endodontic microsurgery without templates or guides that is directed by CBCT location and calibrated surgical burs and instruments tracked by a stereoscopic vision camera (Dianat et al., 2021; Gambarini et al., 2019). All these options provide clinicians with previously unknown precision and safety nets during surgical procedures.
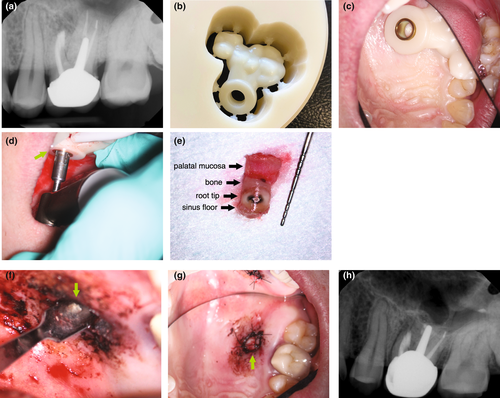
During the same time period that guided surgical techniques took hold, the first CBCT-based endodontic applications for artificial intelligence and its subdomain deep learning were developed. Applications include the development of automated algorithms for computer-aided detection and diagnosis (CAD) of periapical lesions indicative of apical periodontitis (Orhan et al., 2020; Setzer et al., 2020), the identification of cracks (Shah et al., 2018), and the automated segmentation of teeth (Lahoud et al., 2021) and the mandibular nerve canal (Kwak et al., 2020). Early attempts have also been made to use deep learning for the differential diagnosis of cystic periapical lesions from granulomas (Okada et al., 2015). It will likely not be before long that Endodontics will see a coming together of guided surgical techniques with artificial intelligence-based applications. Artificial intelligence may aid in the decision-making if a surgical procedure may be necessary (cyst versus granuloma) and feasible (fracture detection), and in the treatment planning of the approach with automated support for the fabrication of surgical guides (location of the lesion and the mandibular canal, other anatomy and tooth segmentation). Ultimately, this may culminate in the development and application of robotic endodontic microsurgical procedures, which have made their first introduction in implant dentistry (Haidar, 2017; Wu et al., 2019).
CONCLUSION
Endodontic surgery has a remarkable history and is going through a constant evolution with gains in both research developments and clinical applications. Its current iteration of endodontic microsurgery has been demonstrated to have successful short- and long-term outcomes. The technical armamentarium and materials available for the clinician allowed it to become a standard technique for the practitioner specialized in or a focus on Endodontics. The future developments for Endodontic surgery are on the horizon and paint an intriguing picture of the next iterations of this technique.
ACKNOWLEDGEMENTS
We want to thank our friends and colleagues, the ‘Penn Endo Family’, who have contributed to this article, especially Dr. Syngcuk Kim, all of our’s mentor, who has been instrumental in developing Endodontic Microsurgery in its current form.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study




