Present status and future directions: vertical root fractures in root filled teeth
Abstract
Vertical root fracture (VRF) is a common reason for the extraction of root filled teeth. The accurate diagnosis of VRF may be challenging due to the absence of clinical signs, whilst conventional radiographic assessment is often inconclusive. However, an understanding of the aetiology of VRFs, and more importantly, the key predisposing factors, is crucial in identifying teeth that may be susceptible. Thorough clinical examination with magnification and co-axial lighting is essential in identifying VRFs, and although CBCT is unable to reliably detect VRFs per se, the pattern of bone loss typically associated with VRF can be fully appreciated, and therefore, increases the probability of correct diagnosis and management. The prevalence of VRFs in root filled teeth is significantly greater than in teeth with vital pulps, demonstrating that the combination of loss of structural integrity, presence of pre-existing fractures and biochemical effects of loss of vitality is highly relevant. Careful assessment of the occlusal scheme, presence of deflective contacts and identification of parafunctional habits are imperative in both preventing and managing VRFs. Furthermore, anatomical factors such as root canal morphology may predispose certain teeth to VRF. The influence of access cavity design and root canal instrumentation protocols should be considered although the impact of these on the fracture resistance of root filled teeth is not clearly validated. The post-endodontic restoration of root filled teeth should be expedient and considerate to the residual tooth structure. Posts should be placed ‘passively’ and excessive ‘post-space’ preparation should be avoided. This narrative review aims to present the aetiology, potential predisposing factors, histopathology, diagnosis and management of VRF and present perspectives for future research. Currently, there are limited options other than extraction for the management of VRF, although root resection may be considered in multi-rooted teeth. Innovative techniques to ‘repair’ VRFs using both orthograde and surgical approaches require further research and validation. The prevention of VRFs is critical; identifying susceptible teeth, utilizing conservative endodontic procedures, together with expedient and appropriate post-endodontic restorative procedures is paramount to reducing the incidence of terminal VRFs.
INTRODUCTION
A vertical root fracture (VRF) is a longitudinal (axial) fracture involving the cementum, dentine and root canal system of a root. The authors of this paper suggest the following classification of VRF: incomplete if the fracture only involves one side of the root and complete if the VRF extends from one proximal aspect to the opposite side of the root. The term split tooth is used to describe a VRF where there is visible separation of the two components. Although most frequently observed in root filled teeth, VRFs can also occur in teeth with vital pulps. Depending on the cause, they may develop in either a corono-apical or apico-coronal direction. VRFs are typically detected in the bucco-lingual plane of the tooth, and less commonly in the mesio-distal plane (von Arx & Bosshardt, 2017). Sugaya et al. (2015) concluded that VRFs occurred cervically and apically at approximately the same frequency. Furthermore, 57.4% and 90.8% of VRFs were bucco-lingual in orientation when originating in the cervical and apical thirds of the root, respectively.
Vertical root fractures may develop slowly and without any obvious signs and/or symptoms, making (differential) diagnosis challenging. Early detection and appropriate management of (incomplete) VRFs is essential to enhance the chances of retaining the affected tooth and/or minimizing the undesirable consequences of this complication. Timely extraction of teeth with advanced VRFs (i.e., complete VRF or split tooth) will prevent pain/discomfort and limit periradicular bone loss which may have an impact on subsequent implant treatment planning.
For this narrative review, an unrestricted literature search was performed by two evaluators using specified keywords in the PubMed database. Eligibility criteria for included studies required the full text to be available, and to be in the English language, with a publication cut-off date set to March 2022. Keywords relating to the prevalence, aetiology, diagnosis and management of vertical root fractures in root filled teeth were searched using Medical Subject Heading (MeSH) terms. An additional manual search of references in the included papers was also carried out to identify additional relevant research. Following the initial screening process, the abstracts of the included papers were read and considered for suitability. Studies related to ‘cracks’ in teeth, and not specific to vertical root fractures, were only included to discuss possible aetiological factors. Although a number or review articles have been published on the subject of vertical root fractures, these principally relate to diagnosis, and therefore, the purpose of this narrative review is to provide a comprehensive overview of the current literature relating to prevalence, aetiology, diagnosis and management, as well as providing perspectives on future directions on prevention and treatment.
PREVALENCE
Vertical root fracture is more commonly associated with root filled teeth than teeth with (non-)vital pulps (Chan et al.,1999; Cohen et al., 2006; Yoshino et al., 2015). In a retrospective cross-sectional study of 736 extracted teeth, Yoshino et al. (2015) found that 31.7% of teeth were extracted due to VRF, of which 93.6% were root filled teeth. Sugaya et al. (2015) performed a retrospective analysis on 304 teeth with VRFs that had been detected clinically and/or radiographically. The authors reported that 97% of the detected VRFs were found in root filled teeth, whilst 2.3% were detected in teeth with vital pulps and 0.7% in teeth with non-vital pulps which were not root filled. Similarly, a retrospective clinical study conducted in Taiwan demonstrated that 86% of extracted teeth with VRFs were previously root filled (Liao et al., 2017).
The reported prevalence of VRFs in root filled teeth ranges from 4% to 32% (von Arx et al., 2021; Sjøgren et al., 1990; Toure et al., 2011; Yoshino et al., 2015). This wide range in the frequency of VRF may be due to heterogeneity in the method of evaluation of VRFs found in clinical studies. The variations include differences in the diagnosis and categorization of VRF (e.g., misdiagnosis of an isolated periodontal pocketing associated with a VRF as periodontal disease), which makes it difficult to appreciate the true prevalence of VRF in the general population. In some cases, although VRF has been attributed as the reason for extraction of root filled teeth, the VRF may have been present prior to endodontic treatment.
A VRF may be confirmed either by surgical exploration, or by careful examination of the tooth after extraction with high magnification (von Arx & Bosshardt, 2017; PradeepKumar et al., 2016; Tsesis et al., 2010). Surgical exploration is limited to assessment of the buccal, and when accessible, the palatal aspect of the root. The mesial and distal aspects of the root surface cannot usually be visualized during surgical exploration, meaning a VRF may be missed, therefore leading to an underestimation of the prevalence of VRF. Furthermore, it is not possible to unequivocally confirm if the presence of a VRF in an extracted tooth was present prior to extraction or was unintentionally caused during the process of extraction itself, even if performed with an atraumatic technique. As a result, the assessment of extracted teeth may result in an over-estimation of VRF.
From the available evidence, it appears that maxillary (pre)-molar and mandibular molar teeth are most frequently affected by VRF (Cohen et al., 2006; Karygianni et al., 2014; PradeepKumar et al.,. 2016). It has been suggested that the occlusal topography and morphological features of these teeth may make them more susceptible to fracture due to intrinsic points of weakness and higher occlusal loading (Awawdeh et al., 2017; Cavel et al., 1985).
The incidence of VRF increases with age and is most prevalent in patients who are older than 40 years of age (PradeepKumar et al., 2016; Yoshino et al., 2015). This may be due to teeth having undergone several restorative cycles and the cumulative impact of (para)functional activity (Tang et al., 2010).
AETIOLOGY
Although the aetiology of VRFs is multifactorial, several risk factors have been suggested. These factors can be broadly divided into two categories: predisposing and contributory (Table 1).
| Predisposing risks |
|---|
|
| Contributory risks |
|---|
|
Predisposing risk factors
Structural integrity of the tooth
Root filled teeth may fail due to structural failure, which may involve the crown and/or root fracture (Al-Nuaimi et al., 2018; Ng et al., 2011; Salehrabi & Rotstein, 2004). The fracture resistance and survival of root filled teeth is critically linked to the quality and quantity of residual sound tooth structure following endodontic and consequent restorative treatment (Ferrari et al., 2012; Nagasiri & Chitmongkolsuk, 2005; Tjan & Whang, 1985). Residual tooth structure has been assessed in several ways, which include evaluation of the presence of an adequate ferrule effect, the number of coronal walls and remaining tooth volume (height/thickness; Al-Nuaimi et al., 2017; Dammaschke et al., 2013; Fokkinga et al., 2007; Naumann et al., 2012).
A prospective clinical study assessed the outcome of root canal retreatment in relation to the residual volume of coronal tooth tissue of 137 posterior teeth (Al-Nuaimi et al., 2017). At the 1-year recall, the authors reported that teeth with less than 30% residual tooth structure had a significantly higher risk of endodontic failure and suggested that this could be attributed to (pre-)existing fractures in teeth with minimal tooth structure.
Pre-existing (micro-)cracks and fractures
It has been reported that existing cracks in minimally or unrestored vital teeth can ultimately result in pulp infection and necrosis (Berman & Kuttler, 2010). In a case series of 27 teeth (16 symptomatic, 11 asymptomatic), the authors used a dental operating microscope (DOM) to confirm the presence of a mesio-distal VRF. Radiographically, these teeth were associated with discernible widening of the periapical periodontal ligament (PDL) space, and/or periapical radiolucency, and were deemed to be of hopeless prognosis. The teeth were extracted and the presence of a coronal mesio-distal VRF extending into the root was confirmed in all 27 teeth macroscopically and by using micro-computed tomography (micro-CT).
PradeepKumar et al. (2017) found that 7.1% of 633 non-endodontically treated extracted teeth also exhibited pre-existing cracks. These teeth were atraumatically extracted due to endodontic disease. The authors found the prevalence of cracks was significantly higher in older patients (40–70 years) compared to younger patients (20–39 years). 17.8% of cracks were detected in the cervical region, 66.6% of cracks were in the cervical and middle aspect of the roots, 4.4% were present middle third of the root and 4.4% of cracks were located apically. Micro-CT images revealed a complete crack involving the root canal lumen was present in 33% of cases.
The presence of pre-existing cracks is a negative prognostic factor in the outcome of endodontic treatment. A retrospective cohort study evaluating the outcome of root canal treatment for cracked teeth reported VRF as the most common reason for extraction (Kang et al., 2016). Furthermore, 25.2% of the 175 cracked teeth examined in this study had pre-operative probing depths of greater than 6 mm. Although the available data did not distinguish the nature of the periodontal pocket (i.e., isolated or generalized), the survival of cracked teeth following root canal treatment was significantly lower when these teeth were associated with deep probing depths of greater than 6mm. In suspected cases of VRF in endodontically treated teeth, deep periodontal pocketing can be a pathognomonic sign (Tsesis et al., 2010). It is reasonable, therefore, to conclude that a proportion of teeth undergoing root canal treatment may have had existing VRFs prior to endodontic intervention.
The previously discussed study (Kang et al., 2016) was also included as part of a systematic review of endodontically treated cracked teeth reporting a 5-year survival rate of 84.1% (Leong et al., 2020). The authors found that teeth with multiple cracks, radicular cracks and probing depths of greater than 3 mm had an 8%–9% higher risk of extraction. It was stipulated by the authors, propagation of radicular cracks with time may have resulted in a vertical root fracture.
In general, the cause of these pre-existing cracks may include restorative treatment, parafunctional habits, occlusal interferences and the cumulative general wear (Ratcliff et al., 2001). Once a crack has developed within the root dentine, it is accepted that repetitive occlusal loading and flexing of the root (fatigue failure) can lead to propagation of the crack into a VRF (Kishen, 2015).
In the past decade, significant concerns were raised regarding the development of dentinal defects and/or microcracks following root canal preparation and/or filling procedures (Bier et al., 2009; Shemesh et al., 2009). These initial studies based their results on a destructive sectioning method to visualize the inner surface of root dentine in extracted teeth to assess for dentinal microcracks. Both studies concluded that dentinal defects were present only in the roots of prepared canals. Several other researchers utilized similar research methodologies to conclude that various root canal preparation techniques can result in the induction and/or propagation of a dentinal crack (Adorno et al., 2011; Bürklein et al., 2013; Liu et al., 2013).
However, it has been established there are major methodological flaws in sectioning studies. In brief, these include the possibility of creation of a crack during the sectioning process, the inability of using the same specimen as a control before and after canal preparation and the limited analysis of the root surface due to the fact the sectioning method generally yields up to 4 slices per specimen (Versiani et al., 2021).
The methodological flaws are further compounded by the environmental condition of stored and/or extracted teeth. De-Deus et al. (2019) analysed the characteristic of pre-existing microcracks in non-endodontically treated from fresh cadavers. A thorough micro-CT analysis was performed of 65 530 cross-sectional images from a total of 178 teeth present in bone-blocks obtained from the cadavers. No dentinal microcracks were identified during the analysis. The authors suggested that microcracks present within roots of stored and/or extracted can occur as a result of dehydration and/or the extraction procedure. The presence of such microcracks has been described as a laboratory phenomenon which is not replicable in a clinical scenario (De-Deus et al., 2019).
Micro-CT studies performed using extracted teeth have also shown that root canal preparation does not induce any new dentinal microcracks (De-Deus et al., 2014, 2017). Furthermore, micro-CT assessment of extracted teeth has determined that observed microcracks were already present prior to root canal instrumentation (Martins et al., 2021; Miguéns-Vila et al., 2021; Vieira et al., 2020).
These observations corroborate the finding obtained from an in vivo study performed using sound 60 premolar teeth (PradeepKumar et al., 2019). Following hand and rotary root canal preparation in vivo, the teeth were extracted and analysed using micro-CT to assess for the presence of dentinal microcracks. The authors reported no microcracks were detected following root canal preparation. However, the age of the patients included in this study was between 15 and 30 years old. The authors acknowledged that differing results may be obtained in older patients who are more likely to suffer a VRF as discussed previously in the Prevalence section.
There is a lack of available information to assess the correlation between the number of microcracks and the fracture resistance of teeth. Abou El Nasr and Abd El Kader (2014) demonstrated an inversely proportional relationship between the number of cracks generated following rotary instrumentation and the fracture resistance of teeth. The authors performed their analysis using the sectioning method. As discussed previously, sectioning studies are no longer accepted as a valid method to investigate dentinal microcracks due to its inherent risk false positive results.
Conversely, a recent study performed using micro-CT demonstrated no association between the number of root dentinal microcracks and the fracture resistance of teeth (Cavalcante et al., 2020). The specimens used in this study were decoronated non-endodontically treated lower incisor teeth. A compressive axial load was applied to fracture the root using a universal testing machine. Although this study was well designed, it does not provide translatable information regarding the most commonly affected teeth susceptible to VRF.
Well-designed studies are required to determine the importance and/or relevance of microcracks in the development of a VRF.
Once a crack has developed within the root dentine, it is accepted that repetitive occlusal loading and flexing of the root (fatigue failure) can lead to propagation of the crack into a VRF (Kishen, 2015).
Biomechanical properties of dentine
Root filled teeth have an increased susceptibility to VRF due to changes in the biomechanical properties of dentine. Endodontic treatment can result in depletion of the organic components of root dentine and alteration in the chemical composition (Driscoll et al., 2002; Reddington et al., 2003). As the pulp complex is mainly composed of water, root filled teeth will inevitably undergo a reduction in free water content (dehydration) within the dentine matrix and dentinal tubules, with a consequent effect on the viscoelastic properties (Arola & Reprogel, 2005). Dehydration of the dentine can result in lower fracture toughness, decrease in resistance to fatigue failure, as well as reduced microhardness and poorer dissipation of occlusal forces (Arola & Reprogel, 2005; Nadeau et al., 2019). The effects of dehydration may be considered a possible causative factor in the development of a VRF (Shemesh et al., 2018; Winter & Karl, 2012).
In contrast, other studies have suggested that the changes in water content may have no impact on the biomechanical behaviour of dentine (Papa et al., 1994; Sedgley & Messer, 1992). Sedgley and Messer (1992) observed in a laboratory study that there was similarity in the biomechanical properties of root filled teeth and vital teeth. The extracted root filled teeth were stored in saline which may have led to rehydration and reconditioning of viscoelastic properties of the dentine. Papa et al. (1994) found that the microhardness of teeth with vital pulps was only 3.5% greater than that of root filled teeth, and that there was only 2.05% less free water content in root filled teeth compared to vital teeth. In this study, the extracted teeth were wrapped in aluminium foil until the experiment was performed. It has been established, within normal room conditions, 80%–85% of dentinal free water loss occurs within 2 h (Jameson et al., 1993). As a result, the conflicting results of laboratory studies may be accounted for by variations in methodology, particularly, the storage conditions of the tooth samples.
Age-related increases in mineral-to-collagen and cross-linking ratio can lead to a decrease in dentinal strength by roughly 25 MPa per decade (Yan et al., 2017). These changes can impact the mechanical integrity and/or reduce the fracture resistance particularly of radicular dentine (Yan et al., 2017). Kinney et al. (2005) reported increased (transparent) mineralization of the dentinal tubules formed by ageing resulted in a reduction of fracture resistance by 20%. According to Arola and Reprogel (2005), dentine samples taken from patients more than 55 years of age contained a greater mineral content and up to 50% reduced fracture strength when compared to young dentine (<30 years of age). Bajaj et al. (2006) have also demonstrated fatigue crack growth resistance of dentine decreases with age.
Based on these laboratory studies, root dentine appears to become more susceptible to fracture. This may explain in part the reason for increased VRF prevalence in the root filled teeth of older patient groups. However, further research is still required to determine a correlation between the age of dentine and its susceptibility to microcracks.
Anatomy and root canal morphology
The presence of isthmi, hourglass-shaped root canal cross-sections, increased root canal curvature and/or a narrow mesio-distal cross-sections have been shown, using finite element analysis, to be risk factors for VRF due to the natural planes of weakness created within the root (Chai & Tamse, 2015; Lertchirakarn et al., 2003; Sathorn et al., 2005).
A histological analysis performed on 30 extracted root filled posterior teeth revealed that 56.3% of the detected VRFs were associated with the isthmus (von Arx & Bosshardt, 2017). Similarly, in an ex vivo study, Chai and Tamse (2015) determined roots with two canals connected by an isthmus were more vulnerable to VRF than roots with a single canal. This may (in part) explain why the mesio-buccal roots of maxillary molar teeth and mesial roots of mandibular molar teeth have a higher incidence of VRF (von Arx et al., 2021; Chan et al., 1999; PradeepKumar et al., 2016; Tamse, Fuss, Lustig, & Kaplavi, 1999). These roots routinely have two (or more) canals, as well as a high incidence of an isthmus (von Arx, 2005; von Arx et al., 2011; Teixeira et al., 2003). Although less common, immature permanent anterior teeth with necrotic pulps, thin dentine walls and open apices are also susceptible to fracture, most probably due to the fragility of the root (Cvek, 1992).
It is essential for the clinician to appreciate the anatomical and morphological characteristics of each specific case prior to treatment, as this will determine the most appropriate root canal instrumentation technique to minimize unnecessary dentine removal.
Tooth location and occlusal forces
Posterior teeth are significantly more likely to develop a VRF than anterior teeth (Von Arx & Bosshardt, 2017; PradeepKumar et al., 2016). This is most likely because posterior teeth, particularly the last standing molar, are subject to higher functional and non-functional occlusal loading (Hattori et al., 2003; Kumagai et al.,1999). These findings concur with the results of a 4-year prospective study assessing the factors affecting tooth survival following root canal treatment (Ng et al., 2011). The aforementioned study reported 68% of terminal teeth requiring extraction were fractured. Interestingly, 58% of teeth without proximal contacts were extracted due to fracture when compared with 38% of teeth with proximal contacts. Root filled teeth may also undergo higher occlusal loading due to a loss of pulpal proprioception (Randow & Glantz, 1986).
Parafunctional activity and/or non-working side interferences may also lead to excessive lateral forces on posterior teeth, increasing their susceptibility to VRF (Kydd & Daly, 1985; Yang et al., 1995).
Diet
Available clinical data from non-root filled teeth, specifically relating to Asian populations, suggest diets which include hard foods such as betel nut and bones may increase the propensity of a spontaneous VRF (Yang et al., 1995; Yeh, 1997). It can be postulated that such dietary habits will also have an increased negative impact on root filled teeth due to their changes in biomechanical properties and comprised tooth structure. It is unknown if diet associated VRFs are more likely to originate coronally or apically.
CONTRIBUTORY RISK FACTORS
Endodontic treatment
Clark and Khademi (2010a) describe peri-cervical dentine (PCD) as the region of a tooth extending approximately 4mm coronal and apical to the crestal bone level. They propose that the loss of sound dentine, most specifically the PCD, incurred during ‘traditional’ access cavity and root canal preparation may predispose the residual tooth structure to fracture and impact survival. In their narrative commentary and subsequent case series, the authors describe a philosophy to direct access cavity preparation according to the pre-existing caries (caries-driven access) or restoration (restoratively-driven access) in order to minimize overall tooth volume loss. Furthermore, where possible, they advocate incomplete removal of the pulp chamber roof in order to reduce cuspal flexion and transmission of occlusal forces and parafunctional stresses into the root (Clark & Khademi, 2010a; Gluskin et al., 2014; Kishen, 2015). Importantly, these proposals were based on the authors opinions and a series of cases (Clark & Khademi, 2010b), rather than any scientific evidence.
A renewed interest in minimally invasive endodontic techniques has emerged (Marinescu et al., 2020; Saberi et al., 2020; Santosh et al., 2021). However, despite several studies being published on the impact of conservative access cavity preparation on the fracture resistance of root filled teeth, the results are conflicting and do not permit tangible conclusions to be drawn (Plotino et al., 2017; Silva, Lima, et al., 2021). In a recent narrative review (Silva et al., 2022), the inconsistent findings of ex vivo and in vitro studies assessing the effect of access cavity modification on fracture resistance were attributed to study heterogeneity, and also, flaws in experimental design and methodology. A number of ex vivo studies demonstrate poor standardization of the included teeth using either direct external measurement or two-dimensional radiographic techniques; this prevents appropriate matching of the sample selection in respect of pulp chamber volume or the residual thickness, height and volume of dentine (Corsentino et al., 2018; Ivanoff et al., 2017; Krishan et al., 2014; Marinescu et al., 2020; Maske et al., 2021). Furthermore, some studies fail to describe how the included sample selection were chosen at all (Chlup et al., 2017; Makati et al., 2018; Mustafa et al., 2020; Reddy et al., 2020). To highlight the relevance of this point, studies carried out using micro-CT for accurate sample selection matching, as well as pre- and post-experimental assessment of pulp chamber volume, dentine thickness, height and volume, consistently show no difference in the fracture resistance of teeth prepared with minimally invasive access cavities (Augusto et al., 2020; Barbosa et al., 2020; Lima et al., 2021; Rover et al., 2017). Clearly, standardizing the experimental sample selection used in these studies is a challenge, particularly when trying to simulate realistic clinical situations. The majority of ex vivo studies have utilized intact and unrestored teeth to facilitate direct comparison of the effect of modifying the access cavity preparation on fracture resistance (Plotino et al., 2017; Rover et al., 2017; Saberi et al., 2020; Sabeti et al., 2018; Silva, Lima, et al., 2021). Whilst this study design permits more meaningful comparison of the different access cavity designs per se, an occlusal access cavity with intact marginal ridges/axial walls does not represent a commonly encountered clinical situation. Such experimental model designs are likely to influence the study findings, as it has been demonstrated that the loss of one or more marginal ridges has a far more profound impact on cuspal stiffness than the access cavity itself (Reeh et al., 1989). As a result, authors have attempted to overcome this by creating class 2 cavities prior to access, with loss of one or both marginal ridges to represent a more realistic clinical situation (Abou-Elnaga et al., 2019; Corsentino et al., 2018; Ivanoff et al., 2017). However, this creates a challenge in respect of both standardizing and measuring the volume of tooth structure removed for each included specimen (Silva et al., 2022).
Silva et al. (2022) also identified a number of methodological issues with laboratory studies assessing access cavity design which relate to the age of the dentine samples, thermal and cycling simulation and the clinical relevance of finite element analysis.
As discussed previously, the PCD zone encompasses the transition from the access cavity to the coronal part of the root canal system. The effect of dentine removal specifically in the coronal portion of the root canal system on the fracture resistance of teeth is poorly studied. There is a growing trend to favour martensitic rotary files with narrower diameters and reduced tapers to facilitate more conservative root canal shaping (Peters et al., 2015). Root canal preparation techniques carried out with ‘conventional’ austenite Ni-Ti alloys, of larger size and/or taper will lead to greater peri-cervical dentine removal than ‘newer’ controlled memory martensitic files with narrower shanks (Shen et al., 2013; Zupanc et al., 2018). The recent shift in file geometry has developed due to the belief that increased dentine removal during root canal preparation may lead to the development of microcracks (Adorno et al., 2011; Jamleh et al., 2021) and reduce the fracture resistance of root filled teeth (Lang et al., 2006; Sabeti et al., 2018). Furthermore, it has been reported that reciprocating single file systems are more likely to induce dentinal microcracks than continuous rotation file system (Bürklein et al., 2013). However, the results of ex vivo studies, as discussed previously, have been questioned, due to the presence of pre-existing cracks in the sample selection (Arias & Peters, 2022; De-Deus et al., 2014). The results of cadaver studies do not show any causal relationship between root canal shaping and the development of dentinal microcracks (Arias et al., 2014; Bahrami et al., 2017), and these results should be considered as more relevant due to absence of pre-existing cracks (Versiani et al., 2021).
It has also been reported that the use of ultrasonics to remove posts and fractured instruments from the coronal and middle thirds of the root canal system can result in a greater loss of dentine, increasing the risk of VRF, as well as initiating and/or propagating cracks (Fu et al., 2019; Riis et al., 2018).
The fracture resistance of the mesial root of mandibular molars is also influenced by the presence and subsequent preparation of a mid-mesial canal. Keleş et al. (2020) found that the fracture resistance of mandibular molars was reduced when 3 mesial canals (mesio-buccal, mid-mesial and mesio-lingual) were located and prepared when compared with roots with only 2 mesial canals (mesio-buccal and mesio-lingual). Furthermore, flattened roots, with a narrow mesio-distal cross section, such as the mesial root of mandibular molars and mesio-buccal root of maxillary molars, are also more prone to fracture, due to the reduced thickness of dentine on the proximal aspects of the root (Abou-Rass et al., 1980). These have been described as ‘danger zones’ as they are predisposed to excessive thinning of the furcal root canal dentine during instrumentation.
The use of root canal irrigants (e.g., sodium hypochlorite, EDTA solution) and medicaments (e.g., calcium hydroxide) may have deleterious biomechanical effects on dentine, reducing the microhardness, elastic modulus and fracture resistance (Calt & Serper, 2000; Grigoratos et al., 2001). Prolonged exposures to irrigants and medicaments due to lengthy/multiple visits and/or protracted treatment will further compound these weakening effects. These undesirable sequelae may render root filled teeth more susceptible to VRF (Marending et al., 2007; Yassen et al., 2013).
Excessive forces may be imparted on the root during the obturation stage of endodontic treatment, particularly with the use of the (cold) lateral condensation technique. This will predispose the treated roots to VRF (Saw & Messer, 1995; Wilcox et al., 1997). Furthermore, the use of an intra-radicular post following completion of root canal treatment may lead to the additional removal of root canal dentine to facilitate its placement. The post-space preparation for cast posts results in undesirable removal of peri-cervical dentine. In addition, the subsequent lateral forces generated in root dentine post cementation, as well as those which are encountered during function, thereafter, will lead to increased deformation and flexure of the root dentine, predisposing the tooth to VRF (Lang et al., 2006; Li & Kishen, 2018). The use of screw posts generates unfavourable stress concentrations on radicular root dentine, increasing the risk of VRF when compared to ‘passive’ post systems. For example, the use of prefabricated fibre post systems requires minimal, if any, post-space preparation and do not create any undesirable forces during the adhesive cementation process (Maddalone et al., 2018; Standlee et al., 1982).
Several factors may be associated with the development of VRFs, and a single causative factor may be difficult to identify. The combination of factors listed above, in particular, the loss of sound tooth structure, together with the cyclical forces generated by functional and/or parafunctional activity over a period of time, may result in a higher predilection of VRFs in root filled teeth. Carefully designed studies are required to assess the relationship of potential predisposing factors, residual tooth structure and root canal treatment protocols on the development of VRFs over long follow-up periods.
Restorative treatment and occlusal considerations
Studies assessing the survival of root filled teeth are largely retrospective, cohort and epidemiological in design and provide limited insight into the specific prevalence of VRFs as the cause of failure. Despite this, these studies do facilitate the observation of trends and surrogate relationships. Epidemiological studies on the survival of root filled teeth provide data on very large numbers of teeth, clearly demonstrating the higher survival of root filled teeth restored with cuspal coverage restorations (Lazarski et al., 2001; Salehrabi & Rotstein, 2004). The latter study found that of the 1 462 936 observed root filled teeth, 97% survived for 8 years. However, analysis of the extracted root filled teeth revealed that 85% of these did not have cuspal coverage restorations.
Several retrospective studies demonstrate significantly higher estimated survival rates (up to 10 years) for root filled teeth restored with crowns rather than direct restorations (Aquilino & Caplan, 2002; Dammaschke et al., 2013). Dammaschke et al. (2013) retrospectively studied the influence of coronal restoration type, specifically on the fracture resistance of root filled premolar and molar teeth. Observations of the 676 teeth revealed that for root filled teeth restored with full coverage crowns, the mean survival was 15.3 years, whilst for root filled teeth restored with composite resin, amalgam and glass–ionomer cement, mean survival rates were 13.4 years, 11.8 years and 6.6 years, respectively.
Borén et al. (2015) retrospectively assessed the 10-year survival of 420 root filled teeth, treated within a specialist clinic. Root filled teeth restored with crowns had an estimated 10-year survival rate of 91.3% whilst those restored without crowns had a survival rate of 76%. Importantly, the authors provided specific causes of failure for the extracted teeth and found that VRF was the main cause, accounting for 36% of all failures.
An 8-year retrospective study by Pratt et al. (2016) specifically assessed the impact of timing of cuspal coverage on the survival of root filled teeth. The authors found that root filled teeth restored with crowns more than 4 months after endodontic treatment were almost 3 times more likely to be extracted when compared with those restored within 4 months. Interestingly, after 4 months there was no significant increase in failure, until 18 months after the completion of endodontic treatment, at which stage the rate of failure increased dramatically by over nine-fold; the authors suggest this may be a critical time for the presentation of crown and root fractures.
The teeth included in retrospective and epidemiological research are prone to selection bias, where it is possible that a higher proportion of teeth with more favourable prognoses are crowned. As a result, the findings of these studies must be interpreted with caution.
In a prospective study assessing the cause of extraction of 119 root filled teeth recorded by a cohort of general dental practitioners, it was found that 94% of extracted teeth had not been restored with cuspal coverage restorations (Toure et al., 2011). The authors found mandibular first molars without crowns were most frequently extracted. Vertical root fracture was reported as a reason for extraction in 13.4% of all cases. However, in this study, 40.3% of failures were recorded as being due to periodontal disease, and it is feasible to consider that a proportion of these teeth may have included teeth with VRFs presenting as advanced periodontal problems (i.e., isolated periodontal probing defects).
The loss of both marginal ridges of a tooth will result in decreased tooth stiffness as well as increased cuspal flexure (Gokturk et al., 2018; Panitvisai & Messer, 1995), thus rendering extensively restored teeth undergoing endodontic treatment more prone to fracture, particularly when not restored with a cuspal coverage restoration. It is important to appreciate that access cavity preparation alone does not appear to increase fracture susceptibility significantly (Ramachandran et al., 2020).
Concluding remarks
There are a lack of controlled prospective studies comparing the specific cause of failure of root filled teeth, including the specific impact of tooth location and cuspal coverage on VRFs. Whilst the surrogate findings of epidemiological and retrospective studies show inferior survival rates for root filled teeth restored without cuspal coverage restorations, this might be a coincidental finding in some cases. In the studies discussed above, the teeth were not randomized, and therefore, may be susceptible to operator bias. This may have led to root filled teeth with a favourable prognosis being restored with cuspal coverage restorations, whilst those with lesser prognoses, being restored with direct restorations.
Well-designed randomized clinical trials assessing the impact of contemporary cuspal coverage techniques on the specific causes of failure of root filled teeth are required to obtain meaningful conclusions.
PATHOGENESIS OF VERTICAL ROOT FRACTURES
Although the pathogenesis of VRFs has not been clearly established, it has been proposed that they begin as cracks, at either the coronal or apical aspect of the root, and progress in either the apico-coronal or bucco-lingual planes (Sugaya et al., 2015). Unlike traumatic dental injuries, which are acute in nature and usually present immediately after a recognized impact, VRFs usually develop as a result of a dynamic cyclical fatigue process, and therefore, may take months, years or even decades to become evident (Kishen, 2006). Based on the available literature, the authors of this review propose three main stages for the development of VRFs:
Crack initiation
This is characterized by the development of micro-defects and/or microcracks in dentine (stress concentrations), which may first develop coronally or apically. Microcracks may develop as a result of a number of factors, either in isolation or, more likely, in combination. Although the exact aetiology in each case is impossible to establish, Lynch and McConnell (2002) described possible causes as restorative (to include endodontic) procedures, occlusal, developmental or miscellaneous. Stress concentrations within the root have been related to structural and anatomical features (i.e., presence of an isthmus, unfavourable canal geometry, volume of residual root dentine and/or magnitude of stresses sustained by the residual tooth structure; Arola et al., 2012; Kishen, 2015).
Fracture propagation
Several biomechanical factors have been proposed to influence the progression of a micro-crack into a fracture (cleavage). These include localized increases in strain and tensile stress within the residual dentine caused by repetitive mechanical forces (i.e., mastication and/or parafunction), decreased viscoelasticity (i.e., dehydration due to a lack of free water within the dentinal tubules), ageing of dentine and reduced fracture toughness (Kishen, 2015; Nadeau et al., 2019; Shemesh et al., 2018; Yan et al., 2017). The presence of multiple cracks may also lead to structural weakness of the dentine, increasing the chances of propagation of these cracks into a fracture (Gutmann & Rakusin, 1994).
Of all the factors listed above, fracture toughness may be the most important (Arola et al., 2012; Kinney et al., 2005; Kishen, 2015). Fracture toughness can be defined as the ability of the remaining tooth tissue (with or without a pre-existing crack) to absorb energy (without fracturing) when placed under an applied stress (force/surface area) and/or strain (extension/original length) (Kishen, 2006). An ex vivo study on extracted premolar teeth which were subjected to a combination of thermomechanical cycling and continuous loading was performed to determine the resistance of root filled teeth to VRF (Ossareh et al., 2018). Scanning electron microscopy and finite element analysis were conducted post instrumentation. The authors found a greater risk of micro-crack development when significant volumes of tooth tissue were lost. The stress distribution was shifted apically away from the cervical region in a bucco-lingual plane in teeth with greater loss of tooth structure. During this stage, there is a potential for microbial ingress into the root canal complex, through the portal of entry (VRF) created by cleaving of the root canal dentine (Ricucci et al., 2015). As is the case for apical periodontitis lesions, there will be a delay between microbial infection of the root canal system and the manifestation of clinical symptoms (periapical) and signs (periodontal attachment loss associated with the fracture). Radiographic changes are likely to present at even a later stage.
Separation
In advanced cases of VRF (i.e., complete VRF or split tooth), complete separation of the root ensues, following a period of prolonged cyclical loading (fatigue failure), resulting in a continuous ingress of microbes and the formation of a biofilm (Walton et al., 1984). At this stage, periodontal attachment loss will occur, permitting further microbial ingress, contamination with food particles and plaque formation (Walton et al., 1984). The root filling material and/or sealer will essentially be exposed to the oral cavity resulting in further attachment and bone loss. Even in the absence of a periodontal communication (for example, with apically located fractures), if the root canal system has not been adequately cleaned at the time of the endodontic treatment, the residual microbes within the root canal space may act as the trigger for attachment loss following separation, with the ingress of tissue fluid through the fracture providing nutrition for the recalcitrant microbes.
CLINICAL PRESENTATION
Early stage VRFs are a challenge to detect as the patient may not present with symptoms or signs of apical periodontitis (AP) (Table 2). VRF usually has a delayed presentation and can manifest between 2 and 5 years following completion of root canal treatment (PradeepKumar et al., 2016) Clinically, as the VRF progresses, the patient will eventually present with symptoms and/or signs of AP. These may include tenderness to percussion, swelling, tooth mobility, evidence of marginal ridge fractures and/or pain on biting (von Arx & Bosshardt, 2017; Maddalone et al., 2018; Meister et al., 1980; Walton, 2017).
| Clinical features of VRF |
|---|
|
| Radiographic features of VRF |
|---|
|
Early stage VRF
|
| Advanced stage VRF |
|
To detect a VRF, it is essential to use magnification and illumination. Further investigation to enhance visualization of a VRF may be performed using dyes and/or fibre optic transillumination. Removal of existing restorations and root filling materials, including those from the canal entrances, may facilitate confirmation of the presence and/or extent of a VRF (Abbott, 2004). The fracture line may be visible along the pulpal floor, extending down beyond the canal entrances and/or involving the isthmi. Furthermore, removal of interproximal restorations may be necessary to permit a thorough periodontal examination of the mesial and distal surfaces, which may otherwise be inaccessible due to the presence of closely approximated contact areas. If the VRF is still not readily visible, exploratory surgical procedures may be necessary to visualize the presence and nature of an apical fracture (Pitts & Natkin, 1983; Walton, 2017).
A deep, isolated narrow periodontal probing depth may be a sign of a long-standing VRF, and pathognomonic if detected on both sides of a root (von Arx & Bosshardt, 2017; See et al., 2019; Tamse, Fuss, Lustig, & Kaplavi, 1999; Walton, 2017). In some cases, local anaesthesia may be needed in order to carry out periodontal probing comfortably.
It must be stressed that increased probing depths should be interpreted in context of the history of the tooth and distinguished from probing attributed to periodontal disease and/or a sinus tract of endodontic origin (Tsesis et al., 2010). Broader and more gradual increased probing depths are usually attributable to periodontal disease (Page & Schroeder, 1976). ‘Matching’ increased probing depths on opposite sides of a root are less likely to be observed with a ‘true’ periodontal problem.
A narrow and/or flexible periodontal probe, for example, the UNC-15, PCP-12 or Click-Probe™ (Kershaw, Switzerland), should be aligned parallel to the long axis of the root being examined and gently ‘walked’ around the entire circumference of the tooth, to ensure that any isolated periodontal pockets are not missed.
The presence of a sinus tract associated with VRFs has been reported to occur in the range of 18.2%–67% (PradeepKumar et al., 2016). A recent study demonstrated that when a sinus tract is present in relation to a VRF, it may be located more coronally, either at the mid-root level (77.8%) or at the gingival margin (22.2%) (Kasahara et al., 2020). Multiple sinus tracts are also common pathognomonic features of a VRF; taking a radiograph with a gutta-percha tracer inserted into the sinus tract will allow its source to be determined and facilitate diagnosis (Tamse, 2006).
RADIOGRAPHIC PRESENTATION
The radiographic appearance (Table 2) of VRFs on periapical radiographs (PRs) can be highly variable, and may include no obvious pathology, subtle periradicular bone loss, vertical crestal bone defects or frank separation of the root fragments. Vertical bone defects include the ‘classic’ J-shaped radiographic lesion of a long-standing VRF and/or a ‘halo’ radiolucency often involving the furcation region (Figure 1). In certain situations, when the fracture is initiated at the CEJ and progresses in an axial plane, crestal bone loss may be evident mesially and/or distally. Furthermore, root filled teeth restored with cast posts may present with a radiolucency on the lateral root surface, typically at the base of the post; this is indicative of a VRF (Liao et al., 2017; Nicopoulou-Karayianni et al., 1997; Tamse, Fuss, Lustig, Ganor, et al., 1999; Tamse, Fuss, Lustig, & Kaplavi, 1999; Tamse et al., 2006; Testori et al., 1993).
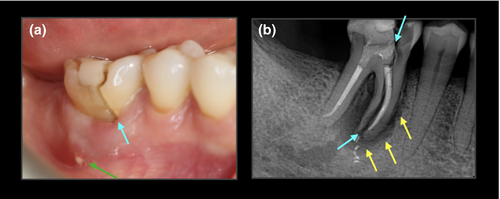
PRs have several well-established limitations in the detection of radiographic signs of endodontic disease, for example, anatomical noise (Bender & Seltzer, 1961) and geometric distortion (Forsberg & Halse, 1994). Early (incomplete) VRFs may not be discernible as the subtle bone defects associated with VRF are masked by the intact cortical plates (anatomical noise) overlying the bone demineralization in the underlying cancellous bone (Brady et al., 2014; Patel et al., 2013). This may result in misdiagnosis and/or inappropriate management (Lustig et al., 2000; Meister et al., 1980).
Cone beam computed tomography (CBCT) overcomes the limitations of PRs by providing undistorted images, which are not susceptible to anatomical noise and enable the clinician to view the tooth from multiple planes and angles (Durack & Patel, 2012). There is clear evidence that CBCT can improve the diagnosis and/or management of complex endodontic problems (Chogle et al., 2020; Ee et al., 2014; Mota de Almeida et al., 2015; Rodriguez et al., 2017).
The literature is replete with studies indicating that CBCT has increased accuracy compared to PRs for the detection of VRFs in root filled teeth. A meta-analysis evaluating data assessing the radiographic detection of root fractures concluded that CBCT provided much higher diagnostic accuracy for the detection of VRFs when compared to PRs (Salineiro et al., 2017). However, the results of this study should be interpreted with caution as there was significant heterogeneity in the methodology between studies, and crucially several of the ex vivo studies included in the meta-analysis had widely displaced fractured segments (0.2–0.4 mm) which would be easily detectable clinically, and therefore, would not require CBCT imaging to confirm the diagnosis (Hassan et al., 2009; Özer, 2011).
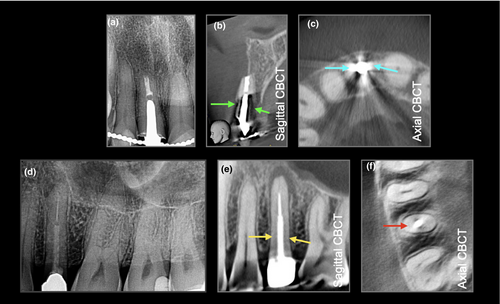
Patel et al. (2013) concluded that CBCT was not accurate for detecting the presence or absence of simulated VRFs in root filled teeth. Imaging artefacts such as beam hardening due to the presence of radio-dense materials (i.e., gutta-percha, metal posts) and/or motion/misalignment artefacts reduce the image quality and can increase the likelihood of false positives (Khedmat et al., 2012; Schulze et al., 2011; Wang et al., 2011). Due to their lower atomic number and reduced radiodensity, fibre (non-metallic) posts have less impact on the diagnostic accuracy when compared to metal posts (Marinho Vieira et al., 2020 et al., 2020; Figure 2). Recent advances in image reconstruction such as motion correction (Spin-Neto et al., 2020) and metal artefact reduction algorithms (MAR) (Queiroz et al., 2018) have been used to reduce imaging artefact, and therefore, improve diagnostic accuracy. However, the results of these studies do not appear to improve the diagnosis of VRFs in root filled teeth (Bechara et al., 2013; Fontenele et al., 2021).
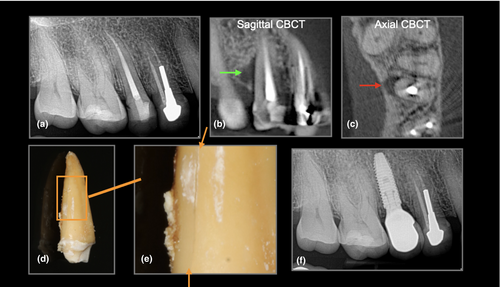
At present, there is insufficient justification to perform a CBCT to detect VRFs within the root of a tooth. However, there is good evidence to indicate that CBCT can detect the subtle radiographic signs of periradicular bone loss indicative of a VRF (Chavda et al., 2014; Dias et al., 2020; G et al., 2021; Zhang et al., 2019). A recent clinical study assessed the accuracy of CBCT for detecting VRFs within roots compared to the accuracy of CBCT in the detection of periradicular bone ( secondary) changes indicative of a root fracture. The authors concluded that although CBCT could not accurately assess VRFs within the root of a tooth, it was significantly more sensitive and accurate in detecting periradicular bone loss patterns suggestive of a VRF (Byakova et al., 2019) Figures 3-5. These findings are in agreement with several other studies and systematic reviews (Chang et al., 2016; Corbella et al., 2014; PradeepKumar et al., 2021; Rosen et al., 2015).
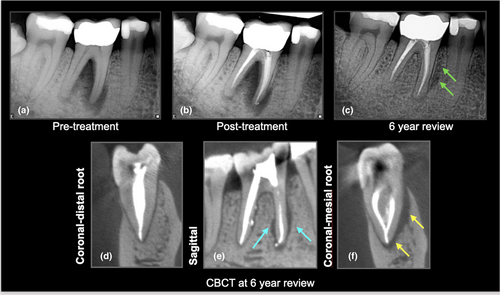
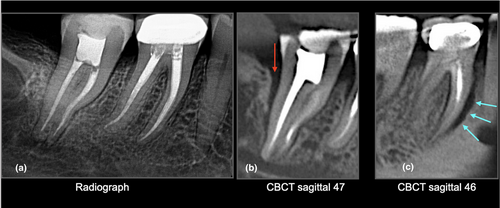
The European Society of Endodontology (ESE, 2019), CBCT position statement and The Joint American Association of Endodontists and American Academy of Oral & Maxillofacial Radiology (AAE/AAOMR) guidelines advocate the use of CBCT when the clinical and conventional radiographic examination are inconclusive in reaching a clear diagnosis in a case of suspected VRF. In these cases, the subtle periradicular bone changes associated with VRF may be detected with CBCT imaging. However, it is essential to tailor and optimize the scanning parameters to the requirement of each individual patient by considering the region of interest, the neighbouring anatomical structures and/or the presence of intracanal materials and/or restorations which could impact the diagnostic quality of CBCT imaging (Gaeta-Araujo et al., 2017).
Of further interest is the emerging research relating to the utilization of Magnetic Resonance Imaging (MRI) and Optical Coherence Tomography (OCT) for the diagnosis of VRFs (Idiyatullin et al., 2016; Shemesh et al., 2008). Although ex vivo investigations are promising, further development of image acquisition and processing is required for MRI (Schuurmans et al., 2019) and improvements in light depth penetration are needed for OCT (de Oliveira et al., 2017).
Future evolution and incorporation of artificial intelligence technology using complex algorithms to assess CBCT images may also enhance the clinical detection of VRFs (Johari et al., 2017; Vicory et al., 2021).
The identification of a VRF may be challenging, as patients are often reluctant to undergo the surgical exploration that is required to permit direct visualization of the root surface(s), as the fracture may be masked by extra-coronal restorations and/or located subgingivally. Moreover, an early stage VRF may share similar pathological signs to post-treatment endodontic disease leading to greater uncertainly in reaching an accurate diagnosis (Tamse, 2006; Tsesis et al., 2010; Walton, 2017).
The presence of multiple pathognomonic clinical and radiographic diagnostic characteristic features increases the likelihood of a tooth being correctly identified with a VRF (Liao et al., 2017; See et al., 2019).
CLINICAL MANAGEMENT
The aim of treatment for teeth with VRF which appear to be restorable is to retain them in a healthy and functional state; this is usually applicable to incomplete VRF only. The desirable objectives of treatment are to eliminate ingress of microbes along the fracture line and prevent destruction of the periodontium.
On diagnosis of a VRF, treatment is recommended as soon as possible to reduce the likelihood of acute apical periodontitis symptoms and/or further periradicular breakdown, as this may complicate the provision of the subsequent prosthodontic replacement, particularly if an implant-retained prosthesis is planned (Walton et al.,1984).
The prognosis of a tooth diagnosed with a VRF is poor (Fuss et al., 2001; Walton, 2017). In the majority of VRF cases, extraction of the tooth is the most predictable treatment of choice.
In certain situations, root resection treatments such as root amputation of the fractured root, or hemi-section, may be appropriate as an alternative treatment option to extraction, permitting retention of the tooth. Prior to root resection procedures, the gutta-percha in the coronal part of the canal to be resected should be replaced with a direct plastic core, for example, glass–ionomer cement or composite resin, which should be placed below the planned level of resection.
Root resection is a well-established treatment option, with retrospective observational studies reporting the survival of teeth following resection of a root as more than 90% over 10 years (Carnevale et al., 1998; Derks et al., 2018). However, there is a lack of clinical research relating specifically to root resection of VRFs in root filled teeth, as the majority of studies observed that root resections were performed for the management of advanced periodontal-endodontic lesions (Setzer et al., 2019). It is feasible to consider that the teeth included in these studies may not be as structurally compromised as those requiring root resection due to VRF, and therefore, the results of these studies should be interpreted with caution.
Several case (series) reports have described novel techniques, and the use of innovative restorative materials, to repair and retain a fractured root. Access to the fractured root can be gained by performing either intentional replantation, raising a surgical flap and/or surgical extrusion of the tooth (Floratos & Kratchman, 2012; Kawai & Masaka, 2002; Nizam et al., 2016). Although recent evidence suggests intentional replantation may be considered as a viable treatment option in specific cases, contraindications to this technique include teeth with advanced periodontal disease and where crown and/or root fracture is highly probably during the course of extraction, as the teeth are usually extensively restored (Becker, 2018; Bender & Rossman, 1993; Cho et al., 2016). The potential complications of surgical intervention to repair the fractured root include the inability to gain full access to the fracture line, gingival recession and/or scarring in the aesthetic zone and removal of healthy crestal cortical bone which may compromise future implant placement (Hadrossek & Dammaschke, 2014).
Repair of the incomplete fractured segment of the root has been suggested with the use of adhesive resins and/or cements, glass–ionomer and bioactive restorative materials such as Biodentine (Septodont, Saint Maur des Fosses, France) (Hadrossek & Dammaschke, 2014; Ozturk & Unal, 2008; Selden, 1996).
There is insufficient evidence in the literature to recommend a specific treatment protocol and/or restorative material for the predictable retention of a root filled tooth with a VRF. The case (series) reports provide limited information due to the small sample sizes, heterogeneous study design and a lack of long-term follow-up data.
PREVENTION
A range of measures can be taken during endodontic treatment to reduce the likelihood of a VRF developing. The clinician must undertake a comprehensive pre-operative assessment of the tooth requiring endodontic treatment by evaluating the factors affecting fracture resistance, such as residual tooth volume, number of remaining walls, presence of pre-existing crack(s) and assessment of root canal morphology.
It is paramount to assess the patient's occlusion, particularly any parafunctional habits (e.g., bruxism), interferences and the overall occlusal scheme (e.g., canine guidance). In cases, where a parafunctional habit has been identified, patient education, as well as the fabrication of an occlusal splint and/or re-establishment of canine guidance, will help to mitigate the biomechanical effects of excessive forces on the teeth (Attanasio, 1997).
Deflective contacts and excursive interferences (particularly in patients with parafunctional activity) may lead to excessive lateral forces on the implicated teeth, and thereby increase the likelihood of a VRF, particularly in heavily restored root filled teeth (Chan et al., 1998; Liao et al., 2017). Both functional and non-functional forces have a more significant effect on terminal teeth as they are subjected to higher loading when compared to anterior teeth (Cavel et al., 1985). The presence of proximal contacts helps to dissipate occlusal forces to prevent catastrophic failure of root filled teeth (Aquilino & Caplan, 2002; Ng et al., 2011). It is therefore desirable to consider the replacement of missing posterior units, where possible, in order to increase the number of occlusal contacts and distribute occlusal and non-occlusal loading.
A pragmatic approach to access cavity preparation and root canal instrumentation, as well as the use of conservative post-endodontic restorative techniques, can be undertaken by a clinician to maintain sound tooth structure. Preservation of peri-cervical dentine during endodontic treatment may theoretically lower susceptibility to VRF by reducing transmission of occlusal forces to the root (Boveda & Kishen, 2015; Clark & Khademi, 2010a; Plotino et al., 2017). It is imperative to note that there is still a lack of strong clinical evidence to support minimal invasive access cavity preparation (Silva, Versiani, et al., 2021, 2022). Despite the recent trend towards more conservative access cavities, the clinician must not compromise on adequate disinfection of the root canal system which remains the core tenet for favourable endodontic treatment outcomes (Al-Nuaimi et al., 2018; Restrepo-Restrepo et al., 2019; Siqueira & Roças, 2021).
Expedient post-treatment placement of an adhesive core, and when appropriate, prompt cuspal coverage restoration to limit cuspal flexure, is essential to reduce the likelihood of VRF (Davis & Shariff, 2019; Pratt et al., 2016).
The use of specific intracanal materials may aid the prevention of microbial leakage and/or propagation of pre-existing cracks in teeth requiring endodontic treatment. A prospective cohort study, performed over a 2–4-year period, assessed the survival of premolar and molar teeth with pre-existing radicular orientated cracks extending up to 5 mm into the canal, and those with associated increased periodontal probing depths (Davis & Shariff, 2019). In this study, a fluoride-releasing resin containing nanoglass particles was used to seal the portion of the canal affected by the crack (and just apical to the base of the crack) using a dental operating microscope. The teeth were taken out of occlusion to eliminate excursive interferences, and patients were instructed to avoid chewing on the side of the cracked tooth. Following the completion of endodontic treatment, patients were advised to proceed with immediate placement of a definitive cuspal coverage restoration. The survival rate for the observed teeth in this study was 96.6% at 4 years.
When considering the cuspal coverage restoration of root filled teeth, the use of cast gold or contemporary monolithic all ceramic materials, in conjunction with a carefully executed bonding protocol, will permit minimal preparation of the residual tooth structure. Depending on the remaining number of walls and residual tooth volume, an onlay should be considered to optimize stress/strain distribution on the tooth and maximize the available bonding substrate following the completion of endodontic treatment. Furthermore, root filled teeth should ideally be avoided as bridge or denture abutments in order to reduce any additional functional forces and/or stresses (Sorensen & Martinoff, 1985).
Novel hybrid nano-ceramic materials such as Cerasmart (GC Corporation, Tokyo, Japan), Lava Ultimate (3 M ESPE, USA) and Enamic (Vita Zahnfabrik, Bad Säckingen, Germany) have recently been developed and may be used in the fabrication of a post-endodontic restoration. These materials have a similar elastic modulus to dentine due to the presence of a homogenously distributed matrix of nano-ceramic particles. As a result, these materials may act as a stress absorber which may reduce stress within the root dentine under load (Gresnigt et al.,2016; Rocca et al., 2016). However, these observations have only been evaluated in vitro, and further clinical studies are required to determine whether these effects are translatable into clinical practice.
A post is only indicated for the retention of the core material when there is minimal sound coronal tissue; it does not improve the strength of the residual tooth structure (Bhuva et al., 2021; ESE, 2021). Wherever possible, posts should be passively cemented into the root canal; mechanical preparation of the root canal walls should be, ideally, avoided (Naumann et al., 2018). Active screw posts should be avoided as these post systems necessitate excessive removal of root canal dentine and result in unfavourable stress generation (Maddalone et al., 2018).
Adhesively cemented endocrowns show promising results; these restorations may be used as an alternative to post-retained crowns for teeth with minimal residual tooth structure. Studies have shown similar survival rates (90%) when compared to conventional full coverage crowns over 7 and 10 years (Fages et al., 2017; Otto & Mormann, 2015).
Educating clinicians to appreciate the importance of expedient placement of conservative cuspal coverage restorations, as well as recognition of undesirable occlusal interferences during both the provisional and definitive restorative procedures, is paramount in the prevention of a VRF.
It is imperative to ensure effective communication between the patient and the clinician to avoid medico-legal issues in relation to the potential consequences of a VRF following endodontic treatment. The patient should be actively involved in the diagnosis and treatment planning processes to ensure they understand the factors which may exacerbate the risk of VRF in root filled teeth.
CONCLUSION
VRFs are most prevalent in root filled molar teeth. There are several putative aetiological and predisposing factors; however, which factors are most critical is unknown and difficult to elucidate.
The symptoms and/or clinical signs of VRF, particularly in the early stages, can make a confident diagnosis of VRF challenging. CBCT may be useful to diagnose the radiographic features of periradicular bone loss pathognomonic of a VRF.
High-level evidence for prevalence, diagnosis and management of VRFs is lacking. There is a need for well-designed clinical studies assessing the presentation, as well as the prognosis of VRFs managed with different treatment protocols.
ACKNOWLEDGEMENTS
We would like to thank Professor John Whitworth, School of Dental Sciences, Newcastle University, Framlington Place, Newcastle upon Tyne, NE2 4BW, UK for his advice on the preparation of this paper.
CONFLICT OF INTEREST
The authors deny any conflicts of interest.




