Present status and future directions: Microbiology of endodontic infections
Abstract
Apical periodontitis has a microbial aetiology and is one of the most common inflammatory diseases that affect humans. Fungi, archaea and viruses have been found in association with apical periodontitis, but bacteria are by far the most prevalent and dominant microorganisms in endodontic infections. Bacterial infection of the root canal system only occurs when the pulp is necrotic or was removed for previous treatment. In some specific cases, including acute and chronic abscesses, the bacterial infection may reach the periradicular tissues. Intracanal bacteria are usually observed as sessile multispecies communities (biofilms) attached to the dentinal root canal walls. Infection in the main root canal lumen can spread to other areas of the root canal system. Although more than 500 bacterial species have been detected in endodontic infections, a selected group of 20 to 30 species are most frequently detected and may be considered as the core microbiome. There is a high interindividual variability in the endodontic microbiome in terms of species composition and relative abundance. Obligate anaerobic species are more abundant in the intraradicular bacterial communities of teeth with primary apical periodontitis, while both anaerobes and facultatives dominate the communities in post-treatment apical periodontitis. Bacterial interactions play an essential role in determining the overall virulence of the community, which has been regarded as the unit of pathogenicity of apical periodontitis. This article reviews the microbiologic aspects of endodontic infections and provides perspectives for future research and directions in the field.
INTRODUCTION
Apical periodontitis is one of the most prevalent oral inflammatory diseases (Eriksen, 2008; Tiburcio-Machado et al., 2021). Observations from studies in rats, monkeys and humans have clearly demonstrated its infectious aetiology (Kakehashi et al., 1965; Möller et al., 1981; Ricucci & Siqueira, 2010a; Sundqvist, 1976). Bacteria are the major infectious agents implicated in causation of apical periodontitis (Siqueira & Rôças, 2009c) and are found usually organized in biofilm communities attached to the canal walls.
Infection establishes in the root canal only after the pulp becomes necrotic, as a result of caries, trauma, periodontal disease or iatrogeny, or the pulp is absent because of previous root canal treatment (Langeland, 1987; Ricucci & Siqueira, 2013). Once infection is established in the root canal, it gradually advances in an apical direction, until eventually bacteria and/or their virulence factors reach the periradicular tissues via apical and lateral foramina, as well as iatrogenic root perforations, and cause inflammation. Apical periodontitis is then established and, depending on a myriad of bacterial and host-related factors, it can be symptomatic (acute) or asymptomatic (chronic). Significant periradicular tissue damage can occur in the form of pus formation (abscess) or bone resorption.
The ultimate goals of root canal treatment are to prevent and treat apical periodontitis (Ørstavik, 2020). Consequently, this means that treatment should prevent bacteria from infecting or re-infecting the pulp space or the periradicular tissues or eradicate or at least control an already existing root canal infection. A thorough understanding of the microbiologic aspects of apical periodontitis is of paramount importance for implementation of high-quality endodontic practice based on solid scientific foundation. This article reviews diverse important microbiologic aspects of apical periodontitis and provides perspectives for future research and directions in the field.
BIOFILM AND THE COMMUNITY-AS-PATHOGEN CONCEPT
One of the most used definitions for biofilms is that they consist of sessile microbial communities attached to a surface and composed of cells enmeshed in a self-produced polymeric extracellular matrix and exhibiting an altered phenotype with regard to growth rate and gene expression (Costerton, 2007; Donlan & Costerton, 2002). The matrix plays an essential role in the morphology, ecology, survival and resistance of the microbial biofilm community (Flemming & Wingender, 2010). Biofilms represent the main form that cellular microorganisms are found in nature and are well recognized as more resistant to antimicrobial treatment (Costerton, 2007; Olsen, 2015).
Morphologic studies have disclosed bacterial organizations in infected canals resembling what currently is termed biofilm (Molven et al., 1991; Sen et al., 1995; Siqueira et al., 2002a). However, Ricucci & Siqueira (2010a) were the first ones to report on the prevalence and association of bacterial biofilms with primary and post-treatment apical periodontitis. They evaluated the occurrence of biofilms in the apical canal and reported an overall prevalence of intraradicular biofilms in 80% of the untreated canals and 74% of the treated canals, with higher frequencies in teeth with large and cystic lesions. That study reported that apical periodontitis fulfilled all the requisites available in the literature to be included in the list of biofilm-mediated disease (Hall-Stoodley & Stoodley, 2009; Parsek & Singh, 2003; Ricucci & Siqueira, 2010a; Figure 1).
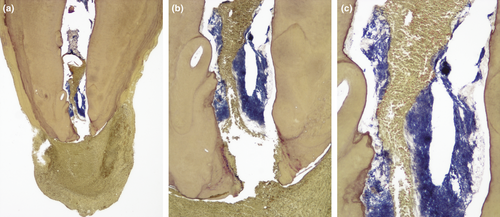
Nevertheless, the cross-sectional nature of morphologic studies can only reveal association, but not causation. Therefore, it remains unknown whether the bacterial organization in biofilm precedes and is a prerequisite for apical periodontitis to develop or is a later event. Whatever the answer, from a treatment perspective, it is important that the clinician be aware that bacterial biofilms occur in the apical root canal segment in the large majority of teeth with primary or post-treatment apical periodontitis, and biofilms usually represent a challenge for proper infection control (Costerton et al., 1999; Svensater & Bergenholtz, 2004).
Biofilm morphology, including thickness, matrix:cell ratio and dominant bacterial morphotypes, has been observed to vary from case to case (Ricucci & Siqueira, 2010a). These findings are in line with the reported interindividual variability in the bacterial diversity of endodontic infections (discussed below). It has been shown that the thickness of the biofilm matrix is dependent upon the species composition and increases with the community age (Abdallah et al., 2014; Flemming & Wingender, 2010; Leriche et al., 2000). The environmental conditions have been shown to affect the biofilm development, behaviour and response to treatment (Chavez de Paz et al., 2007; George et al., 2005; Goller & Romeo, 2008). Factors that influence the endodontic biofilm morphology and resistance to treatment represent an interesting topic for more research in the field.
For many of the human infectious diseases caused by members of the resident microbiota (opportunistic pathogens), the focus has moved from the “single-pathogen” concept, traditionally associated with true classic pathogens, to the realization that the community is the unity of pathogenicity. This concept has been applied to the aetiology of most oral infectious diseases (Jenkinson & Lamont, 2005; Kuramitsu et al., 2007), including apical periodontitis (Siqueira & Rôças, 2009a).
According to the community-as-pathogen concept, the disease is the result of the collective action of the microbial consortium, which is influenced by species composition and relative abundance, and the network of interactions between them (Siqueira & Rôças, 2009a). Therefore, it seems inappropriate to study the pathogenic traits of isolated strains or attribute-specific roles for a given species in disease causation without taking the role context of the community behaviour into account.
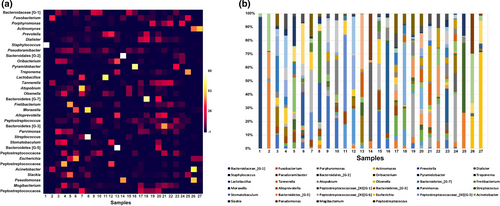
Data from numerous community-profiling studies have shown a great interindividual variability in the diversity of endodontic bacterial communities associated with the same clinical disease (Chugal et al., 2011; Li et al., 2010; Machado de Oliveira et al., 2007; Rôças et al., 2004b; Siqueira et al., 2004b, 2011; Figure 2). This indicates that apical periodontitis has a heterogeneous aetiology, in which different species combinations can lead to the same disease outcome. Thus, irrespective of the individual-to-individual differences in root canal species composition, the bacterial communities possibly exhibit a similar physiologic and pathogenic behaviour. Conserved metabolic gene expression profiles have been reported for disease-associated biofilm communities, regardless of the interindividual variability in species composition (Jorth et al., 2014). Therefore, there may be some redundancy in terms of collective physiology and pathogenicity in disease-associated biofilm communities. In this context of redundancy, the diversity of bacterial products and virulence factors released to the environment is lower than the species diversity. Consequently, it is possible to infer that a bacterial product that plays an important role in the community physiology or pathogenicity may be provided by different species in different consortia.
INFECTION DISTRIBUTION THROUGHOUT THE ROOT CANAL SYSTEM: ESSENTIAL LESSONS TO GUIDE DISINFECTION
Two very important pieces of information for every healthcare professional involved with treatment of an infectious disease include the identity of bacterial species involved and how they are distributed in the infected site. The former is of great relevance to determine the susceptibility of the infective agents to antimicrobial substances. The latter assumes utmost importance to help establish strategies for the antimicrobial treatment to reach and be effective against the infective agents distributed through the tissues. This information has a critical application in root canal treatment, given the remarkable anatomic complexity of the canal system and the location of bacteria commonly associated with treatment failures.
The patterns of bacterial colonization in untreated and treated teeth with apical periodontitis have been extensively evaluated by morphologic studies that reveal a complex distribution of bacterial infection throughout the root canal system (Molven et al., 1991; Ricucci & Siqueira, 2010a; Sen et al., 1995; Siqueira et al., 2002a). Most of the bacteria occurring in the main root canal are attached to the dentinal root canal walls forming biofilm communities. In some teeth, especially those with longstanding infectious processes associated with large apical periodontitis lesions, bacterial biofilms can be seen covering a large area of the main canal walls (Ricucci & Siqueira, 2010a). However, in most cases, biofilm formation is not uniformly observed along the canal walls, with biofilm-covered areas interspersed with areas with discrete or even no bacteria attached (Siqueira et al., 2002a).
In the main canal of untreated teeth, bacteria are usually seen as aggregates (cells with the same morphology), coaggregates (cells with distinct morphologies) and planktonic cells suspended in the fluid phase of the canal lumen (Ricucci & Siqueira, 2013). The most advanced frontline of infection in the main canal lumen of teeth with apical periodontitis can be located anywhere in the canal, from the junction between the middle and apical thirds to the apical foramen, and even occasionally beyond the foramen (Ricucci et al., 2006; Ricucci & Siqueira, 2010a).
Morphologic studies have also shown that the infection can spread from the main canal lumen to other areas of the system, including apical ramifications, lateral canals, isthmuses, recesses and dentinal tubules (Matsuo et al., 2003; Ricucci & Siqueira, 2010a, 2010b). These areas represent a great challenge for proper disinfection of the canal system (Perez et al., 2020; Vera et al., 2012), and studies show that bacterial persistence in the canal before filling is a significant risk factor for post-treatment disease (Sjögren et al., 1997; Waltimo et al., 2005; Zandi et al., 2019). Complex anatomic areas may serve as reservoirs of residual infection to cause persistent apical periodontitis, as demonstrated by morphologic studies evaluating teeth with post-treatment disease (Arnold et al., 2013; Carr et al., 2009; Ricucci & Siqueira, 2008, 2010b; Ricucci et al., 2009, 2013). Therefore, the clinician should be aware that special disinfection strategies may be required to enhance infection control in the canal system and increase the possibility of better treatment outcomes.
In a few cases, infection can also spread to the periradicular tissues as planktonic cells, flocs or biofilms attached to the root surface (Noiri et al., 2002; Ricucci et al., 2005, 2015; Tronstad et al., 1990). Extraradicular infections, when present, are usually associated with symptoms and/or sinus tracts (Ricucci et al., 2018a; Ricucci & Siqueira, 2010a). This issue is discussed in more detail below.
THE INCREASINGLY COMPLEX ENDODONTIC MICROBIOME
Another crucial piece of knowledge for anyone involved with management of an infectious disease is the identity of the microbial pathogens. Research in endodontic microbiology had a long-held desire to identify the main culprit for apical periodontitis. However, since the introduction of anaerobic culture and later sophisticated molecular microbiology methods, the hope of narrowing the list of suspected endodontic pathogens to a single species or at least a few has dissipated. Over the years, with the advances in the analytical accuracy and sensitivity, the list of candidate endodontic pathogens has instead expanded.
A census of the microbial species detected in the different types of endodontic infection (primary, secondary, persistent and extraradicular) by studies published up to 2009 revealed approximately 500 different species, the large majority of each bacteria, but also fungi and archaea (Siqueira & Rôças, 2009c). The bacterial species/phylotypes detected in endodontic infections belong to 9 phyla: Bacteroidetes, Firmicutes, Spirochaetes, Fusobacteria, Actinobacteria, Proteobacteria, Synergistetes, “Candidatus Saccharibacteria” (formerly TM7) and SR1 (Siqueira & Rôças, 2009c). Over the following years, high-throughput sequencing (HTS) technology has been introduced and reported the occurrence of representatives of at least 10 other phyla (Hong et al., 2013; Li et al., 2010; Ozok et al., 2012; Sanchez-Sanhueza et al., 2018; Santos et al., 2011; Siqueira et al., 2016; Tzanetakis et al., 2015; Vengerfeldt et al., 2014; Zandi et al., 2018). Most of the phylotypes from these newly reported phyla are low-abundance bacteria. Actually, a review of 12 HTS studies confirmed that the most prevalent and abundant bacterial species/phylotypes in endodontic infections belong to five phyla: Firmicutes, Actinobacteria, Bacteroidetes, Proteobacteria and Fusobacteria (Shin et al., 2018).
DIFFERENT COMMUNITY PROFILES ACCORDING TO INFECTION TYPES AND GEOGRAPHY
Irrespective of the high interindividual variability (no two infections are the same in terms of species composition and relative abundance), community profiling studies have shown that individuals with the same disease manifestation or from the same geographical location can exhibit more similarities among them when compared to their counterparts. For instance, the community structure significantly differs according to the clinical presentation of the disease, i.e., asymptomatic apical periodontitis, acute apical abscesses or post-treatment apical periodontitis (Sakamoto et al., 2006; Santos et al., 2011; Siqueira et al., 2004b; Tzanetakis et al., 2015). These findings may be a consequence of the different root canal environmental conditions shaping the bacterial diversity. They also suggest that the disease severity (intensity of signs/symptoms) or response to treatment may be associated with the structure (number of species and their relative abundance) of the endodontic bacterial community.
As for geographic locations, significant differences in the prevalence of candidate endodontic pathogens have been reported for individuals residing in different countries (Baumgartner et al., 2004; George et al., 2016; Rôças et al., 2004a, 2006, 2011; Siqueira et al., 2005a). Studies fingerprinting the endodontic communities from different locations have also confirmed these differences (Machado de Oliveira et al., 2007; Siqueira et al., 2008). Geographical differences may not apparently impact the outcome of the nonsurgical endodontic treatment because it is based on unspecific antimicrobial procedures and substances. However, implications of these findings may be more apparent in the treatment of acute abscesses, in which antibiotics with specificity to certain bacterial groups are used as adjuncts to treatment.
THE ENDODONTIC MICROBIOME IN PRIMARY APICAL PERIODONTITIS
Primary intraradicular infection is the infection of the necrotic pulp and is the cause of primary apical periodontitis (Siqueira, 2002). The bacterial species that participate in these infections comprise pioneer species, which started the process of pulp inflammation, necrosis and infection, and latecomers, which took advantage of the environmental conditions in the necrotic and initially infected canal to establish themselves.
Studies using anaerobic culture and molecular microbiology techniques have shown that primary intraradicular infections are characterized by a mixed bacterial community with high prevalence and dominance of obligate anaerobic bacteria (for review see Siqueira & Rôças, 2009c). Although a large number of species have been found in infected canals, many of them were detected in a few teeth and in only one or a few studies (Siqueira & Rôças, 2009c). The most frequently detected species have varied from study to study. However, a group of 20 to 30 species are identified as members of mixed communities in virtually every study of primary infections, indicating that they are the main candidate endodontic pathogens and may compose the core microbiome. They include several Gram-negative (Fusobacterium nucleatum, Dialister species, Porphyromonas endodontalis, Porphyromonas gingivalis, Prevotella species, Tannerella forsythia and Treponema species) and Gram-positive bacteria (Parvimonas micra, Filifactor alocis, Pseudoramibacter alactolyticus, Olsenella uli, Actinomyces species, Streptococcus species, Propionibacterium species and Cutibacterium acnes) (Baumgartner et al., 1999; Fouad et al., 2002; Gomes et al., 1996a; Haapasalo et al., 1986; Keskin et al., 2017; Munson et al., 2002; Rôças & Siqueira, 2008, 2018; Sakamoto et al., 2006; Siqueira et al., 2000, 2007; Sundqvist, 1976, 1992; Vickerman et al., 2007).
The large majority of these taxa are validly named and known species. Moreover, the breadth of bacterial diversity in endodontic infections has been expanded to include several as-yet-uncultivated and/or uncharacterized bacteria, as determined by culture-independent molecular studies (Siqueira & Rôças, 2005a). Approximately 55% of the bacterial taxa found in infected root canals of teeth with primary apical periodontitis are as-yet-uncultivated phylotypes, which represent about 38% of the community members in terms of relative abundance (Sakamoto et al., 2006). Some phylotypes, such as Bacteroidaceae [G-1] bacterium HMT 272 (or Bacteroidetes clone X083) and members of the Synergistetes and Spirochaetes phyla, are amongst the most prevalent bacteria in primary infections (Rôças & Siqueira, 2008, 2009; Sakamoto et al., 2006, 2009). There is a need to successfully cultivate these prevalent and dominant phylotypes so as to evaluate their pathogenic and antimicrobial susceptibility traits.
Bacterial population density in primary infections range from 103 to 108 cells per canal (Blome et al., 2008; Paiva et al., 2013a; Sakamoto et al., 2007; Sundqvist, 1976; Vianna et al., 2006b). The mean number of bacterial species (richness) per canal ranges from 10 to 30 species/phylotypes (Munson et al., 2002; Ribeiro et al., 2011; Rôças & Siqueira, 2008; Siqueira & Rôças, 2009c; Siqueira et al., 2004b), but HTS studies have suggested that these numbers may reach a mean of almost 100 species or even more per canal (Hong et al., 2013; Keskin et al., 2017; Santos et al., 2011; Siqueira et al., 2011; Vengerfeldt et al., 2014). Many of those found in these highly sensitive technologies are conceivably low-abundance species, which cannot be considered as irrelevant because at least an ecologic role in the community is expected (Hajishengallis et al., 2011; Sogin et al., 2006). Bacterial counts and number of species have been demonstrated to be proportionally related to the size of the apical periodontitis lesion (Rôças & Siqueira, 2008; Siqueira et al., 2007; Sundqvist, 1976). In other words, the larger the lesion, the higher the bacterial load and richness. This may be explained by the fact that large lesions conceivably represent long-standing infectious processes in the large majority of cases.
THE MICROBIOME ASSOCIATED WITH ACUTE APICAL ABSCESSES
The typical symptomatic (acute) manifestations of apical periodontitis include symptomatic apical periodontitis and acute apical abscess. The former may in some circumstances evolve into the latter, which is a more severe and advanced form of the disease. In symptomatic infections, bacteria are located not only in the root canal but may have also invaded the periradicular tissues. When an acute abscess is established, infection may spread to other anatomical spaces of head and neck and cause complications (Siqueira & Rôças, 2013).
Pus samples taken from the root canal by paper points or by aspiration through the swollen mucosa have demonstrated a mixed microbiota dominated by obligate anaerobic bacteria (Khemaleelakul et al., 2002; Kuriyama et al., 2000; Nobrega et al., 2016; Rôças & Siqueira, 2018; Sakamoto et al., 2006; Siqueira & Rôças, 2009b; de Sousa et al., 2003). HTS studies have confirmed and expanded the high bacterial diversity in acute apical abscesses (Hsiao et al., 2012; Santos et al., 2011). The species involved are basically the same as reported for primary infections, because most of the abscess cases evaluated in these studies were indeed associated with primary infections (untreated canals). However, the prevalence and counts of certain species as well as the partnerships formed in the mixed communities have been reported to differ between asymptomatic and symptomatic (including abscesses) infections (Gomes et al., 1996a; Griffee et al., 1980; Rôças & Siqueira, 2018; Rôças et al., 2011; Sakamoto et al., 2006; Santos et al., 2011; Sundqvist, 1976), which may partly explain the progression to this more severe type of infection.
As-yet-uncultivated and uncharacterized phylotypes represent 24% to 46% of the taxa in acute abscesses (Flynn et al., 2012; Sakamoto et al., 2006) and account for 6% to over 30% of the community in relative abundance (Riggio et al., 2007; Sakamoto et al., 2006). Bacteroidaceae [G-1] bacterium HMT 272 phylotype has also been one of the most prevalent phylotypes in acute abscesses, being detected in 14% to 36% of pus samples (Rôças & Siqueira, 2009; Siqueira & Rôças, 2009b).
Bacterial counts in acute apical abscesses range from 104 to 109 cells per pus sample (Khemaleelakul et al., 2002; Lewis et al., 1986; Williams et al., 1983). Bacterial richness is more pronounced in abscesses in comparison with canals of teeth with asymptomatic apical periodontitis (Sakamoto et al., 2006; Santos et al., 2011; Siqueira et al., 2004b), suggesting that this is another possible factor that influences the development of symptoms.
THE ENDODONTIC MICROBIOME IN POST-TREATMENT APICAL PERIODONTITIS
Persistence or appearance of signs and/or symptoms of active infection (e.g., periradicular bone radiolucency, sinus tract, swelling, pain, tenderness to percussion) in root canal-treated teeth means that apical periodontitis has persisted, emerged or recurred. This condition is referred to as post-treatment apical periodontitis, which is an infectious problem caused by persistent or secondary intraradicular infection or, in some cases, by an extraradicular infection (Siqueira, 2001).
The causative agents of post-treatment disease have been evaluated in samples taken from the entire extent of the root canal during retreatment or exclusively from the root apices obtained during surgery (Siqueira & Rôças, 2022). The latter is discussed below in the section on apical microbiome.
A high interindividual variability in the structure of the intracanal bacterial communities of treated teeth has also been demonstrated, indicating that different bacterial combinations can lead to post-treatment disease (Rôças et al., 2004b; Sakamoto et al., 2008). Infections are usually mixed, but studies show a less diverse microbiota than teeth with primary disease, especially those with apparently adequate root fillings; teeth with inadequate previous treatments in turn present bacterial richness similar to teeth with primary infections (Pinheiro et al., 2003; Rôças et al., 2004b; Sakamoto et al., 2008; Sundqvist et al., 1998). The total bacterial levels in previously treated canals associated with apical periodontitis range between 103 and 107 cell equivalents, with the higher counts observed in inadequately treated canals (Antunes et al., 2015; Blome et al., 2008; Sedgley et al., 2006; Siqueira et al., 2020).
Enterococcus faecalis is one of the most prevalent species found in canals of teeth with post-treatment apical periodontitis (Gomes et al., 2008; Henriques et al., 2016; Murad et al., 2014; Peciuliene et al., 2000; Pinheiro et al., 2003, 2015; Rôças et al., 2004c, 2008; Schirrmeister et al., 2007; Sedgley et al., 2006; Siqueira & Rôças, 2004; Sundqvist et al., 1998). In terms of relative abundance, this species may account for a high range of less than 1% to 100% of the total bacteria (Rôças & Siqueira, 2012; Sanchez-Sanhueza et al., 2018; Sedgley et al., 2006; Zandi et al., 2016, 2018). This species is much more likely to be found in post-treatment than primary disease (Rôças et al., 2004c), and this may be related to its ability to survive under harsh environmental conditions (Kayaoglu & Orstavik, 2004; Lleo et al., 2001). Actually, E. faecalis has also been found in similar prevalence in treated canals of teeth with no apical periodontitis (Kaufman et al., 2005; Zoletti et al., 2006).
Other bacterial taxa have also been detected in high frequencies and abundance in post-treatment infections. They include Streptococcus species (Antunes et al., 2015; Chavez de Paz et al., 2005; Pinheiro et al., 2003; Rôças et al., 2004a; Siqueira & Rôças, 2004), which have been found as the dominant community members in many cases (Antunes et al., 2015; Rôças & Siqueira, 2012; Zandi et al., 2016, 2018), Actinomyces species, C. acnes, P. alactolyticus, Arachnia propionica (Propionibacterium propionicum), Dialister species, F. nucleatum, P. micra and Prevotella species (Gomes et al., 2008; Pinheiro et al., 2003; Rôças et al., 2008; Rôças & Siqueira, 2012; Sakamoto et al., 2008; Siqueira & Rôças, 2004, 2005b; Sundqvist et al., 1998; Zandi et al., 2018).
As-yet-uncultivated or uncharacterized phylotypes correspond to 55% of the taxa encountered in treated canals with apical periodontitis, with a mean relative abundance of approximately 50% (Sakamoto et al., 2008). As with primary infections, the phylotype Bacteroidaceae [G-1] bacterium HMT 272 is also amongst the most prevalent taxa in post-treatment infections (Sakamoto et al., 2008). An interesting finding was that as-yet-uncultivated bacteria may represent the dominant taxa in some cases, which help explain why culture can fail to detect bacteria in all cases of post-treatment disease.
Detection of bacteria in the root canal at the time of filling is a risk factor for post-treatment apical periodontitis (Sjögren et al., 1997; Waltimo et al., 2005; Zandi et al., 2019). This indicates that persistent infections represent an important cause of treatment failure. Studies have identified the species/phylotypes found after chemomechanical preparation and interappointment medication and revealed a higher frequency of gram-positive species (Byström & Sundqvist, 1985; Chavez de Paz et al., 2003; Gomes et al., 1996b; Sakamoto et al., 2007). As-yet-uncultivated phylotypes have also been detected (Paiva et al., 2013a, 2013b; Rôças et al., 2014; Sakamoto et al., 2007), corresponding to about 40% of the taxa (Sakamoto et al., 2007). Although the general presence of bacteria at the time of filling projects a poor prognosis, no specific species has been linked to it (Siqueira & Rôças, 2008). Actually, there is a scarcity of longitudinal studies that evaluated the antimicrobial effects of treatment and followed up the cases to disclose possible associations between specific persistent species and the treatment outcome.
THE APICAL MICROBIOME: A CRITICAL ISSUE
The main portals of exit of the root canal system to the periradicular tissues are located in the apical part of the root in the form of main foramen and accessory foramina. This explains why inflammation caused by root canal infection is much more frequently located in the periapical tissues. The apical root canal system is a critical area when it comes to treatment because the bacteria that cause apical periodontitis are mostly located in this region.
Given the recognized importance of the apical microbiome to the aetiology of apical periodontitis, it is curious that there are not many studies evaluating the bacterial taxa colonizing that area. This may be partially explained by the technical difficulties to collect samples exclusively from the apical canal segment, which is virtually impossible in the clinical setting using paper points during root canal treatment. For untreated teeth with primary apical periodontitis, the only way to obtain samples exclusively from the apical canal is by using teeth scheduled for extraction. The apical root segment of the extracted tooth is sectioned, and samples are collected from the apical canal by using paper points, burs or files or by cryopulverizing the root specimen (Siqueira & Rôças, 2022). Actually, cryopulverization is the best approach to obtain a sample from the entire apical canal system including the main canal and also other areas not commonly sampled adequately by the other methods, including dentinal tubules, recesses, isthmuses and ramifications (Alves et al., 2009a).
Miller (1894) observed microscopically that the composition of bacterial morphotypes in the apical canal was different from the most coronal regions. Later studies confirmed these differences in terms of predominant morphotypes (Thilo et al., 1986), the anaerobe:facultative ratio (Fabricius et al., 1982) and the bacterial community structure (Alves et al., 2009b; Ozok et al., 2012). A study using community profiling analysis revealed that although the number of different species composing the apical and middle/coronal microbiome were almost similar, the types of species present could be very different or even completely different in some teeth (Alves et al., 2009a). Community profiling studies also disclosed a high subject-to-subject variability in the composition of the apical microbiome (Alves et al., 2009b; Siqueira et al., 2011).
Obligate anaerobic bacteria dominate the apical microbiome, and such a dominance becomes progressively larger in more advanced stages of infection (Fabricius et al., 1982). The most prevalent bacterial taxa detected in the apical canal segment of teeth with primary apical periodontitis include Olsenella uli, P. alactolyticus, Prevotella species, P. endodontalis, Streptococcus species, F. nucleatum, P. micra, T. forsythia and Treponema species (Baumgartner & Falkler, 1991; Dougherty et al., 1998; Ozok et al., 2012; Rôças et al., 2010; Siqueira et al., 2004a, 2009, 2011; Takahama et al., 2018). Bacterial counts in the apical segment of the root canal range from 104 to 106 cells per root apex, as determined by culture (Baumgartner & Falkler, 1991).
Post-treatment apical periodontitis is usually caused by bacteria present in the apical root canal system (Ricucci et al., 2009). In most cases, these bacteria are persistent ones that survived the effects of the previous treatment or retreatment, while in some cases they may be secondary invaders, resulting from coronal leakage or a breach in the aseptic chain during the previous treatment (Siqueira & Rôças, 2008). As with primary apical periodontitis, there are not many studies that identified the bacterial taxa in the apical canal system of teeth with post-treatment disease. For this type of analysis in root filled teeth, the apical root specimen can be obtained by root-end resection during endodontic surgery or tooth extraction (Siqueira & Rôças, 2022).
Complex bacterial communities have been reported by HTS of cryopulverized apical root samples from teeth with post-treatment apical periodontitis, also exhibiting a high variability between individuals (Siqueira et al., 2016). The most dominant taxa were Fusobacterium species and Pseudomonas species, while Enterococcus species were detected in only a few cases, always in low numbers (Siqueira et al., 2016). Streptococcus species, members of the Actinobacteria phylum and P. alactolyticus, were the most prevalent taxa in another molecular investigation of cryopulverized root apexes (Antunes et al., 2015). The high frequency and dominance of Streptococcus and Actinobacteria species were confirmed in another molecular study (Siqueira et al., 2020). The bacterial counts in the apical canal segment of adequately treated teeth with apical periodontitis were approximately 103 to 104 cell equivalents per root apex (Antunes et al., 2015; Siqueira et al., 2020).
Table 1 summarizes the main features of the different types of endodontic infections based on the literature reviewed and cited in this article.
| Feature | Primary intraradicular infection | Persistent intraradicular infection | Persistent/secondary intraradicular infection | |||
|---|---|---|---|---|---|---|
| Asymptomatic apical periodontitis (full root canal) | Asymptomatic apical periodontitis (apical canal) | Acute Apical Abscess (extraradicular infection) | Root canal filling stage (post-preparation/post-medication samples) | Root canal-treated teeth (full root canal) | Root canal-treated teeth (apical canal) | |
| Community | Mixed | Mixed | Mixed | Mixed, occasionally single | Mixed, occasionally single | Mixed, occasionally single |
| Number of taxa/sample | 10 to 30 | 20 to 30 | 20 to 30 | 1 to 5 |
Adequate treatment: 1 to 5 Inadequate treatment: 10 to 30 |
10 to 20 |
| Number of bacterial cells/sample | 103 to 108 | 104 to 106 | 104 to 109 | 102 to 105 | 103 to 107 | 103 to 104 |
| Uncultivated phylotypes (% richness) | 40%–55% | Not Determined | 24%–46% | 42% | 55% | 54% |
| Most frequent taxa |
Fusobacterium nucleatum Porphyromonas spp. Treponema spp. Tannerela forsythia Dialister spp. Filifactor alocis Pseudoramibacter alactolyticus Synergistetes spp. Prevotella spp. Olsenella uli Parvimonas micra Streptococcus spp. Cutibacterium acnes Propionibacterium spp. Actinomyces spp. |
Olsenella uli Pseudoramibacter alactolyticus Prevotella spp. Porphyromonas endodontalis Streptococcus spp. Fusobacterium nucleatum Parvimonas micra Tannerella forsythia Treponema spp. |
Fusobacterium nucleatum Porphyromonas spp. Treponema spp. Parvimonas micra Tannerela forsythia Dialister spp. Prevotella spp. Streptococcus spp. |
Streptococcus spp. Propionibacterium spp. Fusobacterium nucleatum Prevotella spp. Pseudoramibacter alactolyticus Parvimonas micra Olsenella spp. Actinomyces spp. Pseudomonas aeruginosa Enteric rods |
Enterococcus faecalis Candida albicans (yeast) Streptococcus spp. Pseudoramibacter alactolyticus Arachnia propionica Cutibacterium acnes Parvimonas micra Fusobacterium nucleatum Dialister spp. Actinomyces spp. Pseudomonas aeruginosa Enteric rods |
Streptococcus spp. Actinobacteria spp. Pseudoramibacter. alactolyticus Fusobacterium spp. Enterococcus faecalis Pseudomonas aeruginosa |
EXTRARADICULAR INFECTION: NO MORE CONTROVERSIES?
Apical periodontitis is characterized by the development and coexistence of host innate and adaptive immune responses concentrated around the portals of exit of bacteria from the infected canal to the periradicular tissues (Cavalla et al., 2021; Provenzano et al., 2016; Sasaki & Stashenko, 2012). In the large majority of cases, these responses succeed in preventing advance of the infection, which remains confined into the canal space (Ricucci et al., 2006). As discussed above, in the symptomatic (acute) forms of the disease, especially when an abscess is formed, the infection has been shown to cross the boundaries of the apical foramina and reach the periradicular tissues (Siqueira & Rôças, 2013). No wonder the acute apical abscess is the main indisputable example of extraradicular infection. The occurrence of extraradicular infection in asymptomatic (chronic) forms of the disease is one of the most controversial issues in endodontics (Bergenholtz & Spångberg, 2004).
Studies evaluating teeth with apical periodontitis, especially post-treatment disease, and excluding acute abscesses, have reported extraradicular infections in the form a biofilm attached to the outer apical root surface (Noiri et al., 2002; Ricucci et al., 2015, 2016; Tronstad et al., 1990) or as flocs (cohesive colonies or “planktonic biofilms”), often resembling actinomycotic-like colonies, occurring in the lesion body (Happonen, 1986; Ricucci et al., 2018b; Ricucci & Siqueira, 2008; Sakellariou, 1996). However, in the large majority of cases with extraradicular infection, symptoms (pain, tenderness to percussion) or sinus tracts were present (Ricucci et al., 2018a; Ricucci & Siqueira, 2010a).
Actually, the most common form of asymptomatic (chronic) periradicular disease that is often associated with an extraradicular infection is the chronic apical abscess. A recent histobacteriologic evaluation of teeth with this condition, exhibiting a sinus tract but no symptoms, found extraradicular bacteria in 83% of the cases; most of them in the form of bacterial biofilms attached to the root surface around the exit of the apical foramen (Ricucci et al., 2018a).
Several studies using culture (Signoretti et al., 2013; Sunde et al., 2002; Tronstad et al., 1987; Wayman et al., 1992) and molecular microbiology methods (Gatti et al., 2000; Handal et al., 2009; Saber et al., 2012; Sunde et al., 2000; Zakaria et al., 2015) have identified extraradicular bacteria in post-treatment lesions and reported the occurrence of many anaerobic bacteria that are commonly found in intraradicular infections. In most of these studies, samples were taken during endodontic surgery. The difficulties in preventing sample contamination by bacteria from saliva and the gingival crevice during surgery were recently addressed (Siqueira & Rôças, 2022). Even if the contamination issue is disregarded, the possibility exists that the extraradicular bacteria were there only transiently before being eliminated by the host defenses.
Nevertheless, morphologic evaluation of specimen blocks containing both the root apex and the lesion, preserving their spatial relationship, can provide more reliable information as to the occurrence of extraradicular bacteria and their pattern of tissue colonization, also helping to preclude the possibility of external contamination (Ricucci et al., 2020). Histobacteriologic studies have reported the occurrence of extraradicular biofilms in approximately 6% of the teeth with apical periodontitis (Ricucci & Siqueira, 2010a), some exhibiting diverse levels of mineralization – apical calculus (Ricucci et al., 2005, 2016). In general, extraradicular biofilms have been associated with symptoms in untreated teeth (Ricucci & Siqueira, 2010a), as well as persistent symptoms (Ricucci et al., 2015) and persistent exudation (Ricucci et al., 2016) in teeth under treatment.
When present, the extraradicular biofilm forms a continuum with an intraradicular biofilm (Ricucci & Siqueira, 2010a; Subramanian & Mickel, 2009), suggesting that the former may be sustained by (and dependent on) the latter. Conceptually, these cases may be successfully handled by root canal treatment. There are only a few cases reported in the literature in which the extraradicular infection was not apparently associated with intraradicular bacteria and were the alleged cause of persistent post-treatment disease (Ricucci et al., 2015, 2018b). These cases were only resolved by surgery.
CHANGING GEAR: FROM IDENTITY TO ATTITUDE
The microbial species composition of different types of endodontic infections has been extensively deciphered by culture and molecular identification methods. Therefore, the “who's there” question has been widely addressed. Time has come to determine the behaviour of those species in terms of physiology, function and pathogenicity, moving the research interest to address the question “what are they doing there”. Identification data permit only inferences to be made in this regard. The microbial behaviour can be predicted by using genetic information generated by metagenomics or metatranscriptomics analyses, which have not so far been performed for endodontic infections. Another way is by evaluating the products (e.g., proteins, metabolites) secreted by the microbial community in situ.
- Proteins involved with metabolic and housekeeping processes were commonly detected in the root canal as well as in apical periodontitis and abscess samples, indicating that the bacterial communities were viable and active (Francisco et al., 2019; Nandakumar et al., 2009; Provenzano et al., 2013, 2016);
- Many proteins associated with virulence were found, including proteases, adhesins, exotoxins, invasins and biofilm formation (Francisco et al., 2019; Nandakumar et al., 2009; Provenzano et al., 2013, 2016);
- Proteins involved with resistance to antibiotics were identified, including beta-lactams and tetracyclines (Francisco et al., 2019; Nandakumar et al., 2009; Provenzano et al., 2013);
- Numerous proteins related to survival in stressful conditions were found (Provenzano et al., 2013, 2016);
- Abscess samples resulted in higher amounts of bacterial proteins than asymptomatic cases (Provenzano et al., 2013);
- Archaeal proteins were occasionally found (Provenzano et al., 2013).
Host proteins related to the innate and adaptive immune responses were also detected in abscesses and apical periodontitis lesions (Alfenas et al., 2017; Francisco et al., 2019; Provenzano et al., 2016).
FUTURE DIRECTIONS
Basic science is of fundamental importance to explain natural phenomena, contributing to the knowledge and understanding of nature and its processes. Most scientific theories are essentially based on data from basic science. The studies evaluating the microbial cause of apical periodontitis, the species involved and how they colonize the canal system are typical examples of basic science that can be applied to help solve clinical problems. This is exemplified by the recognition of the need of asepsis and proper infection control for a satisfactory treatment outcome, the choice for antimicrobial substances that are effective against endodontic pathogens and the development of strategies to enhance disinfection in difficult-to-reach areas of the canal system. Basic science can also set the foundation for the development of new technologies, substances and materials, which is another example of applied science.
The past two decades have witnessed a substantial expansion in the knowledge of the microbial diversity in endodontic infections, brought about by technologic advances in microbiologic methods. The recognition of the bacterial biofilm community as the unit of pathogenicity of apical periodontitis (Siqueira & Rôças, 2009a) has prompted studies to profile the communities associated with different clinical conditions. These studies generally show a core of species that are found in the majority of cases and also a high interindividual variability in species composition and relative abundance (Siqueira & Rôças, 2009c). The first information may help drive antimicrobial protocols that are more effective against the most commonly found species. The second information may be the basis for a customized approach to treat root canal infections on an individual basis.
Endodontic bacterial communities are composed of multiple species, resulting in a high number of potential interactions between the community members that define their overall behaviour. This makes some communities be more aggressive (virulent) than others and may play a significant role in causing severe host inflammatory response and symptoms (Siqueira & Rôças, 2013). Actually, studies have shown that the community structure significantly differs when comparing symptomatic and asymptomatic infections (Rôças et al., 2011; Sakamoto et al., 2006; Santos et al., 2011; Siqueira et al., 2004b). The fact that some species have not been confirmed as the main pathogens associated with symptoms does not mean that they cannot act as “keystone pathogens” (Hajishengallis et al., 2012) and orchestrate the community towards to a more virulent phenotype. This seems to be an interesting avenue for future investigations. Moreover, still in the field of symptomatic infections, the specific role, if any, played by herpesviral/bacterial interactions still remains to be clarified (Jakovljevic & Andric, 2014).
One important limitation of the microscopic studies investigating the patterns of bacterial colonization of the root canal system is that they provide no information on species identity. This type of information would be very useful to help identify the tissue location of the major candidate pathogens involved with primary and post-treatment apical periodontitis, as well as to reveal the species architecture and arrangements in multispecies biofilms, especially in the apical canal. Fluorescent in situ hybridization (FISH) and its variants could be explored to provide these pieces of information. One limitation of the method is its closed-ended nature, but it might help confirm the status of some candidate endodontic pathogens and shed some light on the role of as-yet-uncultivated phylotypes.
The study of the endodontic community behaviour is still incipient and limited to a few metaproteomic studies (Francisco et al., 2019; Nandakumar et al., 2009; Provenzano et al., 2013, 2016) and evaluations of secreted bacterial metabolic products (Maita & Horiuchi, 1990; Provenzano et al., 2015). This is an area with a great potential for expansion, including extending the use of metaproteomics to answer more questions, as well as exploiting other approaches not yet used in endodontic microbiology research, such as global gene expression by transcriptomics and building comprehensive inventories of released metabolites by metabolomics. Because of functional redundancy, identification of released products may help disclose patterns that might serve as biomarkers and be useful for the development of chairside tests to guide proper treatment and project the treatment prognosis or the risks of disease aggravation.
Qualitative data from culture (Engström et al., 1964; Sjögren et al., 1997; Sundqvist et al., 1998; Waltimo et al., 2005) and molecular studies (Zandi et al., 2019) have demonstrated that attainment of negative results for bacteria in root canal samples taken at the time of filling can project a better long-term outcome for teeth with apical periodontitis. Therefore, findings from microbiologic studies have been regarded as adequate surrogate endpoints for long-term treatment outcome. However, most information available refers to bacterial presence/absence. Because of the wide range in bacterial counts persisting after treatment, qualitative data are not accurate. It has been established that the main microbiologic goals of endodontic treatment are to promote reduction in bacterial counts to levels that may be compatible with healing of apical periodontitis (Siqueira, 2011). Therefore, looking for the association of quantitative findings for persisting bacteria and treatment outcome is required. A molecular study revealed that canals with residual bacterial levels below 3 × 103 cells at the time of filling had a satisfactory retreatment outcome characterized by healing of apical periodontitis (Zandi et al., 2019). Other studies using sophisticated highly sensitive methodologies are required to confirm these findings and expand them for teeth with primary apical periodontitis.
Moreover, there is no consistent study searching for specific species, bacterial association patterns or bacterial virulence factors or metabolites detected at the time of filling that might be potential risk factors for post-treatment apical periodontitis and serve as outcome predictors. Host factors related to apical periodontitis activity, such as cytokines and other mediators of inflammation, have also been detected and quantified before and after root canal treatment (Maia et al., 2020; Tavares et al., 2012, 2013) and represent other potential targets to predict prognosis.
Many of the areas of future research suggested above might serve as the basis for development of chairside tests to predict the treatment outcome or the risk of flare-ups and other complications. Some chairside tests for rapid bacterial detection have been proposed in the literature (Herzog et al., 2017; Sato et al., 2012; Tan et al., 2015). However, they have yet to be introduced in the clinical routine, and there is a need for them to be validated by long-term longitudinal follow-up studies showing a correlation between the rapid chairside results and treatment outcome.
Although bacteria are by far the most frequent and dominant microorganisms in endodontic infections, other microbial types have been reported in much lower prevalence and sometimes only in special circumstances. Fungi are only occasionally found in primary infections (Egan et al., 2002; Lana et al., 2001; Möller, 1966; Siqueira et al., 2002a, 2002b), but some molecular studies have detected them more frequently than previously reported (Baumgartner et al., 2000; Miranda et al., 2009; Persoon et al., 2017). As for teeth with post-treatment apical periodontitis, Candida species have been encountered in up to 18% of the cases (Cheung & Ho, 2001; Egan et al., 2002; Molander et al., 1998; Möller, 1966; Peciuliene et al., 2001; Pinheiro et al., 2003; Siqueira & Rôças, 2004; Sundqvist et al., 1998). Although archaea have not been detected in some studies (Rôças & Siqueira, 2011a, 2011b; Siqueira et al., 2005b), others have reported the occurrence of methanogenic archaea (Ozok et al., 2012; Paiva et al., 2012; Vianna et al., 2006a; Vickerman et al., 2007) or archaeal proteins (Provenzano et al., 2013) in teeth with apical periodontitis. Viruses, especially herpesviruses, have been detected in periapical samples of teeth with symptomatic apical periodontitis lesions (Sabeti et al., 2003a, 2003b), acute apical abscesses (Chen et al., 2009; Ferreira et al., 2011a, 2011b), large radiolucent lesions (Sabeti & Slots, 2004; Sabeti et al., 2003b), and lesions from HIV-positive patients (Saboia-Dantas et al., 2007). Currently, the role of fungi, archaea and viruses in endodontic infections remains to be clarified and represent an important research line in the field.
Extraradicular infection is one of the causes of post-treatment apical periodontitis (Siqueira et al., 2014). With current diagnostic technologies, it is impossible nowadays to determine in the clinical setting whether the cause of a persistent disease is intraradicular or extraradicular. This information might help guide proper case selection and treatment. Developing proper diagnostic tests to make such a distinction, preferably chairside, should be a goal of future research.
With the advances in molecular microbiologic diagnosis, rapid, sensitive and accurate assays are now available to identify bacteria and other microorganisms in a matter of minutes to a few hours. In cases of disseminating and complicated abscess infection of endodontic origin, the clinician and the patient might benefit from a rapid microbiologic diagnosis to adhere to the best therapy. Rapid detection of antibiotic resistance genes may also help guide prescription of the best antibiotic for each individual case (Jungermann et al., 2011; Rôças & Siqueira, 2013).
Evidence has mounted that in spite of the variability in the bacterial diversity of endodontic infections, individuals from the same geographical location have more similarities between them when compared to those residing in distant locations (Machado de Oliveira et al., 2007; Siqueira et al., 2008). With this knowledge comes the inevitable question as to whether the best treatment approach to treat infection in a study population can be applied to others. This may be probably more significant in infections treated by antibiotics, which are drugs that affect a specific spectrum of species. Multicenter studies evaluating the efficacy of antimicrobial protocols in different geographic populations are required.
There is a low-to-moderate evidence in the literature that apical periodontitis can exert systemic effects in the host and predispose to systemic diseases (Berlin-Broner et al., 2017; Caplan et al., 2006; Cotti et al., 2011; Cotti & Mercuro, 2015; Garrido et al., 2019; Gonzalez-Navarro et al., 2020; Joshipura et al., 2006; Pasqualini et al., 2012; Segura-Egea et al., 2015). So far, there is no scientific reason to regard endodontic infections as segregate events that have no effects on other body sites. Such systemic involvement is more likely to be related to endodontic infections as part of the total oral infectious burden or to bacteremia stemming from endodontic treatment or acute abscesses. There are numerous questions without answers, such as which species or strains of certain species are more likely to survive bacteremia and establish themselves at distance sites; what is the amount of bacteria in the canal and bloodstream during bacteremia that might influence a systemic response; and what are the effects of antimicrobial root canal treatment on systemic health.
CONCLUDING REMARKS
The infectious aetiology of apical periodontitis is well established, and the microbiome involved with the different types of endodontic infections have been extensively examined. New analytical technologies may help fill in the existing gaps in knowledge about disease pathogenesis and response to treatment. Personnel and financial investment in microbiology research should be stimulated to foster further advances in the field. Several perspectives for future research are proposed herein. There is an urgent need for efforts to translate the extant substantial basic knowledge into improvements in clinical practice and treatment outcome.
ACKNOWLEDGEMENTS
This study was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazilian Governmental Institutions.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.




