PRIASE 2021 guidelines for reporting animal studies in Endodontology: explanation and elaboration
Abstract
Laws and ethics require that before conducting human clinical trials, a new material, device or drug may have to undergo testing in animals in order to minimize health risks to humans, unless suitable supporting grandfather data already exist. The Preferred Reporting Items for Animal Studies in Endodontology (PRIASE) 2021 guidelines were developed exclusively for the specialty of Endodontology by integrating and adapting the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines and the Clinical and Laboratory Images in Publications (CLIP) principles using a validated consensus-based methodology. Implementation of the PRIASE 2021 guidelines will reduce potential sources of bias and thus improve the quality, accuracy, reproducibility, completeness and transparency of reports describing animal studies in Endodontology. The PRIASE 2021 guidelines consist of a checklist with 11 domains and 43 individual items and a flowchart. The aim of the current document is to provide an explanation for each item in the PRIASE 2021 checklist and flowchart and is supplemented with examples from the literature in order for readers to understand their significance and to provide usage guidance. A link to the PRIASE 2021 explanation and elaboration document and PRIASE 2021 checklist and flowchart is available on the Preferred Reporting Items for study Designs in Endodontology (PRIDE) website (http://pride-endodonticguidelines.org/priase/).
Introduction
The need for the Preferred Reporting Items for Animal Studies in Endodontology (PRIASE) 2021 guidelines
Animal testing is important for evaluating the preclinical safety and effectiveness of new dental materials, drugs or devices to help identify and eliminate potential health risks to humans. However, the translation of research observations from animal studies to humans has always been challenging (Yoneda et al. 2017). Sometimes the most promising products developed using animal research can fail when used in human trials and never become incorporated in daily clinical practice (Hackam & Redelmeier 2006, Pound & Bracken 2014). Furthermore, poorly designed and executed animal studies can produce unreliable and inaccurate preclinical results (Pound & Bracken 2014, Singh et al. 2016), which can defeat the purpose of animal testing, rendering it useless.
The ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines (Kilkenny et al. 2010, Percie du Sert et al. 2020) and the SYRCLE (Systematic Review Centre for Laboratory animal Experimentation) risk of bias tool (Hooijmans et al. 2014) were developed to guide researchers and ultimately improve the quality of animal studies. However, animal studies in Endodontology often need information related exclusively to the specialty. Hence, the PRIASE 2021 guidelines were developed with the objective of improving the standard of manuscripts submitted to journals describing animal studies linked to the specialty of Endodontology. It is anticipated these guidelines will be of value to researchers, editors and peer reviewers of scientific journals (Nagendrababu et al. 2021).
The use of experts to develop the PRIASE 2021 guidelines
The PRIASE guidelines were developed by building a consensus within a group of experts in the field of Endodontology (Nagendrababu et al. 2021) and followed the Guidance for Developers of Health Research Reporting Guidelines (Moher et al. 2010). The project leaders (VN, PD) identified the need for reporting guidelines for animal studies in Endodontology. A steering committee was formed with nine members (PD, VN, AK, PM, MN, JF, EP, JJ and SP), that included the project leaders. The steering committee drafted a preliminary checklist and flowchart which included the essential items required to be included in peer-reviewed manuscripts of animal studies within the specialty of Endodontology. This initial draft checklist and flowchart were developed by integrating and adapting the ARRIVE guidelines (Kilkenny et al. 2010, Percie du Sert et al. 2020) and Clinical and Laboratory Images in Publications (CLIP) principles (Lang et al. 2012).
The steering committee formed a PRIASE Delphi Group (PDG) consisting of 31 experts from around the world with the aim of building consensus through the use of the Delphi methodology to revise the items of the preliminary PRIASE guidelines. The revised PRIASE checklist and flowchart were then discussed during an online meeting conducted via Zoom on 9 September 2020 with a PRIASE Online Meeting Group (POMG) made up of 28 individuals (19 academics/clinicians, two postgraduate students, seven steering committee members). The details of each item were discussed, and collective feedback was obtained that allowed the steering committee to further refine the items in the checklist and flowchart. The revised guidelines were then tested by several volunteer authors who drafted hypothetical manuscripts describing animal studies in Endodontology when following the revised PRIASE guidelines. The final version of the PRIASE 2021 guidelines consists of a checklist with 43 items under 11 sections and a flowchart (Nagendrababu et al. 2021).
PRIASE 2021 explanation and elaboration document
This explanation and elaboration document provides a comprehensive explanation for each of the items in the checklist and for the contents of the flowchart. In addition, it reproduces extracts from reports of published animal studies to provide further help for authors and enhance understanding. In some of the real examples, citations or website addresses have been removed, and abbreviations entered in full.
Item 1a: Title – The specific animal species and its health or disease status (sometimes called ‘animal model’) must be provided
Explanation
The type of animal (rat, mouse etc.) must appear in the title to help readers identify the animal model used (Examples 1a.1, 1a.2). This information facilitates indexing in databases and may improve search results, for example Knockout mice with periapical lesions, or Wistar rats with exposed molars etc. Other details of the animal model need only be included in the title if they are a focus of the study, for example age, gender, root canal disinfection, healing of periapical lesions etc.
Example 1a.1
From Conti et al. (2020) – ‘Relationship between apical periodontitis and atherosclerosis in rats: lipid profile and histological study’.
Example 1a.2
From Cotti et al. (2017) – ‘The Influence of Adalimumab on the Healing of Apical Periodontitis in Ferrets’.
Item 1b: Title – The specific test, field, subject, treatment of interest within the animal model must be provided
Explanation
The title must specify the treatment or study intervention using descriptive terms and words for readers to identify the focus and key elements of the study (Examples 1b.1, 1b.2), for example biocompatibility, regenerative endodontics, sealer microleakage, pulp capping, tooth replantation resorption, apexification, apexogenesis, periapical healing, root canal disinfection, irrigation, analgesic effectiveness, stem cell therapy, etc. An exception can apply when the animal experiment is only a small part of a larger multiphase study with several other components, for example animals were used to test the biocompatibility of a newly developed sealer along with several other laboratory-based tests. In this scenario, it may not be essential to include the animal model and specific test in the title because of title word count limitation.
Example 1b.1
From Lin et al. (2019) – ‘Dental Pulp Stem Cell Transplantation with 2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside Accelerates Alveolar Bone Regeneration in Rats’.
Example 1b.2
From Xu et al. (2016) – ‘Systemically Transplanted Bone Marrow-derived Cells Contribute to Dental Pulp Regeneration in a Chimeric Mouse Model’.
Item 2a: Keywords – Keywords such as ‘animal model or ‘in vivo model’ and the specific area(s) of interest must be provided
Explanation
The inclusion of between two and five relevant keywords can help to identify peer-reviewed manuscripts of specific interest to readers, facilitate the indexing in databases and improve the results of electronic literature searches. One of the keywords must be ‘animal model’ or ‘in vivo model’. Other keywords should include terms from the medical subject headings (MeSH) terminology of the National Library of Medicine (NLM; Examples 2a.1, 2a.2).
Example 2a.1
From Altaii et al. (2016) – For the animal study entitled ‘Endodontic regeneration and tooth revitalization in immature infected sheep teeth’, the key words used were ‘animal model, dental pulp necrosis, immature teeth, regeneration treatment, revitalization treatment’.
Example 2a.2
From Chang et al. (2020) – For the animal study entitled ‘Regeneration of Tooth with Allogenous, Autoclaved Treated Dentin Matrix with Dental Pulpal Stem Cells: An In Vivo Study’, the key words used were ‘autoclaved, dental pulp stem cells, in vivo study, tissue engineering, treated dentin matrix’.
Item 3a: Abstract – The Introduction of the Abstract must explain the significance of the study
Explanation
The introduction of the abstract (if provided) must identify the gap in knowledge and mention the significance and relevance of the study (Examples 3a.1, 3a.2). The significance is an explanation of how the study fills a gap in current knowledge, and the reasons why the use of an instrument, device, material or treatment may be beneficial or controversial. The information should be succinct, not confusing and focus on the important details.
Example 3a.1
From Chang et al. (2020) – ‘Biomaterials designed for tissue engineering should be nontoxic and nonimmunogenic and should achieve their intended functions. Treated dentin matrix (TDM), a bioactive extracellular matrix, is promising for tooth regeneration. However, the effect of sterilization on the surface properties of allogenous TDM in the animal model is unclear’.
Example 3a.2
From Tohma et al. (2020) – ‘Pulp capping materials allow healing of injured pulp with a layer of reparative dentin. Glucose is needed to cure the injured area. Glucose is transported by glucose transporter (Glut) 2 and Glut4, which are transmembrane proteins that act as gatekeepers. We hypothesized that the transport of glucose via Glut2/Glut4 might contribute to the production of a dentin bridge during wound healing. Therefore, we explored Glut2 and Glut4 expression during reparative dentinogenesis after mineral trioxide aggregate capping’.
Item 3b: Abstract – The unambiguous aim(s) and objective(s) of the study must be provided
Explanation
The aim and objectives must be clearly described using terms that do not confuse readers (Examples 3b.1, 3b.2). Terms that have more than one clear assumed meaning must be defined by using a specific criterion, such as success or failure. For example, success can mean several things: survival of teeth, no pain, radiographic healing or something else entirely. Failure can mean several things: lack of healing, flare up, pain, missed canal, irreversible pulpitis, necrotic pulp, loss of teeth, or something else entirely. To help authors improve the clarity of their aims and objectives, the use of PICO(T) elements: Problem/Patient/Population, Intervention/Indicator, Comparison, Outcome, and (optional) Time element or Type of Study, is recommended.
Example 3b.1
From Zaccaro Scelza et al. (2010) – ‘The present study aimed to evaluate the inflammatory response of 17% EDTA, 17% EDTA-T, and 10% citric acid in bony defect created in rat jaws’.
Example 3b.2
From Tawil et al. (2009) – ‘The purpose of this study was to assess the healing of periapical tissues using three different materials (IRM [L.D. Caulk Inc, Dentsply International Inc, Milford, DE], Geristore [Den-Mat, Santa Maria, CA], and MTA [ProRoot MTA; Dentsply Tulsa Dental Specialties, Tulsa, OK]) after endodontic microsurgery in an animal model’.
Item 3c: Abstract – The most important details of the animal and the experimental model must be provided
Explanation
The abstract must describe the details of the species, strain and health/disease status of the animals with enough specificity for a reader to identify the animal model. The type of model employed must be mentioned (Examples 3c.1, 3c.2).
Example 3c.1
From Saito et al. (2020) – ‘A groove-shaped cavity was prepared on the mesial surface of the upper first molars in wild-type and Opn knockout (KO) mice’.
Example 3c.2
From Azevedo et al. (2019) – ‘Experimental periapical lesions (C57Bl/6 wild-type mice) were evaluated regarding endogenous vasoactive intestinal peptide (VIP) expression correlation with lesion development and the effect of recombinant vasoactive intestinal peptide (VIP) therapy in lesion outcome’.
Item 3d: Abstract – Key details of the methodology must be provided
Explanation
The Methodology section within the Abstract must briefly explain what materials, devices, instruments, motors, solutions, drugs and treatments were investigated, including the criteria used to describe the outcomes (Examples 3d.1, 3d.2).
Example 3d.1
From Conti et al. (2020) – ‘Atherosclerosis was induced using a high-lipid diet associated with a surgical ligature in the carotid artery and a super dosage of vitamin D3. Apical periodontitis was induced via pulp exposure to the oral environment. At 45 and 75 days, serum levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured. The maxillary and mandibular jaws and carotid artery were collected and processed for histological analysis’.
Example 3d.2
From Jara et al. (2018) – ‘A standard serial root canal preparation technique was performed in the molar of one side, whilst the opposite side was the control group. Rats were randomly divided into three experimental groups (n = 8), according to the diameter of apical enlargement during root canal preparation: K-files size 20 (EG1), size 25 (EG2) and size 30 (EG3). Each animal was its own positive control, because the opposite arch remained untreated. Root canals were filled with a standard technique. After 3 weeks, the animals were euthanized. The main outcome of apical periodontitis healing was evaluated radiographically (mm2) and histologically (ordinal scores of inflammation) using a HE staining technique’.
Item 3e: Abstract – The most relevant and important results must be presented succinctly including differences amongst the means, medians or modes of the dependent variables (treatment outcome and test results) and any significant P-values
Explanation
The mean, median or mode outcome(s) of the treatments should be reported, along with the differences, and P-value significance (Examples 3e.1, 3e.2).
Example 3e.1
From Alves et al. (2018) – ‘There was no significant difference in the bacterial penetration among groups A, B, and C at 45 days (P = 0.903) and 120 days (P = 0.211). No statistically significant difference was found (P = 0.608) between the exposure time intervals’.
Example 3e.2
From Berlin-Broner et al. (2020) – ‘Both groups developed a similar degree of atherosclerosis (mean lesion area 7.46 ± 0.44% in the Tx group compared with 7.65 ± 0.46%, in the Sham group, P = 0.77), and a similar degree of inflammation’.
Item 3f: Abstract – Succinct conclusions supported by the results must be provided
Explanation
The Conclusions of the Abstract must be based only upon the results (Examples 3f.1, 3f.2). The best abstracts have memorable ‘take-away’ messages and give advice on future practice and research; however, over-generalizing the conclusions or speculation must be avoided.
Example 3f.1
From Saito et al. (2020) – ‘These results suggest that the expression of dentin matrix protein 1 (DMP1) is up-regulated in osteopontin (OPN) knockout mice both in vivo and in vitro, and DMP1 compensates for the lack of OPN in regulating odontoblast like cell differentiation after tooth injury’.
Example 3f.2
From Conti et al. (2020) – ‘Apical periodontitis influenced triglyceride levels, increasing them even in the absence of atherosclerosis, and influenced the increase in the thickness of the carotid artery intima tunic in the presence of atherosclerosis. Atherosclerosis intensified the inflammatory reaction and increased bone resorption in periapical lesions’.
Item 4a: Introduction – The relevant background information must be provided using terminologies consistent with professional standards and previous publications
Explanation
The Introduction must accurately describe the relevant background information, using terminologies consistent with professional standards and previous publications (Examples 4a.1, 4a.2). Professional terminologies (tooth number, root canal morphology, treatment etc.) must be used to avoid causing reader confusion. New terminologies should not be invented, or old terminologies defined incorrectly. Multiple professional terminologies to describe the same issue must not be used, terminologies must be consistently used throughout the manuscript, that is avoid inventing a new term, novel root-end maturogenesis, to describe apexogenesis; avoid confusing root canal treatment with other endodontic treatments (e.g. partial-pulp capping, Cvek pulp capping, apexification, apexogenesis and regenerative endodontics).
Example 4a.1
From Choi et al. (2019) – ‘Vital pulp therapy such as direct pulp capping, indirect pulp capping, and partial or full pulpotomy can be used to preserve the health status of teeth (1) because healthy pulp tissue is very important for tooth longevity (2, 3). Up-regulation of odontogenic differentiation, dentin formation, and angiogenesis of human dental pulp cells (hDPCs) are key factors in vital pulp therapy (4). Materials used in vital pulp therapy should have adequate biocompatibility and bioactivity to promote dental pulp stem cell activity and pulp healing in permanent teeth (5). Although mineral trioxide aggregate (MTA) provides a very good sealing (6), acceptable biocompatibility, and dentin bridge formation in animal teeth (7) and human teeth 8, it has some drawbacks such as discoloration potential, the presence of heavy metal, difficult handling characteristics, a long setting time (9), and high material cost. Thus, several new brands of MTA products such as Biodentine (Septodont, Saint-Maur-des-Fosses, France) and EndocemZr (Maruchi, Wonju, Korea) have been introduced into the market in an attempt to overcome these shortcomings. These MTA products have shown a relatively fast setting time, good biologic outcomes, and acceptable color stability’.
Example 4a.2
From Lin et al. (2019) – ‘Dental pulp stem cells (DPSCs), the first identified dental stem cell source, have inherent mesenchymal characteristics and osteogenic potential. DPSCs are harvested from adult tooth pulp tissues after enzyme treatment (3). Although gene profiles of DPSCs are similar to those of bone marrow mesenchymal stem cells, DPSCs showed higher colony-forming units and proliferation rates (4). In addition to a higher proliferation rate, DPSCs also possess the ability of mutilineage differentiation, such as osteogenic (5), neurogenic, and adipogenic lineages (6). In the past few decades, DPSCs for bone tissue regeneration have been widely reported in tissue engineering and regenerative medicine (7, 8). To begin with, significantly increased alkaline phosphatase (ALP) levels and up-regulation of osteogenic markers, such as Runx2, osteopontin, and osteocalcin, were observed in DPSCs when cultured in osteogenic medium, suggesting that DPSCs were undergoing osteogenic differentiation on the one hand (9, 10) After 40 days of culture, human DPSCs were reported to form a structure similar to a woven fibrous bone with physical qualities of in vitro and in vivo bone on the other hand (11). Furthermore, osteogenic induction of DPSCs and their combinations with various biomaterials such as collagen were also investigated and resulted in positive outcomes (12, 13, 14). Although the therapeutic potential of DPSCs has been studied for bone regeneration, the therapeutic efficiency needs further consideration and examinations for clinical applications (15)’.
Item 4b: Introduction – The appropriateness of the selected animal model to address the aims and objectives of the study must be explained
Explanation
In the context of the general public opposition to animal testing on nonhuman primates (monkeys, apes etc.) and pets (dogs, cats etc.), a statement describing the appropriateness of the animal model must be provided (Examples 4b.1, 4b.2, 4b.3). Extremely painful and extensive traumatic testing on some types of animals that maybe distressing to readers is generally not acceptable for publication. Painful animal tests must include pain monitoring and appropriate pain relief measures. The animal model must have appropriate tissues, cells, lesions, infections, anatomy, physiology and an immune system to accomplish the aim and objective of the study. Ideally, animals must have a fully functioning immune system to study healing responses and disinfection, for example root canals must be infected to study disinfection. There should be a clear justification for using a particular animal test method. For example, subcutaneous implantation of dental materials to study biocompatibility in accordance with ISO 10993 and 7405 standards.
Example 4b.1
From Altaii et al. (2016) – ‘The possibility of endodontic regeneration/revitalization treatment of immature infected teeth is a recent development offering considerable biological advantages; but a more complete understanding of the treatment requires in vivo research in a suitable animal model. Primates have been used in many endodontic regeneration studies because of their anatomical similarity to human, but these animals are expensive, not readily available and can be difficult to manage. Dogs are seen as pets in many cultures, and have substantially different tooth anatomy to humans. Rodent incisor teeth are small with wide-open apices and have a continuous growth. Larger animal models such as pigs offer an alternative, but they can grow to an unmanageable size and can be very unpleasant and uncooperative. Sheep, on the other hand have been used in many medical and dental studies due to their teeth being similar to humans in many anatomical and histological aspects. Sheep are widely available, easy to handle and are comparatively cheap to keep and maintain as they can be released to fields’.
Example 4b.2
From Kim et al. (2019) – ‘To achieve pulp-dentin complex regeneration with tissue engineering, appropriate candidate substances have been proposed and tested in animal models. Unlike an in vitro environment in which several factors can be easily controlled, in vivo experiments with animal teeth require particularly advanced skills and techniques. Because of these difficulties, in vivo studies on pulp-dentin complex regeneration to date have usually involved ectopic transplantation of the candidate substance into the subcutaneous tissue or renal capsule rather than orthotopic transplantation directly into the teeth. Only several studies have been performed the orthotopic transplantation of a candidate substance in large animals such as dogs, pigs, ferrets, and monkeys. However, before applying these candidate substances in clinical trials, their treatment efficacies and safeties should be evaluated using in vivo orthotopic transplantation in a sufficient number of animals. Experiments using sufficient numbers of animals are restricted by breeding, costs and ethical issues involved in securing a sufficient number of experimental animals. In contrast, mice are relatively inexpensive, reproduce quickly, and can be easily manipulated genetically. Despite these advantages of mice, most pulp-dentin complex regeneration studies have used large animals because the mouse tooth, of which the diameter is only 1.5–2 mm, has been considered too small’.
Example 4b.3
From Simon et al. (2008) – ‘To date, several animal models of reparative dentinogenesis, including the rat, dog, monkey and ferret, have been used; however, to our knowledge, a mouse model has yet to be reported. The mouse represents an interesting and well-characterized laboratory model, specifically with regard to transgenics. These models are predicted to be extremely informative in studies on the molecular signaling involved in pulp healing. The small size of the animal, however, complicates surgical procedures during pulp capping, as traditional instrumentation is not suitable for use on molar teeth whose diameter is approximately 1.4mm. Miniaturization of these procedures is therefore necessary to exploit the mouse as a laboratory model for pulp-capping research’.
Item 4c: Introduction – A justification of the reasons why the investigation was necessary using an animal model must be provided
Explanation
The Introduction must justify the use of an animal model and adequately describe the background for using each of the treatments, materials, instruments and devices to allow readers to understand the reasons for performing the investigation and to understand any controversies or knowledge gaps that exist. All factual statements must be supported by relevant literature citations (Examples 4c.1, 4c.2). That is, it is acceptable to cite reviews, but it is preferable to cite facts from original scientific publications. Whenever there are guidelines for a professional standardized approach relevant to the study, these should be described and conformed with: Institutional Animal Care and Use Committees (IACUC), Institutional Review Board (IRB), International Organization for Standardization (ISO), American National Standards Institute (ANSI), American Dental Association (ADA), Food and Drug Administration (FDA), etc. Any ignorance of the applicable standards will reflect poorly on the authors’ depth of knowledge of the article topic.
Example 4c.1
From Berlin-Broner et al. (2020) – ‘In spite of the numerous epidemiological studies suggesting a link between apical periodontitis (AP) and cardiovascular disease (CVD), causality has yet to be demonstrated. Performing a longitudinal study in humans to demonstrate causality is challenging due to the complexity of the systemic conditions influencing inflammatory status and the difficulty in controlling all potential confounders along with AP. The absence of animal studies may be attributed to the complexity of the experimental setting, which requires microsurgical techniques and long-term follow-up. Thus, there is a gap in knowledge regarding the causality of the relationship between AP and atherosclerosis, and the mechanism(s) by which they may be linked, and an animal model is essential to study the role of AP as a separate risk factor. The overall goal of this study was twofold: first, to determine the feasibility of using the low-density lipoprotein receptor knockout (LDLR KO) mouse, a classic and recognized model in the field for reliably mirroring aspects of human atherosclerotic disease, to study AP’.
Example 4c.2
From Leite et al. (2010) – ‘It was previously shown that dental pulp from diabetic rats stimulated catalase activity, suggesting an increase in oxidative stress in the dental pulp tissue of diabetic rats. The oxidative stress could cause damage to biomolecules such as DNA, proteins and lipids, compromising the functions of dental pulp. Astaxanthin can be an adjunct in the treatment of diabetes, because it might restore some important cellular functions or at least prevent oxidative damage caused by a ROS-overproduction. Considering the excessive generation of ROS in diabetes mellitus, it has been proposed that the supplementation of diabetic rats with astaxanthin might antagonize, or at least improve, the defect in their antioxidative status’.
Item 4d: Introduction – The unambiguous aim(s) and objectives(s) of the animal study must be provided
Explanation
The aims and objectives must consider all the PICO(T) elements (such as the Problem/Patient/Population, Intervention/Indicator, Comparison, Outcome, and (optional) Time element or Type of Study) (Examples 4d.1, 4d.2). In the interests of continuity and avoiding reader confusion, the aim(s) and objective(s) in the Introduction must be identical to the wording of the text of the Abstract. That is, to avoid having two different aim(s) and objective(s) for the same manuscript, in different sections.
Example 4d.1
From Tawil et al. (2009) – ‘The purpose of the present study was to evaluate the postsurgical periapical healing response of three retro filling materials after 6 months using a modern endodontic surgical protocol in beagle dogs’.
Example 4d.2
From Zaccaro Scelza et al. (2010) – ‘The aim of this study was to verify the inflammatory response of three decalcifying substances (17% EDTA, 17% EDTA-T, and 10% citric acid) using an animal model in which critically sized mandibular defects were created that communicated from the buccal to the lingual surfaces in rats’.
Item 5a: Materials and Methods – The reference number of the approval granted by the ethics board, such as an Institutional Review Board or Institutional Animal Care committee, must be provided along with a reference to the applicable institutional and/or national regulations that were enforced. Any identifying details about the authors institution should not be disclosed during the blind peer review
Explanation
The reference number of the approval granted by the ethics board must be provided (Examples 5a.1, 5a.2, 5a.3). Ethical review board or Institutional Animal Care and Use Committee (IACUC) approval memos should be submitted as supplemental materials with the manuscript to ensure ethical compliance with regulatory standards. The animal care methods and treatments described in the ethical approval must match precisely the words within the manuscript. The manuscript should also describe the housing, handling, diet, veterinary care and experimentation using animals, and how these care standards were regulated, for example Animal [Scientific Procedures] Act, U.K. (1986) or similar. The authors should need not name the institution who granted approval to maintain the blind peer review, the institution details can be added after the peer-review has been completed.
Example 5a.1
From Palma et al. (2017) – ‘The study protocol was approved by the Animal Welfare Committee of the Direção-Geral de Veterinária of Portugal (no. 0420/2011) and complied with the International Guiding Principles for Biomedical Research Involving Animals (Geneva, 1985)’.
Example 5a.2
From Mena-Álvarez et al. (2019) – ‘The present research project has been approved by the Ethics Committee for Research of University Alfonso X el Sabio, by the Ethics Committee of the Animal Research Service of the Hospital Militar Gómez Ulla of Madrid (Ref. ES280790000187) and also by the Environment, Local Administration and Territorial Organization Office of Madrid Autonomy (order number PROEX 201/15). All sections of this report adhere to the ARRIVE guidelines for reporting animal research 18. A completed ARRIVE guidelines checklist is included in Checklist S1’.
Example 5a.3
From Silva et al. (2020) – ‘All procedures were carried out in accordance with conventional guidelines in the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health 85-23, revised 1996). The local Institutional Animal Care and Use Committee (register no. 1041) approved all experimental protocols. This study is reported according to the ARRIVE guidelines (Animal Research: Reporting of in vivo experiments) [28] and PREPARE guidelines (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence) [29] with regard to the relevant items. All efforts were made to minimize animal suffering and to reduce the number of animals used with adherence to the 3Rs principles (replacement, reduction, and refinement)’.
Item 5b: Materials and Methods – The sample size must be justified by citing prior similar studies and/or be estimated by using statistical power calculations to ensure an adequate sample size is used to detect any significant differences and answer the research questions. This is to avoid making any type I and type II errors
Explanation
Large numbers of animals are not always required to obtain reliable results whilst using only a few animals should be avoided. In other words, the sample size should ensure a high probability of detecting a significant P-value difference, if one truly exists. A reference to a similar study may be relevant provided that the experimental design, the outcome and the minimal relevant difference are the same (Examples 5b.1, 5b.2, 5b.3).
Example 5b.1
From Berlin-Broner et al. (2020) – ‘The number of mice in each group was based on a power calculation from a previous periodontal disease study. In that study, to achieve a P value < 0.05 with 90% power, the sample size was 11 mice per group. Based on the length of the study and the degree of dermatitis experienced in the current facility, the number of mice was increased; 17 in the treatment group (Tx) and 22 in the Sham group completed the 16-week regimen’.
Example 5b.2
From Conti et al. (2020) – ‘Sample size was estimated based on data from previous studies. Considering an alpha error of 0.05% and 95% power to recognize a significant difference of 1 in the median scores, a minimum of seven animals per group was necessary. Considering possible animal deaths, three more animals were added in each group, resulting in ten rats per group’.
Example 5b.3
From Pappen et al. (2019) – ‘The minimum number of samples needed to identify differences between groups was determined using the G * Power 3.1 programme for Mac (one-way ANOVA test from the F family of tests). Due to the absence of previous studies that correlated the volume of extruded dentine with inflammatory tissue reaction, an average effect size of 0.7 was chosen. Other parameters included were: alpha-error = 0.05, beta-power = 0.8 and correlation between the repeated values of 0.5. The result indicated a minimum of 5 samples per group and per experimental time’.
Item 5c: Materials and Methods – Details of how animal pain and disability was monitored and how animal suffering was prevented during all aspects of experimentation must be provided
Explanation
It is entirely unacceptable to cause preventable suffering to animals during experimentation. Researchers must never ignore pain, distress, discomfort, suffering, disability, death, mayhem, excessive bleeding, gangrene, necrosis, hunger, thirst, lack of hygiene and general lack of animal care during experimentation. At the time of occurrence of an adverse event, corrective measures, pain relief, anaesthesia or euthanasia must be provided to prevent animal suffering (Examples 5c.1, 5c.2). Details of pain monitoring and the pain relief measures taken to prevent animal suffering and disability must be described. In addition to providing details of animal housing conditions, bedding, light, food and temperature settings, to assure readers that the animals were being adequately cared for (Examples 5c.3, 5c.4).
Example 5c.1
From Silva et al. (2020) – ‘The animals were anesthetized intraperitoneally with 1 mL/100 g of a solution containing 10% ketamine (1 mL/kg; Virbac; São Paulo, SP, Brazil), 2% xylazine (0.5 mL/kg; FortDodge; Rio de Janeiro, RJ, Brazil), 5% midazolam (0.6 mL/kg; Roche; Rio de Janeiro, RJ, Brazil), tramadol (0.2 mL/kg; Sun; Goiânia, GO, Brazil), and 0.9% saline solution (8.5 mL). During the postoperative period, the rats received analgesia with 5 mg/kg of meloxicam (Eurofarma; São Paulo, SP, Brazil) subcutaneously every 24 h, starting immediately after the surgical procedure and for 2 additional days’.
Example 5c.2
From Alves et al. (2018) – ‘The dogs were sedated with an intramuscular injection of xylazine (Abbott, São Paulo, SP, Brazil) associated with a 10% ketamine hydrochloride solution (Aster, São Paulo, SP, Brazil), and were anesthetized with a 5% solution of thionembutal (Abbott), injected intravenously at a dose of 0.1 mL/kg. During the operative procedures, the animals received an infusion of saline solution and intravenous anesthetics, as required. The dogs were monitored throughout the entire experiment to ensure that no clinical signs of infection or pathology were present’.
Example 5c.3
From da Fonseca et al. (2019) – ‘Sixty male Holtzman rats (Rattus norvegicus albinus) weighing ± 220 g were housed in polyethylene cages under 12-h light/12-h dark cycle at controlled temperature (23 ± 2 °C) and humidity (55 ± 10%), with water and food (Guabi rat chow, Paulínia, SP, Brazil) provided ad libitum’.
Example 5c.4
From Reyes-Carmona et al. (2011) – ‘The experiments were conducted using 55 male Swiss mice aged 5 to 7 weeks old (35–40 g) housed in polycarbonate cages placed in a ventilated, temperature-controlled room. Animals were kept in a 12-hour light/dark cycle, with controlled humidity (60% ± 5%) and temperature (25°C ± 1°C). The commercial pellet diet and distilled water were available ad libitum. Experiments were performed during the light phase of the cycle. The animals were acclimatized to this environment for 5 days before testing’.
Item 5d: Materials and methods – The job titles and qualifications of the animal caretakers must be provided
Explanation
The qualifications and job titles of the animal caretakers (e.g. certified animal technicians) must be described to ensure all animal care personnel were adequately qualified (Example 5d.1). The amount of supervising veterinary care should be described, for example the animal housing facility had 24-h animal care with emergency veterinarian support; the health and welfare of each animal was monitored every hour for 3 days following surgery/intervention, and thereafter every 8 h. As good practice, the animal monitoring roles of the animal caretakers can be provided in a supplementary document.
Example 5d.1
From Silva et al. (2020) – ‘A senior veterinarian conducted all the nutritional recommendations and was in charge of the care and pre- and postoperative fasting of the animals, carried out in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and with the current international legislation on animal use in experimental research’.
Item 5e: Materials and methods – Specific details of the animals must be provided, including their species, strain, immune system, breeding programme, age, weight, health status and any special characteristics
Explanation
The source or supplier of animals must be identified. If the animals were sourced from a breeding programme, it should be described. The manuscript should describe the animals using the international strain nomenclature, genetic modification status such as ‘knock out’ or ‘immunodeficient’, because these specific variables could influence the results. The manuscript should describe the animal’s average weight, species, strain, sex, age and tooth developmental stage to help readers adequately comprehend and replicate the study (Examples 5e.1, 5e.2).
Example 5e.1
From De Rossi et al. (2008) – ‘Male C57BL/6 wild-type mice (WT) and mice deficient in IFN-γ, IL-4, IL-10, ICAM-1, and CCR5, 6 to 8 weeks old in the beginning of the experiments, were used. The mice were bred and maintained in microisolator cages in the animal housing facility of the Department of Pathology, Faculty of Medicine of Ribeirão Preto, University of São Paulo. Mice with targeted disruption of IFN-γ, IL-4, IL-10, ICAM-1, and CCR5 were obtained from Jackson Laboratories (Bar Harbor, ME). All knockout mice were originally generated in a mixed 129-B6-DBA background and then backcrossed to the C57BL/6J background for more than 8 generations’.
Example 5e.2
From Garlet et al. (2010) – ‘In this study, C57BL/6 wild-type (WT) and CCR2 knockout (CCR2-KO) mice, obtained from Jackson Laboratory (Bar Harbor, ME), were used. CCR2-KO animals are generally healthy and do not express any significant phenotype. All experiments were performed with 8-week-old mice, weighing around 22 g, with at least 5 animals in each experimental group. Mice were bred and maintained in FMRP animal house facilities’.
Item 5f: Materials and methods – The experimental design must include details of the numbers of animals, numbers of experimental units (e.g. teeth) and timelines (e.g. 5, 30 and 60 days) used
Explanation
Precise details of the experiments must be provided and include the following: the numbers of animals, the numbers of experimental units (defined as the smallest unit to which a level of the treatment factor can be administered, e.g. root canals) and post-treatment timelines for each treatment data collection and for the sacrifice of animals (Example 5f.1). In some studies, the experimental unit is the animal, in other it may be the tooth (e.g. a split-mouth study; Example 5f.2) or perhaps the root canal. That is, ‘After the six animals reached two months of age (60 days ±3 days), the root canals were accessed and infected/disinfected, after 7 days (±1 day) three animals were sacrificed, after 30 days (±4 days) the final three animals were sacrificed’. Any animals removed from the results or which died during the study (if any) must also be reported with an explanation.
Example 5f.1
From Reyes-Carmona et al. (2011) – ‘The animals were divided into seven groups, with n = 10 for the 12 hours and 1, 3, and 7-day experimental periods and n = 5 for the 15-, 30-, and 60-day time points. Mice were anesthetized with 80 mg/kg of ketamine hydrochloride (Dopalen; Division Vetbrands Animal Health, Jacareí, SP, Brazil) and 10 mg/kg of xylazine (Anasedan; Agribrands do BrasilLtda, Paulínia, SP, Brazil). Then, four separate 1-cm incisions were made in the backs of mice at 1-cm intervals. The skin was deflected to create four subcutaneous pockets by a blunt dissection on one side of each incision, two in the cranial portion and two in the caudal portion. Each mouse received three dentin tubes, two filled with each material and one empty, whereas no specimen was inserted in the fourth pocket (sham). After 12 hours and 1, 3, 7, 15, 30, and 60 days after implantation, the animals were euthanized, the tubes with surrounding tissues were removed, and the surrounding tissues were collected. Half the samples (n = 5) from the 12-hour to the 7-day time points were fixed in 4% paraformaldehyde, at 4°C, for histological and immunohistochemical staining’.
Example 5f.2
From Cotti et al. (2017) – ‘Three animals provided the positive controls (control group) for the histologic evaluation of AP and 9 ferrets were randomly divided into 3 groups: Systemic group = RCT with systemic adalimumab (Humira): 3 ferrets (12 teeth) received conventional RCT and systemic subcutaneous administrations of 0.2 mL adalimumab (50 mg/mL) every other week for the remainder of the study; local group = RCT with local adalimumab: 12 teeth randomly distributed in 6 ferrets received RCTs, and before canal obturation, had 0.1 mL adalimumab administered via the root canal to the periapical areas; conventional RCT only (CRCT) group = RCT only: 12 teeth randomly distributed in 6 ferrets received conventional RCT… Postoperative CBCT scans of the ferrets were obtained every 4 weeks for 3 months, following completion of RCTs to monitor the healing of AP (ie, 30 [T2], 60 [T3], and 90 [T4] days post-RCT scans)’.
Item 5g: Materials and methods – The primary outcome data measures or categories as well as any other secondary outcome data measures or categories that will be assessed must be provided
Explanation
The Materials and Methods must mention the unit of analysis for the outcome, for example single animal, group of animals, samples/teeth/root canals/wounds/surgical sites/lesions/infections within animals. In connection with the outcomes, the outcome measure(s) should also be mentioned, for example measured as X - Y or %X - %Y. If the outcomes were categories of healing, pulpitis, vitality, regeneration, success or failure etc., the published standardized criteria used must be described and cited (Example 5g.1). Creating or inventing new criteria must be avoided when suitable standards exist such as ISO 7405 and ISO 10993. Adherence to standardized methods used by prior high-quality peer-reviewed publications will help increase the reproducibility, comparability, validity and reliability of the data.
Example 5g.1
- Degree of tooth re-eruption: the degree of tooth re-eruption was divided into 3 categories: complete re-eruption, partial re-eruption and no re-eruption. A completely re-erupted tooth was when the occlusal surface of the intrusive tooth reached the occlusal level of the first molar. A no re-erupted tooth referred to those whose occlusal surface was still at the cervical level of the first molar, and partially re-erupted referred to a state between completely re-erupted and no re-erupted.
- Pulp calcification: radiopaque in the pulp cavity and root canal.
- Ankylosis: the loss of periodontium space and the integration of acellular cementum and alveolar bone.
- Replacement root resorption: the root resorbed and replaced with bonelike tissue.
- Marginal bone loss: the marginal bone in the buccal side of the intruded tooth healed but recessed; marginal bone loss was evaluated from 30 days after injury’.
Item 5h: Materials and Methods – Details must be provided on (1) steps in the interventions and treatments, (2) instruments, medicaments or device allocation, and (3) concealment and randomization prior to data collection
Explanation
Sufficient details of each step of the interventions or treatments must be described, including sterilization, disinfection, aseptic handling, the type of injury, infection, or disease created, followed by the intervention/surgery. All test materials, supplies, assays or equipment handling should be used according to the manufacturer’s directions to counter potential criticism of a lack of conformity. If image analysis software was used to collect data from histology, radiographs or micro-CT images, how the data collection was calibrated and validated must be explained. The wavelengths of spectrophotometers, light curing units, flow cytometers and measurements from Instron machines and microscopes must be calibrated, to ensure accurate data collection. If any subjective results were collected, the steps taken to prevent bias must be described. The manuscript should describe how the investigators were not aware of the treatments, materials or assignments of specimens (histology, photographs, micrographs, radiographs, assays etc.) by randomizing them and concealing them with blind codes during data collection. Animal studies evaluating pain must describe how the pain was monitored, minimized, relieved and ended (Example 5h.1).
Example 5h.1
From Wu et al. (2010) – ‘Sixteen female wistar rats weighing approximately 100g were obtained from Experimental Animal Center of Guangxi Medical University. This study was approved by the animal care and use committee of Guangxi Medical University. The animals were divided randomly into two groups: Group I, Controls, animals were given tap water containing 0.16 mg F − per L; Group II animals were given sodium fluoride (NaF) in their drinking water at a concentration of 100 mg F − per L. Each group consisted of eight female rats. Rats were fed regular laboratory rodent diet and were allowed water ad libitum. After 3 months, the characteristic enamel striations were apparent, which are indications of dental fluorosis and altered mineralization of dentine and enamel. Rats were killed humanely by cervical dislocation, and a pair of mandibular central incisors was dissected from each animal, and the unmineralized proximal portion of the incisor was removed. The incisors were split longitudinally into two halves, and the pulp tissues removed using a spoon excavator. One pulp of each rat was used for microarray analysis. Every four pulps of each group were pooled into one tube. Then, each group samples was divided into two pools for microarray analysis. Control pulps were divided into two pools of 4(c1), 4(c2); fluoride treatment group into two pools of 4(f1), 4(f2). The remaining pulps of each group were pooled into one tube, 8(c3, control group), 8 (f3, fluoride treatment group), which were submitted to RNA isolation for validation of microarray experiment data’.
Item 5i: Materials and methods – Details regarding postdisease and postoperative care of the animals must be provided
Explanation
It is not ethically acceptable to mistreat animals used in experimentation, by ignoring any severe pain, suffering or disability. To ensure adequate care and welfare of animals, the manuscript should provide details about the postinjury, postdisease and postoperative care and monitoring to ensure steps were taken to guarantee that animals were not disabled, disfigured and did not experience severe pain as a result of the experimentation. Pain relief medications and antibiotics should always be used (Examples 5i.1, 5i.2, 5i.3, 5i.4, 5i.5). Withholding care and medicaments from animals is always unacceptable.
Example 5i.1
From Paras et al. (2019) – ‘During the 30 days of the experiment, the health status of the animals (behaviour, changes in the skin and hair, food and water consummation, urinating and defecation) was checked daily’.
Example 5i.2
From Verma et al. (2017) – ‘An analgesic (carprofen, 3 mg/kg, subcutaneous, every 24 h) was given immediately prior to the procedure and continued until the following day to manage postoperative pain’.
Example 5i.3
From Silva et al. (2020) – ‘During the postoperative period, the rats received analgesia with 5 mg/kg of meloxicam (Eurofarma; São Paulo, SP, Brazil) subcutaneously every 24 h, starting immediately after the surgical procedure and for 2 additional days’.
Example 5i.4
From Pappen et al. (2019) – ‘After the experimental procedures, the animals were placed in cages until their recovery. Two animals were kept per house, with a cycle of 12 h day night − 1, temperature between 19–23 °C, relative air humidity between 40%–70%. To aid recovery, paracetamol (0.06 mg g − 1 day − 1) was added to their drinking water for 72 h’.
Example 5i.5
From Altaii et al. (2017) – ‘Experimental animals were subjected to a treatment protocol comprising four sessions. After each session, animals were given analgesics (50 mg mL − 1 Rimadyl IM injection, Pfizer, West Ryde, Australia). Postoperatively, animals were given analgesics (2.2 mg kg − 1 Rimadyl tablet) and visually monitored to check for signs of distress’.
Item 5j: Materials and methods – Details on the statistical analysis, statistical tests, the type of software used, and the steps taken to control, interpret success or failure, and to validate the accuracy of the data must be provided
Explanation
All too often a single P-value is all that is given from a multiple-group statistical analysis. Additional statistical information is essential, including details of the statistical tests used for analysis, the type of software used, and any steps taken to validate or control the accuracy of the data. Preferably, the steps of the statistical analysis should be described in the same order as used when the results are presented. A complete statistical analysis must include an analysis of the significance of the differences between each of the test/treatment group means, and if relevant confidence intervals (Examples 5j.1, 5j2, 5j.3). Nowadays, when used correctly, the commercially available statistical software are highly reliable, so using two different software packages is not always necessary to validate statistical analyses. The main problem with the statistical analyses submitted to journals is not the quality of the software package, but the statistical ignorance of the user. To prevent potential evaluator bias and to validate the accuracy of data, automated data collection by machine using values or image analysis should be considered, or by using two-independent data collectors. The use of methods of analysis that enable quantification and parametric statistical methods, when possible, should be described. It is good practice to have the statistical analysis performed by someone who did not collect the data, such as a statistician or colleague. If the statistics is unfamiliar or complex, a statistician should be consulted to validate the analysis to avoid reporting errors and artefacts. Datasets should be designed to include positive and negative control samples/groups to help validate the accuracy of the data (e.g. antibody specificity) and to identify problems such as a failure to completely sterilize biomaterials prior to testing, or the use of contaminated cultures of E. faecalis. If using absorbance or light curing methods for material setting, all light sources and machines must be calibrated prior to experimentation.
Example 5j.1
From Jara et al. (2018) – ‘The distributions of the radiographic and histological parameters were analysed, and descriptive statistics (mean and standard deviation) were calculated. Normality was ascertained by Kolmogorov–Smirnov test. The measurement of effect was obtained between the three experimental groups (EG1, EG2 and EG3) by carrying out generalized estimating equations, with Poisson regression with robust variance, pairing each EG with its respective CG within animals, and adjusted for the mean within animal differences (∆ = CG side - EG side), with α = 5%. Data were analysed using SPSS software (SPSS Statistics for Windows, version 20.0, SPSS Inc., IBM, Chicago, IL, USA)’.
Example 5j.2
From Ma et al. (2016) – ‘All data are expressed as mean ± standard deviation (SD). One-way analysis of variance (anova) was performed to examine the effect of differing concentrations of LPS on cell proliferation, and least significant difference test was used for paired comparisons. For independent sample, t-test was performed to compare the expression of Notch signalling genes between LPS group and control group in vitro. All statistical analyses were performed using the SPSS software package (version 17.0; SPSS, Inc., Chicago, IL, USA). A value of P < 0.05 was considered as statistically significant’.
Example 5j.3
From Pappen et al. (2019) – ‘Categorical data were analysed statistically using SPSS statistical software (version 24.0, IBM, Chicago, IL, USA). Histopathological events were considered as the dependent variables, whilst debris (infected, noninfected and no-dentine), amount of debris (0, 5, 10 and 20 mg) and time of evaluation (7, 30 and 60 days) were considered as the independent variables. The effect of each independent variable over the dependent variables was studied individually by using a nonparametric test, Kruskal–Wallis with the due Bonferroni corrections. Pair-wise comparisons were also studied by means of a Mann–Whitney U test also with Bonferroni correction. All significances were accepted at P = 0.05. Box-plot graphs were constructed following interaction of the independent variables displaying significance at individual evaluations’.
Item 6a: Results – Average baseline characteristics of the animals (e.g. age, weight, gender, microbiological status) at the beginning of the experiment must be provided
Explanation
The average baseline characteristics of the animals (e.g. age, weight, microbiological status) at the beginning of testing will help readers understand the health, sickness, disease and care status of animals prior to experimentation (Examples 6a.1, 6a.2, 6a.3).
Example 6a.1
From Berlin-Broner et al. (2020) – ‘Mice in both groups gained weight similarly during the experimental period (mean weight gain percentage in Tx: 22.16 ± 3.06%, Sham: 22.58 ± 2.39%, P = 0.9139). The final absolute weights were also similar (Tx: 35.59 ± 1.2 g, Sham: 34.14 ± 1.15 g, P = 0.3269). There was no difference in plasma total cholesterol levels (Tx: 1007 ± 74.57 mg dL − 1, Sham: 996.9 ± 46.17 mg dL − 1, P = 0.9014)’.
Example 6a.2
From Cosme-Silva et al. (2019) – ‘The general health condition of the animals remained constant throughout the experimental period. At the end of the experimental period, no significant difference was observed in mean body weight or food and water consumed by the animals (Table 1) (P > 0.05)’.
Example 6a.3
From Alexandria et al. (2019) – ‘In the in vivo study, we observed that all rats gained weight and remained apparently active and healthy until the end of the experiment; no statistically significant difference was observed for weight gain among the groups (data not shown). The data was normally distributed’.
Item 6b: Results – The results for each group of primary and secondary outcomes should describe the means, median or mode, as well as differences and their statistical significance
Explanation
The results should describe the mean, median or mode, for each group, condition, category, treatment or intervention, along with the magnitude in difference (%) and its statistical significance in terms of probability (P) value, at each end-point or time interval for each figure. In general, P-values larger than 0.01 should be reported to two decimal places, and those between 0.01 and 0.001 to three decimal places; P-values smaller than 0.001 should be reported as P < 0.001. If relevant, the estimation of effects and the 95% confidence intervals can be given together with these estimates (Example 6b.1). For example, ‘Three months after apexogenesis, the mean root lengths of immature teeth (X mm) had increased by X% compared to the immature teeth after apexification (Y mm) (n = Z, P < 0.001), whereas there was little difference between these two treatments (X mm vs. X mm) after 1 month (n = 12, P > 0.05) or at the time of treatment as a control (X mm vs. Y mm) (n = Z, P > 0.05)’. These details must be provided in the text within the Results section and graphs and figures (e.g. bar chats, pie charts and photographs identifiers) should be used to complement the written information.
Example 6b.1
From Wei et al. (2011) – ‘The mean marrow:bone ratios, in descending order were: 0.57 (±0.04), 0.55 (±0.04) and 0.15 (±0.03) for the inter-bone control, intra-bone control and test groups, respectively. The marrow:bone ratios of the inter-bone group was significantly (P = 0.04) greater than the intra-bone control group, although the difference (mean difference 0.03; 95% CI 0.01, 0.05) was small (Table 2). The marrow:bone ratios of inter-bone control and intra-bone control groups were significantly (P < 0.001) greater than the test group. The mean differences in ratio were 0.42 (95% CI 0.40, 0.44) and 0.39 (95% CI 0.37, 0.41), respectively (Table 2)’.
Item 6c: Results – All adverse events during the animal experimentation and the method of euthanasia must be reported
Explanation
The numbers of animals effected by any adverse health events, handling accidents, welfare problems, medication overdoses, underdoses or contraindications, or unexpected deaths sometimes called the ‘drop-out rate’ must be reported (Examples 6c.1, 6c.2). The explanation should include the reason for the adverse events for readers to judge the safety and health hazards of the treatments and interventions. This promotes better care of research animals during testing to avoid reporting these undesirable consequences.
Example 6c.1
From Wolle et al. (2012) – ‘Of note, the treatment with tempol (50 mg/kg) was able to significantly reverse the body weight loss (Fig. 2B; P < .05), an effect that was accompanied by a general improvement of locomotion, although the reduction of catalase activity was not significantly altered (Fig. 2C and D; P > .05)’.
Example 6c.2
From Aubeux et al. (2020) – ‘No animal died during the experiments and no specific side effects due to HCA were noted. No necrosis and or serious side effects were reported’.
Item 6d: Results – Any changes made to the experimental protocols to prevent the occurrence of animal adverse health events, analgesic or other medication overdoses or underdoses, or unexpected deaths must be provided
Explanation
The steps taken to prevent animal adverse events must be reported, because it promotes improvements in animal welfare, care and handling during experimentation.
Example 6d.1
From Stewart & Martin (2003) – ‘Analgesic regimens included buprenorphine (0.025, 0.05, and 0.1 mg/kg subcutaneously [s.c.]; 1 ml/kg), fentanyl (0.01 and 0.1 mg/kg intraperitoneally [i.p.]; 1 ml/kg), flunixin meglumine (1.1 and 2.5 mg/kg, s.c.; 1 ml/kg) and acetaminophen (100 and 300 mg/kg orally; approximately 3 & 10 ml/kg). Drugs were administered once daily on days 0, 1, and 2 postoperatively’.
Example 6d.2
From Ohishi et al. (2008) – ‘Sudden deaths of F344 rats (F344/Du Crj (Fischer)) have occurred frequently in the late stage of carcinogenicity studies using stomach tubes. To reduce the sudden deaths, the incidence of sudden deaths was compared in the control groups from 104-week carcinogenicity studies using two different stomach tubes (metal and Teflon) and feeds (pellet and powder)’.
Item 7a: Discussion – A discussion on how the methods and results are relevant to the study aims, and how the results support or dispute prevailing theories advocated in prior publications must be provided
Explanation
The methods and results must be discussed using terminology consistent with professional standards and relevant peer-reviewed literature. The discussion should evaluate how the methods and results are relevant to the study aims, and how these results support or disprove prevailing theories advocated by previous publications (Examples 7a.1, 7a.2).
Example 7a.1
From Frozoni et al. (2012) – ‘In this study, to stimulate the differentiation of odontoblast-like cells from progenitor or stem cell population, exposure on maxillary first molars of 3.6-GFP transgenic mice was used as described before (Simon et al. 2008) with some modifications. Utilization of transgenic animals allowed a better insight into many aspects of this reparative process including destruction of odontoblasts after pulp exposure, presence of dentine chips at the healing pulp, the fate of the pre-existing odontoblasts around these chips, recruitment of progenitors to the injury site and their subsequent differentiation and the formation of different patterns of tertiary dentine’.
Example 7a.2
From de Oliveira et al. (2017) – ‘The progression of the periapical lesion was evaluated for 7, 21, and 42 days in mice with or without rosiglitazone for 2 weeks. TZD administration was used as a form of osteocyte apoptosis induction because this effect has already been reported previously in the literature (13, 16, 17). The initial hypothesis was that the animals that received TZD would present larger periapical lesions because the apoptosis of osteocytes leads to a greater recruitment of osteoclasts to the region, triggering greater bone destruction (29). It was possible to observe a gradual increase in the area of the lesions with their progression in the control and rosiglitazone groups. A trend toward greater lesions in the groups receiving rosiglitazone was observed but without a statistically significant difference (P > .05). Thus, it is noteworthy that the rate of osteocyte apoptosis observed in jaws induced by oral rosiglitazone for 2 weeks was not sufficient to statistically alter the size of the periapical lesion. From this finding, 2 hypotheses seem to arise. The first is that apoptosis of osteocytes does not actually interfere in the development of the periapical lesion and, second, that the rate of osteocyte apoptosis observed in the present study was not sufficient to influence the development of the periapical lesion. In addition, it is worth mentioning that osteocyte death could stimulate, besides alteration in the cytokine profile expressed by this cellular type, compensatory mechanisms in the context of periapical lesion development’.
Item 7b: Discussion – An objective presentation of the strengths and limitations of the animal model, study design, methods, materials, instruments, drugs and devices, and outcomes must be provided, including any biology/functional variability between the animal model and humans
Explanation
The strengths and limitations of the animal study must be reported (Examples 7b.1, 7b.2, 7b.3, 7b.4). As a hypothetical example: ‘The human-sized endodontic instruments had to be used with modified protocols in the animals, due to the miniscule sizes of root canals’. It is never acceptable to extrapolate the results from an animal study directly to clinical use, without first requiring a clinical trial. However, it is often necessary to discuss the similarity and differences in physiology and anatomy between animals and humans to explain how these could influence the significance of the results.
Example 7b.1
From Berlin-Broner et al. (2020) – ‘The present study has limitations that preclude the unequivocal conclusion that there is no causality. The variability in the number, size and clinical aspects of periradicular lesions (PALs and FL) amongst the Tx group may have contributed to the outcome. Although the four 1st molars were included, it may be that even four PALs are not sufficient to increase systemic inflammation above the threshold to influence atherosclerosis. As opposed to the periodontal study, in the current study, there was no introduction of exogenous pathogenic bacteria (exposed pulps were naturally infected by endogenous oral flora). Patients with AP often present with chronic periodontal disease, which is prevalent in 46% of the population. Since both are common oral diseases in the adult population, it may be that CVD correlates more strongly when both conditions are present, and this accounts for the positive association findings in epidemiologic human studies. Although pathogens are part of the natural oral flora of mice, they might not be present in enough quantity “to push” the system towards significant inflammation that leads to changes in atherosclerosis. It would be interesting in future studies to introduce a periodontal disease pathogen at the time of pulp exposure, mimicking the common situation in humans’.
Example 7b.2
From Simon et al. (2008) – ‘A limitation of the model presented is that it currently uses healthy teeth, whilst in the clinical situation pulp inflammation is generally present. However, future experiments could simulate caries-like situations by incorporating bacterial infection models using whole live bacteria or bacterial components. In addition, the presence of dentinal chips or debris arising from the creation of a pulp exposure may contribute to reparative responses in the pulp. Although this may complicate data interpretation, it does reflect the clinical situation where dentine fragments and dissolution productscontribute to overall pulpal responses. Reproducibility of the pulp-capping procedure was regarded as an important element in the viability of the model especially in view of the small size of the mouse tooth. The histological observations confirmed the reproducibility of our surgical procedure in size and position of the pulp exposure’.
Example 7b.3
From Kopper et al. (2003) – ‘Although the current literature contains many reports in leakage, there is no consensus about the sealing ability of endodontic sealers. One of the drawbacks is the fact that investigations do not follow a similar methodological pattern, which leads to contradictions. The present study is closer to clinical reality, and its results may be more easily extrapolated to dental practice’.
Example 7b.4
From Mena-Álvarez et al. (2019) – ‘One of the limits of this study was that it does not match the clinical situation, because healing events are completely different in a healthy rat skull bone compared to the infected perirradicular bone of a human tooth, rat calvaria defects have been used to evaluate the biologic potential of various devices, as well as osteoinductive and/or osteoconductive biomaterials and biologics to promote bone regeneration’.
Item 7c: Discussion – The potential influence of the results on future research plans must be discussed
Explanation
The results of the study must be carefully analysed to extrapolate future research goals and to identify any knowledge gaps (Examples 7c.1, 7c.2). If further animal testing is necessary to evaluate the risks of toxic, allergic and adverse health events, this should be explained. Only if the results indicated no adverse events, and no toxic, or allergic risks, pursuant to dental device evaluation standards ISO 7405 and ISO 10993 can future clinical trials be advocated.
Example 7c.1
From Wei et al. (2011) – ‘This present study showed a significant increase in bone generation upon local bisphosphonate application for a short period of time, independent of the carrier used, although the pattern of effect may have been different. The local delivery of bisphosphonates could be beneficial in promoting bone regeneration after endodontic treatment or surgery. In endodontics, bisphosphonates could potentially be delivered locally in conjunction with grafting procedures involving periapical lesions and in root canal filling materials. The osteoconductive property of the bisphosphonate used suggests that it could be used as a surface-coating material for bone grafting materials, root ends and root fillings. Clinical use of bisphosphonates for aiding bone regeneration may only be recommended once the biological basis of their action is fully understood. Future research should focus on clarifying the mechanisms of biological actions, and their critical delivery profiles’.
Example 7c.2
From Kim et al. (2017) – ‘This study provides baseline data for surface characteristic behavior of the NiTi PathFile system when subjected to limited applications ex vivo and in vivo. Further studies would be necessary to evaluate the efficacy of the PathFile system in severely curved canals and its usability for more than 3 canals after subjecting them to sterilization protocols. Profilometric analysis after each successive use would provide valuable data in the reusability of these NiTi file systems’.
Item 7d: Discussion – If appropriate, the impact the findings have on human health, treatments or healthcare must be explained
Explanation
The relevance of the findings to humans must be discussed in the knowledge that few animal studies, particularly rodent studies are directly relevant to endodontic treatment in humans, due to some animal teeth continually growing throughout their life, and greater physiological repair and regeneration potentials (Examples 7d.1, 7d.2).
Example 7d.1
From Cotti et al. (2017) – ‘Among the limitations of the current work, it is important to underline that ferrets are a different species from humans and their ability to modulate the immune response with a TNFα blocking drug may be different. This must be considered before extrapolating the results to humans, yet this study opens the way to further assessment of TNFα modulation on the development and healing of AP. As stated before, the clinical implications of altered immunity on AP need to be clarified. Epidemiologic studies need to follow’.
Example 7d.2
From Long et al. (2017) – ‘This study confirmed that the newly developed BG pulp capping materials can induce reparative dentin bridge formation at the injury sites of rat pulps. Research performed on rat molar teeth is reproducible in humans’.
Item 8a: Conclusion – A rational basis for the conclusion(s) must be provided, that is, they must be directly supported by the results of the study
Explanation
The conclusions must be supported entirely by the results. Investigators must never conclude something they did not investigate. The conclusion must be explicit, without an over-generalization to clinical practice and be based on a general interpretation of the results without any unsupported bias (Examples 8a.1, 8a.2).
Example 8a.1
From Torabinejad et al. (2018) – ‘Based on the results of this animal model, it appears that regeneration of the pulp-dentin complex is possible when 1–4 mm of pulp remains in the apical segment of immature teeth’.
Example 8a.2
From Altaii et al. (2017) – ‘An endodontic regeneration/revitalization protocol using a blood clot scaffold in immature infected sheep teeth showed further development and maturation of the teeth confirmed radiographically. Histological analysis of the revitalized tissues showed vital tissue developed in the root canal and hard tissues deposited on the dentinal walls. The structure and the maturation degree of the newly formed tissue indicated that they likely progressed from the apical to the coronal portion of the root’.
Item 8b: Conclusion – Explicit conclusion(s) from the study, including appropriate follow-up research ideas, must be provided
Explanation
A good conclusion will guide the reader about the future directions of the research, by suggesting follow-up ideas. This will often be further animal testing or a clinical trial. Authors are encouraged to suggest ideas for future research that will have a broad appeal to clinicians, patients and researchers (Examples 8b.1, 8b.2). Keep in mind that this part of the conclusion is likely to be the most cited sentence from a publication.
Example 8b.1
From Torabinejad et al. (2018) – ‘This mechanistic approach provides a potential foundation for future vital pulp therapy and pulp regenerative procedures. Future studies are needed to investigate the potential of residual inflamed pulp on the regeneration of the pulp-dentin complex in immature and mature teeth’.
Example 8b.2
From Sasaki et al. (2019) – ‘This model will be a valuable tool not only for the further elucidation of the pathobiology of osteomyelitis but also for the development of new therapies to accelerate bone and wound healing’.
Item 9a: Funding and support – All funding, donations, assistance and support provided for the study must be reported
Explanation
The name of the funding source for the study must be provided, as well as the names of individuals or vendors who provided or donated custom-made instruments, materials, chemicals, antibodies or devices. Thanks and credit should also be given by name to individuals who translated or edited the manuscript or helped to draw the figures and calculate the statistics (Examples 9a.1, 9a.2, 9a.3, 9a.4, 9a.5). Authors should not include grant numbers or university details that can be used to reveal their identity during the blind peer-review process. However, these details must be included in the revised manuscript after the peer-review has been completed.
Example 9a.1
From Gu et al. (2019) – ‘This study was supported by the Japan Society for the Promotion of Science (grants-in-aid no. 26293405 and no. 25670808 to T.O. and no. 24592862, no. 15K11110 and no. 18K09594 to T.K.)’.
Example 9a.2
From Altaii et al. (2016) – ‘The authors greatly acknowledge the support from Gilles Plains Large Animal Research and Imaging Facility (LARIF), Adelaide Microscopy Centre, Dr John Berketa from the department of Forensic Odontology, the University of Adelaide, Babylon University and the Iraqi Ministry of Higher Education and Scientific Research’.
Example 9a.3
From Berlin-Broner et al. (2020) – ‘This study was supported by the Canada Foundation for Innovation; Alpha Omega Foundation of Canada Research; University of Alberta, Faculty of Medicine and Dentistry Motyl Graduate Studentship in Cardiac Sciences and Department of Dentistry Fund for Dentistry. We also acknowledge the use of the Department of Dentistry MicroCT, Members of Daniel Graf Lab. and the Alberta Diabetes Institute “HistoCore” (Lynette Elder)’.
Example 9a.4
From Sasaki et al. (2019) – ‘The authors thank Drs YoshimitsuAbiko (Nihon University School of Dentistry at Matsudo, Matsudo, Chiba, Japan), KiichiHirota (Kansai Medical University, Hirakata City, Osaka, Japan), and Akio Ohta (The Institute of Biomedical Research and Innovation, Kobe City, Hyogo, Japan) for their advice on the experimental design and for helpful discussions’.
Example 9a.5
From Kim et al. (2018) – ‘The authors thank Seung-Hee Kwon for providing technical assistance’.
Item 10a: Conflicts of interest – An explicit statement on conflicts of interest must be provided
Explanation
The specific interest(s) of the researcher or clinician associated with a research project can include financial, commercial, legal, professional or personal relationships (Example 10a.1). These relationships can lead to bias and are referred to as conflicts of interest. Authors should explicitly declare the absence of a conflict of interest (Example 10a.2). Authors should not include details that can be used to reveal their identity during the blind peer-review process. However, these details must be included in the revised manuscript after the peer-review has been completed.
Example 10a.1
From Walsh et al. (2018) – ‘Carolyn M. Primus was formerly affiliated with Avalon Biomed Inc and maintains a consultancy with NuSmile Ltd’.
Example 10a.2
From Berlin-Broner et al. (2020) – ‘The authors have stated explicitly that there are no conflicts of interest in connection with this article’.
Item 11a: Quality of images – Details of the equipment (model, supplier, city, country), software (version, supplier city, country) and settings used to acquire image(s) must be described in the Methods and/or figure legend
Explanation
Authors need to provide information about the equipment, software and settings used to capture and process image(s) as well as the manufacturer and the model/version of the device(s) used for recording and reproduction of images, that is city, country. For software, the name of the programme, the developer and version etc. is essential (Examples 11a.1, 11a.2).
Example 11a.1
From Berlin-Broner et al. (2020) – ‘Three-dimensional (3D) micro-CT scans of mouse heads were taken at 25 μm, 360°, 75 MSec, 50 kV and 0.24 mA (Milabs U-CT, Utrecht, Netherlands). Scans were reconstructed in MiLabs Software and analysed with Amira software (Thermo Fisher Scientific, Ottawa, ON, Canada). Any periradicular radiolucency wider than the size of the width of the periodontal ligament (PDL) was recorded’.
Example 11a.2
From Okamoto et al. (2019) – ‘The animals were sacrificed at 4 weeks after direct pulp capping. The induced tertiary dentine was analysed using a micro-CT scanner (R_mCT2; Rigaku, Tokyo, Japan) with a scanning resolution of 10-μm intervals in an individual image. The basic parameters of the scanner were as follows: voltage 90 kV, current 160 μA and exposure time about 3 min in drying conditions. After scanning, 512 consecutive tomographic slice images were obtained. Then, image data were reconstructed using three-dimensional reconstruction imaging software (TRI/3D-BON; Ratoc System Engineering, Tokyo, Japan)’.
Item 11b: Quality of images – The reason why the image(s) was acquired and the rationale for its inclusion in the manuscript must be provided in the text
Explanation
An explanation is required if an image is special or is included (Example 11b.1) because it is representative of all the animals, treatments or results for that treatment group. Images should include a legend with sufficient text to describe what the image is showing to an inexperienced reader. The worst images are pixilated, unfocused, distorted or show artefacts. Poor-quality images can create an impression of sloppily performed research. Only images that meet the quality standards of the journal should be submitted.
Example 11b.1
From Chen et al. (2019) – ‘Current methods for analysing alveolar bone in animal models of chronic apical periodontitis include histomorphometry and 2D radiography. However, these methods can only provide 2D or linear data. These 2D projections of 3D structures often fail to provide an adequate representation of the region of interest, resulting in a loss of quantification accuracy. Micro-CT analysis can image and evaluate the 3D structures of hard tissues in a highly accurate manner. Through specific 3D parameters, bone mass and bone internal microstructure can be evaluated by nondestructive, rapid and very accurate methods. Studies have confirmed that micro-CT analysis of chronic apical periodontitis is highly correlated with traditional histology’.
Item 11c: Quality of images – The circumstances (conditions) under which the image(s) was viewed and evaluated must be provided in the text
Explanation
The method used to capture, analyse and interpret the image(s) should be clearly described. The examiners involved in interpreting and assessing the images should be identified with their credentials and eligibility to be able to do so. Authors should not include details that can be used to reveal their identity during the blind peer-review process. However, these details must be included in the revised manuscript after the peer-review has been completed. If applicable, the equipment and viewing conditions used by the examiners should be reported in the manuscript. If needed, the training provided and the level of agreement between the examiners should be provided in the text. The level of agreement can be given as inter/intra-rater agreement by K statistic or proportion of concordant interpretations (Examples 11c.1, 11c.2).
Example 11c.1
From Chen et al. (2019) – ‘In order to evaluate the resorption of dentine/cementum of root at the interface, the methodology of Estrela et al. (2009) was used. Their scoring criteria were based on the sites (apical, middle and cervical) and number of surfaces (mesial, distal, buccal, palatal (lingual) and root apex) affected by inflammatory root resorption (IRR) in human tooth roots. The degree of root resorption severity was recorded by IRR extension. In the present study, the 5-point scoring system of IRR extension was modified according to the proportion of rat tooth root to human tooth root (Table 1). IRR indexes were measured on three-dimensional micro-CT scans of rat tooth roots with Mimics software as follows: axial, transverse and tangent. The examined roots (80 roots per group) were from the first and second molars in the right maxilla and mandible of five rats. All images were evaluated by two calibrated blinded examiners’.
Example 11c.2
From Wang et al. (2013) – ‘Root development, dentinal deposition, and periapical lesions were evaluated by radiography (Focus; Instrumentarium Imaging, Milwaukee, WI) every 4 weeks. All periapical radiographs were taken by the same technician, who used the bisecting angle technique with a projection angle of − 37° and an exposure time of 0.1 second. The completion of root development was defined as the closure of the apical foramen and the thickening of the root canal wall. The length of the root, the thickening of the dentin wall, and the width of the apical foramen were measured on the radiographs by 2 different dentists who were trained and who passed the consistency test (kappa value > 0.8)’.
Item 11d: Quality of images – The resolution, magnification and any important manipulation(s) on any image (e.g. brightness, image smoothing, staining etc.) must be described in the text or legend
Explanation
All too often images have no scale, and no details are provided about any editing, or cropping manipulations that were performed with image editing software. For those reasons, the resolution, original magnification and any modifications, such as cropping, changes in brightness, image sharpness, smoothing and colour enhancement made to the image using software must be provided either in the legend or in the text. A scale bar should be included with magnified images (Examples 11d.1, 11d.2). Please keep in mind that minimal image manipulation is acceptable to the extent that it does not change or misrepresent the results. Any intentional distortion, misrepresentation or concealment of problematic results is never acceptable (Rossner & Yamada 2004, Lang et al. 2012).
Example 11d.1
From Liu et al. (2019) – ‘Fig. 2 provides the key information in the legend with scale bars on images. (Figure 2. Postoperative inflammatory cell infiltration in the root canal after RET. (a) Histological observation showed inflammatory cells in the root canal of the RET group; most were lymphocytes. (b) Magnified view of the boxed region in (a). (c) Histological observation showed no inflammatory cells in the pulp of the control group. (d) Magnified view of the boxed region in (c). Blue arrowheads indicated lymphocytes. D, dentine. P, pulp. PDL, periodontal ligament. Scale bars = 200 μm (a, c); and 100 μm (b, d)’.
Example 11d.2
From Lai et al. (2018) – ‘Fig. 4 provides the key information in the legend with scale bars on images. (Figure 4. Intracanal metformin treatment attenuated oxidative stress and apoptotic activity of osteoblasts in periapical lesions. (A and C) The hematoxylin-eosin sections show multiple bone resorption lacunae, a zigzag osseous outline, and obvious infiltration of inflammatory cells in the vehicle group. (B and D) In contrast, lesions treated with metformin have a smoother osseous outline and mild inflammatory cell infiltration. (E) Immunohistochemical staining shows numerous 8-OHdG + osteoblasts (arrowheads) in the vehicle group, and (F) metformin treatment results in fewer osteoblasts positive for 8-OHdG. Correspondingly, TUNEL-positive osteoblasts (arrowheads) are plentiful in the periapical lesions of (G) vehicle-treated teeth but significantly fewer in the (H) metformin group. Magnification: A and B, 100×; C–H, 200×. Ap, root apex.)’.
Item 11e: Quality of images – An interpretation of the findings (meaning and implications) from the image (s) must be provided in the text
Explanation
Sometimes, it is unclear to readers precisely what an image or radiograph is showing. Readers should not be left to interpret images by themselves. The text and figure legend should contain all relevant details and information derived following the expert evaluation and interpretation of the images by the authors (Examples 11e.1, 11e.2).
Example 11e.1
From Liu et al. (2019) – ‘Pulp-like tissues and free cellular cementum-like tissues in the root canal and cellular cementum-like tissues on the root wall were observed. Inflammatory cells were present in the periapical area and root canal together with newly formed tissue, and most were lymphocytes (Fig. 2a,b). Fisher's exact test was used to compare the difference between the ratio of lymphocytes infiltration in the RET and the control group. The ratio of lymphocyte positive specimens was 89% (26 of 29 specimens in the RET group had inflammatory cells). No inflammatory cells were observed in the control group (Fig. 2c,d). The difference is significant (P < 0.001). This result showed that the immune cells and/or the local immune microenvironment might participate in the tissue regeneration after RET’.
Example 11e.2
From Lai et al. (2018) – ‘Microscopically, active bone resorption was found in the vehicle group as evidenced by the presence of multiple Howship lacunae, zigzag osseous outline surrounding the periapical tissues, and intensive infiltration of inflammatory cells (Fig. 4A and C). In contrast, metformin alleviated bone destruction as manifested by the smoother osseous outline circumscribing the periapical areas and mild inflammatory cell infiltration (Fig. 4B and D). Immunohistochemical staining showed that the percentage of 8-OHdG–positive osteoblasts (Fig. 4E) in the periapical areas of vehicle-treated roots was significantly higher than that in the metformin group (Fig. 4F), indicating that metformin treatment alleviated oxidative stress in osteoblasts of the periapical lesions. Correspondingly, TUNEL-positive osteoblasts were more numerous in the vehicle group (Fig. 4G) compared with the metformin-treated group (Fig. 4H), denoting a reduction of apoptotic activity in osteoblasts by metformin’.
Item 11f: Quality of images – The legend associated with each image must clearly describe the subject matter specific feature(s) illustrated. Images of animals must describe their age and test duration, and other relevant features such as important anatomical landmarks and relevant features
Explanation
Legends for images should be written in such a manner as to be so comprehensive that a reader does not need to refer back to the text for information (Example 11f.1). The legend should include relevant demographic information and identify the important anatomical landmarks with arrows such as a root canal perforation, root apex, periapical lesion, access preparation, lateral accessory canal, extruded sealer, etc.
Example 11f.1
From Cintra et al. (2014) – ‘Figure 3 Representative histological findings 60 days following diabetes mellitus induction in group 8: (a1-a4) right upper first molar with apical periodontitis and (b1-b4) left upper second molar with periodontal disease. (a1) A sagittal section of a FM that shows periodontal and periapical tissues, a surgically induced crown opening (#) and total necrosis of the pulp tissue (N), alveolar bone (AB) and AP (haematoxylin and eosin, 259 magnification). (a2) Magnification of boxed section of a1; microscopic aspect reveals severe acute inflammatory cell infiltration near the tooth apex region (arrowheads) with severe disorganization of the periodontal ligament, cementum (C) and dentine (D) (haematoxylin and eosin, 1009 magnification). (a3) Increased magnification of boxed section from a2, which shows polymorphonuclear cells (haematoxylin and eosin, 10009 magnification). (a4) Increased magnification of boxed section from a1, which shows AB trabeculae and the lacunae of active bone resorption (▼) (haematoxylin and eosin, 4009 magnification). (b1) A sagittal section of a SM that shows periodontal and periapical tissues, AB, pronounced bone loss, root biofilms (B) and bone sequestration (*) (haematoxylin and eosin, 259 magnification). (b2) Magnification of a boxed section from b1, which shows histological evidence of intense inflammatory cell infiltration (arrowheads), cementum (C) and dentine (D) (haematoxylin and eosin, 1009 magnification). (b3) Magnification of the boxed section from b2; microscopic aspect reveals the presence of polymorphonuclear cells (haematoxylin and eosin, 10009 magnification). (b4) Magnification of another boxed section from b1, which shows the various lacunae of dentine resorption (♦) (haematoxylin and eosin, 4009 magnification). Abbreviations and symbols: AP, apical periodontitis; PD, periodontal disease; AB, alveolar bone; #, surgically induced crown opening; N, total necrosis; C, cementum; D, dentine; arrowheads, inflammatory infiltrate; ▼, lacunae of active bone resorption; B, root biofilms; *, bone sequestration; ♦, lacunae of dentine resorption’.
Item 11g: Quality of images – Arrow markers and relevant labels must be provided in image(s), if relevant, in order to identify key information
Explanation
The important information about images should be identified using arrows and labels along with a key within the corresponding legend (Example 11g.1). For example, images can illustrate the severity of a disease condition, a diagnosis, a treatment procedure or demonstrate treatment effectiveness/outcomes. Arrow markers in images must be provided to identify features of interest, such as cell types, lesions, membranes, smear layer, margins, microleakage etc. Care is needed to position the labels and markers in such a manner as to ensure they do not obscure important information.
Example 11g.1
From Shrestha et al. (2018) – Figure 1: Arrows (black/white) have been used to identify key information and are mentioned in the legend.
Item 11h: Quality of images – The legend of each image must include an explanation whether it refers to pretreatment, intratreatment, post-treatment or postsacrifice, and if relevant, how images were standardized over time
Explanation
The labels and legends for images should inform readers whether the animal was still alive and shows post-treatment, or pretreatment information, or if the images were taken after sacrifice (Examples 11h.1, 11h.2). By labelling images with alive or dead animals, it is hoped that this will promote the greater use of noninvasive imaging of live animals for data collection over time, and so reduce the total numbers of animals sacrificed in each study. Details on how sequential images were standardized to allow comparisons to be made must be provided.
Example 11h.1
From Jensen et al. (2010) – ‘Figure 1, a) Standardized monocortical bone defects in the rabbit calvarium before application of haemostatic agents. Example of photograph used for visual assessment of initial bleeding score. b) Schematic illustrations used for visual assessment of bleeding. c) Presentation after application of haemostatic agents. Example of photograph used for visual assessment of final bleeding score’.
Example 11h.2
From Chen et al. (2015) – ‘Figure 6. Examples of healing on different imaging modalities. In all panels, 1 is postsurgery periapical radiograph, 2 is 6-month follow-up periapical radiograph; 3 is mesiodistal (MD) section and 4 is buccolingual (BL) section of CBCT images at 6-month follow-up; 5 is MD section and 6 is BL section of micro CT image at 6-month follow-up. (A) Periapical radiographs were not able to detect periapical bony defect effectively. (A1 and A2) Comparing postsurgery and follow-up radiographs, it was diagnosed as complete healing. (A3 and A4) Periapical radiolucency was clearly shown on CBCT images. The root was diagnosed as absence of hard tissue covering the root-end surface (score 0) and absence of trabecular bone in the periapical area (score 0). (A5 and A6) Micro CT image demonstrated same results. (B) Root filled with RRM. (B1 and B2) Periapical radiographs showed complete healing. (B3 and B4) Diagnosed as hard tissue completely covered the root-end surface (score 2) and normal trabecular pattern in the periapical area (score 2) on both sections. (B5 and B6) Micro CT images showed the same results. (C) Root filled with MTA. (C1 and C2) Diagnosed as complete healing by using periapical radiographs. (C3 and C4) CBCT showed hard tissue completely covered the root-end surface (score 2) and normal trabecular pattern in the periapical area (score 2) on both sections. (C5 and C6) Micro CT images showed the same results except the finding of less dense trabecular bone in the periapical area (score 1) on BL section. (D) Root filled with MTA showed uncertain healing on periapical radiographs. (D1 and D2) Size of radiolucency has decreased but was larger than twice the width of PDL space. (D3 and D4) CBCT showed no hard tissue forming on the resected root-end surface and in the periapical area (score 0) on both MD and BL sections. However, intact cortical plate was observed (score 2). (D5 and D6) Micro CT images showed absence of hard tissue on the resected root-end surface (score 0), but some trabecular bone was observed in the periapical area (score 1). F/U, follow-up’.
PRIASE 2021 flowchart
Explanation
The steps that are followed during the conduct of animal studies are summarized and presented visually in the PRIASE 2021 flowchart. The flowchart provides a diagrammatic sequence of the various components of a study that can be used as a template by authors to prepare their manuscripts. The flowchart can be customized and used to fit the information that needs to be included in the submission of the manuscript. The template of the flowchart is freely accessible from the ‘Preferred Reporting Items for study Designs in Endodontology (PRIDE) website’ – http://pride-endodonticguidelines.org/priase/.
Example
Figure 2: PRIASE 2021 flowchart illustrating the steps involved in conducting animal studies*.
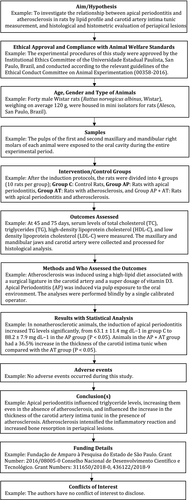
Discussion
Animal testing is required by the laws in many countries, which conform to the international testing standards, for example ISO 10993 and ISO 7405 (Dammaschke 2010) to evaluate the safety and efficacy of novel materials and treatments before using them in human clinical trials (Stanley 1992). Animal testing plays an important role in the development of novel treatments that are safe and effective and which could be used to alleviate human pain and suffering.
The PRIASE 2021 guidelines provide a framework for authors when publishing high-quality animal studies in Endodontology. This PRIASE Explanation and Elaboration document supports the guidelines and adds further information to enhance their understanding, uptake and dissemination. The process that led to the development and overall structure of the PRIASE 2021 guidelines was similar to other guidelines, for example Preferred Reporting Items for Case reports in Endodontics (PRICE) 2020 (Nagendrababu et al. 2020). In the current document, examples from the literature are provided to complement each item in the PRIASE 2021 checklist.
Images provide important visual evidence for both researchers and clinicians to authenticate and support the findings provided in a report (Kotz & Cals, 2013, Polepalli Ramesh et al. 2015). Images are often important in the reporting of animal studies in Endodontology and several items in the checklist are designed to improve the quality of images submitted with manuscripts.
It has been reported that the quality of randomized controlled trials and systematic reviews improved with the use of flowcharts (Egger et al. 2001, Vu-Ngoc et al. 2018). The PRIASE 2021 guidelines include a flowchart to help readers understand the various stages within animal studies.
The following sentence should be included in manuscripts when reports of animal studies followed the PRIASE 2021 guidelines: ‘This animal study was reported according to the PRIASE 2021 Guidelines (Nagendrababu et al. 2021)’. The adoption and endorsement of the PRIASE 2021 guidelines by relevant journals in their ‘Author guidelines’ will ensure the guidelines are used. Readers can then critically and systematically evaluate the reported animal studies using the guidelines as a benchmark. The reporting of an animal study can follow its unique and logical flow and need not follow strictly the sequential order of the reporting checklist as given in the guidelines. Adherence to the PRIASE 2021 guidelines might increase the word count in manuscripts; however, this can be considered as an advantage for readers as it will allow them to understand the details of the study more clearly.
Conclusion
Animal studies are arguably amongst the most criticized, controversial, complex, challenging, expensive, labour-intensive and most highly regulated of all the types of studies within Endodontology. Therefore, the demand-driven PRIASE 2021 guidelines are complemented and supported by this explanation and elaboration document, which is aimed to help authors reduce potential sources of bias and thus improve the quality, accuracy, reproducibility, completeness and transparency of reports describing animal studies in Endodontology. Because of these advantages, we advocate for the more widespread adoption of the PRIASE 2021 guidelines to ultimately transfer the benefits of improved research into the specialty of Endodontology, to help its practitioners to meet the needs of patients more effectively.
Acknowledgments
First author (V Nagendrababu) was associated previously with the School of Dentistry, International Medical University, Kuala Lumpur, Malaysia, where the ethical clearance was obtained (No: IMU 450/2019) and where the study was conducted. The first author (V Nagendrababu) is now associated with the University of Sharjah, Sharjah, United Arab Emirates, where a further ethical clearance was obtained (REC-20-11-06-01). The authors are most thankful to Professor Michael Væth, Department of Public Health, Aarhus University, Aarhus, Denmark, for his valuable advice and suggestion with the statistical terms. We would like to thank those who helped in sharing their views on the PRIASE 2021 Explanation and Elaboration document: Brenda Gomes, Brazil; Josette Camilleri, UK; Juan J. Segura-Egea, Spain; Meetu Kohli, USA; Renato M Silva, USA; Shaul Lin, Israel. The authors gratefully acknowledge the contributors whose work has been reproduced in this publication. The authors deny any responsibility for any potential errors, omissions or artifacts that may be discovered outside of their control.
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- * Adapted from Conti LC, Segura-Egea JJ, Cardoso C, Benetti F, Azuma MM, Oliveira P, Bomfim S, Cintra L (2020) Relationship between apical periodontitis and atherosclerosis in rats: lipid profile and histological study. International Endodontic Journal, 10.1111/iej.13350. Advance online publication. https://doi.org/10.1111/iej.13350