Divergent foraging habitat preferences between summer-breeding and winter-breeding Procellaria petrels
Abstract
Foraging niche specialization is thought to occur when different members of speciose communities divide resources in either time or space. Here we compared habitat preferences of the congeneric Grey Petrel Procellaria cinerea and White-chinned Petrel Procellaria aequinoctialis, tracked in the same calendar year using GPS loggers from Gough Island and Bird Island (South Georgia), respectively. We identified periods of active foraging and determined habitat characteristics using remote-sensing data. Although these highly pelagic species could potentially overlap at sea across large areas, they showed markedly different foraging preferences during their incubation periods, which are temporally offset because Grey Petrels breed during the austral winter. Grey Petrels foraged mostly in pelagic cold-water areas to the north-west of South Georgia, whereas White-chinned Petrels foraged almost exclusively in the warm, shallow waters of the Patagonian Shelf. Within each species, foraging habitat characteristics were highly consistent. Our results demonstrate the diversity of habitat preferences within genera, and provide further evidence that colony-specific information on habitat preference is crucial to identify important feeding areas for pelagic predators.
Foraging in dynamic environments challenges predators to locate and capture prey that are temporally and spatially unpredictable. Although optimal foraging strategies are complex and often variable, their key objective is to maximize prey consumption while minimizing the effort required to travel and feed (Orians & Pearson 1979, Stephens et al. 2008, Waggitt et al. 2018). A wide variety of environmental and physiological factors may constrain foraging behaviour (Tucker et al. 1995, Gilchrist et al. 1998, Spaethe et al. 2001). Although we expect foraging animals to target habitats that yield the highest prey capture rates, this involves trade-offs between resource abundance and levels of inter- and intraspecific competition, which in speciose communities often leads to high levels of spatial and temporal segregation in habitat use (Masello et al. 2010, Navarro et al. 2013). Understanding habitat specialization in closely related species can provide insights into how these communities are maintained (Vilchis et al. 2006, Granroth-Wilding & Phillips 2019).
Coexistence is often promoted in highly diverse communities via specialization, which allows a greater number of species and individuals to partition resources (Schoener 1974, Phillips et al. 2017). This has been shown across diverse taxa, including reptiles and amphibians (Toft 1985), mammals (Aldridge & Rautenbach 1987) and birds (Feinsinger & Colwell 1978). Given their high diversity and abundance, coupled with the central-place foraging constraints imposed by breeding on land, seabirds provide ideal models for investigating specialization. During breeding, they must balance travelling to access the best resources with the needs of incubation and chick-rearing (Phillips et al. 2017). The depletion of resources around their nesting islands has long been discussed (Ashmole 1963, Birt et al. 1987) and specializations on particular prey, habitats or in other aspects of foraging behaviour have been shown to occur among species, populations, sexes and age-classes (Bearhop et al. 2006, Thiebot et al. 2012, Wakefield et al. 2013, Mendez et al. 2017, Votier et al. 2017). When interspecific competitors are morphologically similar, specialization is often via behavioural variation, such as in timing of foraging or location (Nicholls & Racey 2006). Partitioning of resources is a potential driver of speciation if behavioural or phenotypic changes ultimately lead to reproductive isolation (Bolnick et al. 2007). If selection leads to differences in timing of breeding of related taxa (allochrony), speciation is possible even in sympatry (Friesen et al. 2007a, 2007b, Taylor & Friesen 2017).
Extreme allochrony in Antarctic seabirds can lead to the phenomenon of winter breeding (Poupart et al. 2019). The underlying ecological drivers remain unclear, particularly because chick provisioning is energetically expensive (Welcker et al. 2015) and so we expect reproduction to coincide with the most favourable foraging conditions; for the great majority of temperate and polar seabirds, this is during the austral summer, when longer days and warmer conditions enhance phytoplankton blooms, in turn supporting abundant primary and secondary consumers (Poupart et al. 2019). By comparison, productivity in the Southern Ocean is reduced in autumn and at its minimum in winter (Alvain et al. 2008). As such, the shift during speciation to winter breeding is counter-intuitive, particularly as many summer breeders avoid the seasonal decline in food availability by migrating to lower latitudes.
The Procellaria petrels are long-lived, highly K-selected species which often forage at sites long distances from their colonies (Bugoni et al. 2009, Rollinson et al. 2018, Frankish et al. 2020). Two of the five species (Grey Petrels Procellaria cinerea and Westland Petrels Procellaria westlandica) are winter breeders. Westland Petrels, Spectacled Petrels Procellaria conspicillata and Black Petrels Procellaria parkinsoni are each endemic to only one or two breeding islands, whereas Grey Petrels and White-chinned Petrels Procellaria aequinoctialis are much more abundant, breeding in highly speciose seabird communities at several island groups around the Southern Ocean (Phillips et al. 2016).
Tracking studies in the last one to two decades suggest that the contrasting distribution, abundance and phenology among Procellaria petrels may partly be explained by differences in foraging habitat availability and preferences. Habitat use also determines fisheries overlap, which has major implications for conservation; Grey and White-chinned Petrels are listed as Near-threatened and Vulnerable, respectively, by the IUCN because of high bycatch rates in longline fisheries (Phillips et al. 2016). Here we compare habitat preferences of Grey Petrels and White-chinned Petrels from the largest populations in the South Atlantic: Gough Island and South Georgia, respectively. Spectacled Petrels also breed in the region (at Inaccessible Island) but feed in much warmer waters than Grey Petrels and White-chinned Petrels (Bugoni et al. 2009, Reid et al. 2014). Previous tracking of White-chinned Petrels from South Georgia indicated that they target warm, shallow waters at the Patagonian Shelf during the non-breeding season and incubation, then switch to colder waters around and south of the Antarctic Polar Front during chick-rearing (Berrow et al. 2000, Phillips et al. 2006). Grey Petrels are known to target particular broad-scale oceanographic features during the non-breeding season (oceanic ridges with moderate current velocities and average surface temperatures of 7–13 °C), but preferences vary among study colonies (Kerguelen, Antipodes and Prince Edward Islands) to such an extent that habitat models are not transferable across ocean basins (Torres et al. 2015). In our study, we classified behavioural states during trips to sea by these two species tracked in the same region, breeding stage and calendar year, and identified oceanographic features of key habitats. We predicted that habitat choice would be consistent within species, based on previous indications of high site fidelity in these species (Rollinson et al. 2018, Delord et al. 2019). We also predicted that foraging would be more likely at locations furthest from the colony. By comparing results from closely related species we can better understand the extent to which habitat preferences are fixed or flexible, which has important implications for the capacity of organisms to adapt to environmental change. Further, understanding the types of foraging habitat targeted by winter-breeding Grey Petrels can help to explain the evolution of this uncommon strategy.
METHODS
Device deployments and initial processing
Grey Petrels are winter breeders, attending colonies from February to September, whereas White-chinned Petrels breed in the summer, attending colonies from September to May (Phillips et al. 2006, Torres et al. 2015). GPS tags (IgotU; Mobile Action Technology Inc., Taiwan), weighing c. 25 g including heat-shrink packaging, were attached by Tesa® tape to the mantle feathers of 16 White-chinned Petrels and 20 Grey Petrels. All White-chinned Petrels and eight Grey Petrels were also fitted with either a geolocator-immersion logger (Intigeo C250, Migrate Technology, Cambridge, UK; 2.6 g) attached to a plastic band on the tarsus, or a Time Depth Recorder (TDR) (G5, Cefas Technology; mass 2.7 g) housed in heat-shrink with the GPS logger, respectively. Grey Petrels were tagged during the incubation period in April–May 2014 (austral winter) and White-chinned Petrels in December 2014 to January 2015 (austral summer). Mean mass of the tracked Grey Petrels was 1152 g. To minimize handling time, the White-chinned Petrels were not weighed, but the mean mass of other birds weighed during the deployment period was 1307 g (n = 32 birds). GPS devices were retrieved after an average of 22.4 and 30.8 days, respectively, from 18 (90%) of the Grey Petrels (all 20 birds were recaptured but two had lost the GPS logger) and from 13 (81%) of the White-chinned Petrels, possibly because some of the other three birds were non-breeders, or the had chick hatched, so the adult was missed during burrow checks. Thirteen of the GLS devices on White-chinned Petrels were retrieved along with the GPS loggers, and the three others in the following austral summer. The maximum combined mass of the two devices and tape or ring attachments was < 3% of mean body mass for both species, which is below the threshold at which deleterious effects are more common in pelagic seabirds (Phillips et al. 2003), but this does not guarantee there will be no impact (Geen et al. 2019). Devices were set to record at 30-min intervals and removed after a single foraging trip (most birds) or after two foraging trips (two birds only). Fourteen (Grey Petrel) and 12 (White-chinned Petrel) devices downloaded successfully. There were insufficient good-quality locations for four Grey Petrels and one White-chinned Petrel. The retained tracks were interpolated to 30-min intervals to ensure consistency between the datasets using the redisltraj function from the R package adehabitatLT (Calenge 2006).
Behavioural classification
Behavioural states were determined using expectation–maximization binary clustering (EMbC), an algorithm which uses speed and turning angles to categorize behaviour into four states (Garriga et al. 2016). High turning angles were presumed to be associated with foraging behaviour regardless of speed (thus merging two of the states together), low turning angles at high speed with transit behaviour, and low tortuosity at low speed with resting (Garriga et al. 2016). This algorithm is suitable for modelling behavioural responses to dynamic environmental variables, and is robust for use on data of our temporal scale (Bennison et al. 2018). For White-chinned Petrels, the immersion data were used to check whether the locations classified as foraging using EMbC corresponded to landings on the water (Supporting Information Fig. S1). Locations classified as transit or resting were pooled as ‘not foraging’ and compared with foraging points in binomial analyses (clustering for all categories is shown in Supporting Information Fig. S2).
Habitat modelling
- Sea-surface temperature (SST, indicating cold fronts and water mass: CMEMS/Copernicus Marine) measured daily, 0.083° grid;
- Chlorophyll-a concentration (chl-a, a proxy for marine productivity: CMEMS/Copernicus Marine) measured daily, 0.25° grid and log-transformed;
- Sea-level anomaly (height above geoid (m), index of mesoscale oceanic activity: CMEMS/Copernicus marine) measured daily, 0.083° grid;
- Eddy kinetic energy (EKE, index of mesoscale oceanic activity calculated from eastward and northward sea water velocities: CMEMS/Copernicus Marine) measured daily, 0.083° grid and log-transformed.
- Bathymetry (identifying shelf and pelagic zones, 0.00833° grid, GEBCO);
- Euclidean distance from colony (as a proxy for the effect of accessibility).
All layers were resampled to the coarsest scale (0.25°) using the package raster in R (Hijmans & van Etten 2012). Sea-level anomaly was removed from final models as it was 94% correlated with sea-surface temperature. A binomial (presence/pseudoabsence) generalized additive model (GAM) was used to assess the influence of these environmental variables on habitat selection in each species. Model terms were initially selected via the dredge function (Bartón 2020) using adjusted Akaike information criterion (AICc) values, and unique and total deviance explained for each model term was calculated to help contextualize biological significance. The influence of environmental variables (as above) on behaviour (foraging or not foraging, classified using EMbC) was assessed using binomial GAMs with cubic spline smoothing. Model fitting and selection was undertaken using AICc values.
RESULTS
Trip characteristics and habitat selection
Primary foraging areas were highly consistent within species. During the winter, the Grey Petrels (n = 10) tracked during incubation travelled over 3000 km from Gough Island to forage predominantly west-northwest of South Georgia, in a region that was overflown with minimal foraging or resting by White-chinned Petrels in the following austral summer. Instead of utilizing this area, the incubating White-chinned Petrels (n = 11) travelled over 2000 km from South Georgia to forage primarily on the Patagonian Shelf (Fig. 1). Grey Petrels were tracked for a mean of 9.56 ± 3.69 days, but batteries in the loggers depleted before the bird returned and the tracking data were incomplete. It is likely that these loggers depleted quickly due to the impact of low temperatures on the batteries, and potentially because the GPS devices had been used in previous studies. While the GPS loggers collected data on average for 67% of the duration of foraging trips, the vast majority of dives (682 of 775; 88%) recorded by TDRs (which ran for the entire trip) were within the period for which there was GPS data, suggesting that the area to the west-northwest of South Georgia is indeed the key foraging area for this species (full dive details reported in Rollinson et al. 2016; see Supporting Information Fig. S4 showing the last available GPS location from each bird). White-chinned Petrels were tracked for 13.83 ± 3.57 days, which represented their entire foraging trip.
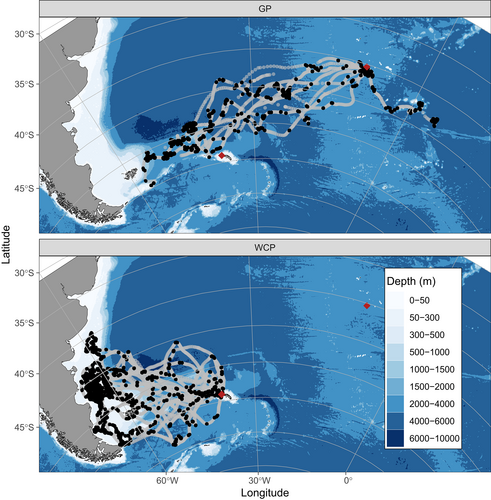
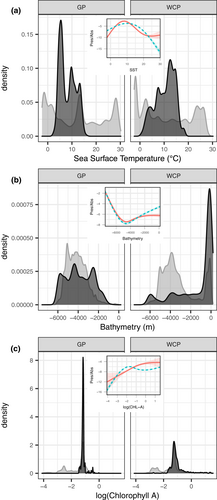
Comparison of presences and pseudoabsences from real and rotated tracks indicated that both species selected habitat in a narrower band of sea surface temperature than that available, with Grey Petrels mostly foraging in water ~ 5 °C and White-chinned Petrels ~10–15 °C (Fig. 2a). Additionally, White-chinned Petrels targeted a specific bathymetric profile, the shallow shelf area < 500 m (Fig. 2b), and both species preferred the upper range of available chlorophyll-a values (Fig. 2c).
Foraging habitats
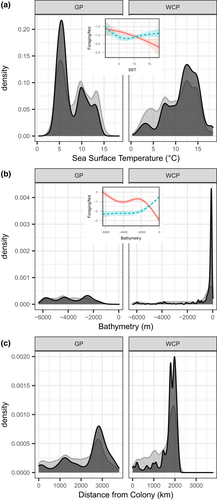
Foraging behaviour, in comparison with transit or resting behaviour, was observed at sea surface temperatures of 0–18 °C, with a marked peak around 5 °C for Grey Petrels, and a broader peak between 10 and 15 °C for White-chinned Petrels (Fig. 3a), reflecting their greater range in latitudes on the Patagonian Shelf. Grey Petrels foraged across a wide depth gradient in colder, deeper waters, whereas White-chinned Petrels foraged almost exclusively in shallow shelf waters < 500 m (Fig. 3b). In both species, foraging behaviour was more likely to occur at maximal distances from the colony (~ 3000 km for Grey Petrels and ~ 2000 km for White-chinned Petrels; Fig. 3c).
DISCUSSION
Habitat preferences and flexibility in habitat use are fundamental to our understanding of ecological processes, community structure and population dynamics, and critical for effective conservation and management in a world where human impacts are pervasive. In this study, we compared the oceanographic characteristics of incubation foraging trips by the winter-breeding Grey Petrel and their summer-breeding congener, the White-chinned Petrel. We identified the habitats where feeding behaviour occurred, as opposed to transit or resting. We observed a clear divergence in habitat preference between congeners, despite the potentially large area of overlap of birds from the two populations. Grey Petrels tracked from Gough Island targeted a specific cold-water area to the northwest of South Georgia, probably related to the relatively high primary productivity at that time of year. In contrast, White-chinned Petrels transited that region, preferring to feed in the shallow (< 500 m) waters of the Patagonian Shelf, without a specific preference for temperature regime. Both species targeted areas of relatively high productivity.
The preference for foraging in cold, pelagic habitat by Grey Petrels from Gough was consistent among individuals, whereas evidence from fisheries bycatch in New Zealand shows that, at least for part of the breeding season, some Grey Petrels from Antipodes Island travel to the north coast of New Zealand, where temperatures are probably much higher than the ~ 5 °C peak observed in our study (Mischler & Bell 2017). The Antipodes population also appears to show sexual segregation in foraging areas during breeding (Mischler & Bell 2017), which was not evident at Gough Island (Rollinson et al. 2016). To our knowledge, the Grey Petrels from Gough represent the only population tracked with GPS devices during breeding. Fine-scale habitat preferences of Grey Petrels at Kerguelen, Antipodes and Marion Islands differed markedly during the non-breeding season (Torres et al. 2015) and hence tracking at other colonies is needed to determine whether the same applies during the breeding season.
Contrasting habitat preferences among colonies are also apparent in the White-chinned Petrel. In our study, birds did not target a specific temperature profile, although all travelled to shallow, productive waters on the Patagonian Shelf. This contrasts with White-chinned Petrels from Iles Kerguelen, which foraged during the entire breeding season in Antarctic and sub-Antarctic waters of 1–5 °C (Péron et al. 2010); those from Marion Island, which tended to forage either in waters close to the colony or off the southern coast of South Africa (Rollinson et al. 2018); and those from Iles Crozet, which foraged both north and south of the colony during incubation, and targeted cold waters to the south while rearing chicks (Weimerskirch et al. 1999, Catard et al. 2000). White-chinned Petrels from South Georgia also forage in cold, southerly waters, as far as the ice edge, but not until the chick-rearing period (Phillips et al. 2006). It could be argued that the consistent targeting of the Patagonian Shelf by White-chinned Petrels during the incubation period might be a new behaviour since the advent of industrial fishing, but it seems unlikely given the huge numbers of other predators – many of which do not scavenge behind vessels – that also use this highly productive region (Song et al. 2016). Our study also reaffirms that this area is critical habitat for White-chinned Petrels during breeding every year, as our new data indicate the use of similar foraging areas to White-chinned Petrels tracked in incubation over a decade earlier (Phillips et al. 2006).
Differences among congeners in habitat use can vary from subtle to distinct, and can involve temporal segregation when niches are very similar. Both MacGillivray's Pachyptila macgillivrayi and Broad-billed Prions Pachyptila vittata target similar foraging areas at the same points in their breeding cycle, but do not compete due to a temporal offset of breeding by around 3 months (Jones et al. 2020). The same mechanism also reduces competition between Northern Macronectes halli and Southern Giant Petrels Macronectes giganteus, which lay on average around 6 weeks apart and which also show sexual segregation (Brown et al. 2015, Granroth-Wilding & Phillips 2019). In contrast, the oceanographic characteristics of foraging areas chosen by Grey and White-chinned Petrels are much more distinct, and the temporal offset in timing of breeding is much longer (approximately 3–4 months). The high consistency in foraging habitat preferences within Grey and White-chinned Petrels, and its strong divergence between species, as well as the markedly different phenology, may have originally developed during sympatric speciation. Further study focusing on islands where these two species breed in sympatry (Marion, Crozet, Kerguelen and New Zealand) would help determine the mechanisms by which this is maintained. The observed flexibility in habitat choice of Procellaria species across populations and between breeding stages suggests that temporal segregation of peak resource-demand (i.e. breeding allochrony) is as effective for partitioning resources as habitat specialization, and also more likely to lead to reproductive isolation and therefore speciation.
The most extreme example of breeding allochrony in seabirds is winter breeding, which in Procellaria occurs in both Grey and Westland Petrels. Although most seabirds appear to time reproduction such that the most energetically intensive phase (chick-rearing) coincides with peak resource availability, there may be a benefit to instead aligning the non-breeding period with the productivity peak. Grey Petrels and Westland Petrels have unusually long chick-rearing periods, and laying is more protracted than in other petrels and shearwaters, which has been attributed to the scarcity and variability of food in the austral winter (Zotier 1990). This long breeding period means that the slowest parents have < 80 days for post-breeding moult to restore their body condition prior to the onset of the subsequent season (Zotier 1990, Chastel 1995). While such a short period between fledging and expected return would usually result in biennial breeding, as in the Wandering Albatross Diomedea exulans, this is not the case for Grey Petrels. It has been argued that such a quick recovery is possible for winter breeders due to the abundant resources available in the summer, which they can exploit without the central-place restrictions experienced by breeding birds (Chastel 1995).
Alternatively, allochrony may develop simply because winter resource levels are high enough to support alternative breeding strategies. Seven reciprocally monophyletic clades of band-rumped storm-petrels (Hydrobates spp.) have now been revealed, which probably comprise cryptic species or sub-species that show strong allochrony caused (or at least maintained) by multiple temporal peaks in resource availability (Monteiro & Furness 1998, Taylor et al. 2019). It may be that despite lower overall productivity in winter, there is a secondary peak of food accessibility for Grey Petrels, due to a reduction in interspecific competition in waters around South Georgia. Grey Petrels from Gough Island show temporal segregation in peak demand for resources from both White-chinned Petrels at South Georgia, and Spectacled Petrels, which breed on nearby Inaccessible Island (Reid et al. 2014). Demand for resources other than food may also contribute to breeding allochrony; indeed, it has been suggested that winter breeding in seabirds emerged in response to competition for burrows (Harrison et al. 1983). Winter breeding at South Georgia is not possible for burrow-nesting species due to frozen ground and persistent snow cover; however, at lower-latitude sites such as Marion Island, there is strong evidence for competition (in the form of chick evictions) between Grey and White-chinned Petrels at the start or end of their respective breeding seasons (Dilley et al. 2019). Winter breeding may have evolved in sympatry as a mechanism to reduce such competition and then carried over to islands where these species breed in allopatry. In addition, because zooplankton remain abundant in the area north of South Georgia well into winter (Atkinson et al. 2001), the absence of local breeders may more than compensate for the commuting costs borne by Grey Petrels coming from Gough. It has been suggested that winter breeding in Westland Petrels is sustained by sufficient prey abundance in the absence of summer-breeding competitors (Poupart et al. 2020). It may be that winter breeding also provides Grey Petrels with a dual advantage, allowing access to sufficient prey resources in the south, while avoiding peak competition for burrows on their temperate breeding islands.
Our study highlights the importance of tracking for identifying key foraging areas and habitats for pelagic predators, which can be located thousands of kilometres from the nest. Here we report results for two congeners with distinct foraging preferences, but we infer that their strategies are also influenced by the highly speciose communities in which they breed: specialization is therefore likely to occur at even smaller scales and be further influenced by individual preferences (Navarro et al. 2013, Phillips et al. 2017). Such comparisons help develop a deeper understanding of the relationships between foraging behaviour, niche partitioning and life history.
We are grateful to all the fieldworkers involved in the device deployment and retrieval and to Andy Wood for database support. This study represents a contribution to the Ecosystems component of the Polar Science for Planet Earth Programme, and NC-ODA (grant NE/R000107/1) funding from the Natural Environment Research Council through the British Antarctic Survey funded by NERC. Tracking devices were funded by the Government of South Georgia and the South Sandwich Islands (GSGSSI). The fieldwork on White-chinned Petrels was carried out under permit from the Government of South Georgia and the South Sandwich Islands (SCI/2014/14 and WPA/2014/16) and approval for animal handling was granted by the British Antarctic Survey Ethical Review Committee (ERC #1010). Permission to undertake the work on Gough Island was provided by the Tristan Conservation Department. Logistical and financial support was provided by the South African Department of Environmental Affairs, through the South African National Antarctic Program, the National Research Foundation and the University of Cape Town. Long-term monitoring on Gough Island was established with a grant from the UK Foreign and Commonwealth Office with further support over the years from the UK Government's Overseas Territories Environment Programme, the Royal Society for the Protection of Birds, and the Agreement on the Conservation of Albatrosses and Petrels. Animal ethics review for bird handling was provided by the University of Cape Town Animal Ethics Committee (permit SFAEC 2014/V10/PR). This study has been conducted using E.U. Copernicus Marine Service Information. L.K.B. was funded by the Gates Cambridge Trust.
AUTHOR CONTRIBUTIONS
Lily K. Bentley: Conceptualization (equal); data curation (equal); formal analysis (lead); investigation (lead); methodology (equal); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Andrea Manica: Conceptualization (supporting); formal analysis (supporting); methodology (equal); supervision (lead); writing – review and editing (supporting). Ben J. Dilley: Data curation (equal); investigation (equal); project administration (equal); resources (equal); writing – review and editing (supporting). Peter Ryan: Data curation (equal); funding acquisition (equal); project administration (equal); resources (equal); writing – review and editing (supporting). Richard Phillips: Conceptualization (equal); data curation (equal); funding acquisition (equal); investigation (lead); methodology (supporting); project administration (equal); resources (equal); supervision (lead); writing – review and editing (supporting).
CONFLICT OF INTEREST
All authors declare they have no conflicts of interest.
FUNDING
Government of South Georgia and the South Sandwich Islands, South African National Antarctic Program, Foreign and Commonwealth Office, British Antarctic Survey, Gates Cambridge Trust.
ETHICAL NOTE
None.
Open Research
Data Availability Statement
The tracking data can be downloaded from the Seabird Tracking Database (BirdLife International 2022) (http://seabirdtracking.org; dataset ids: 1288 and 1386).




