Late-breeding Great Cormorants Phalacrocorax carbo sinensis produce fewer young of the more vulnerable sex
Abstract
We examined the brood sex ratio and offspring body mass in relation to the timing of breeding and brood size in the Great Cormorant Phalacrocorax carbo sinensis. The brood sex ratio was not related to brood size but it was significantly related to the hatching date, with a decreasing proportion of males in the brood in the course of the season. Male chicks had significantly lower body mass if they hatched later in the season, whereas there was no such relationship for female offspring. Assuming that environmental conditions deteriorate with progress of the breeding season, and male offspring may be more vulnerable to poor environmental conditions, the observed decline in the proportion of male offspring late in the season may be adaptive.
Sex ratio theory predicts that brood sex ratio in birds may be subject to selection under certain ecological conditions. In sexually size-dimorphic species, where one of the sexes may be more vulnerable to unfavourable rearing conditions (Fiala & Congdon 1983, Røskaft & Slagsvold 1985, Teather 1987, Teather & Weatherhead 1988, Griffiths 1992, Torres & Drummond 1997, Kalmbach et al. 2005), the parents may benefit from producing more of the less vulnerable sex when conditions are unfavourable. This might be the case, for instance, when birds breed late in the season, when their timing of breeding is not in synchrony with maximum food availability and/or if their food resources have already been depleted by earlier breeders (e.g. Stempniewicz et al. 2003, Němečková et al. 2008). In the same context, sex differences in hatching order may be subject to selection (Mock & Parker 1997, Velando et al. 2002). This may be particularly important in species with large, asynchronously hatching broods, where differences in offspring size caused by age and sex can produce competitive feeding hierarchies with disadvantages to the smaller or later-hatched chicks (Mock & Parker 1997). Thus, brood sex ratio may be adaptive if variation in brood sex ratio is related to differences in reproductive success.
Indeed, some studies have reported significant variation in primary offspring sex ratio that appears to be adaptive (e.g. Nager et al. 1999, Whittingham & Dunn 2000, Velando et al. 2002, Rutkowska & Badyaev 2008). Other studies have shown that brood sex ratio may also be influenced by post-zygotic differential mortality of male and female offspring, in particular under unfavourable ecological conditions (e.g. Røskaft & Slagsvold 1985, Torres & Drummond 1997, Velando et al. 2002, Kalmbach et al. 2005). On the other hand, some studies have found no evidence for an adaptive variation of the offspring sex ratio (e.g. Verboven et al. 2002, Postma et al. 2011) and negative results are probably under-reported. The reason for this apparent inconsistency between studies may be that selection on male and female offspring may vary with species-specific life-history traits and ecological circumstances. Thus, to evaluate the ability to adjust offspring sex ratios, studies need to consider both variation in offspring sex ratio with respect to specific selection factors and differences between the two sexes with respect to the same selection factor.
In this study we examined the hatching sex ratio and body mass of male and female offspring in relation to hatching date and brood size in the Great Cormorant Phalacrocorax carbo sinensis to evaluate the female parent's ability to adjust the sex ratio of her offspring. The Great Cormorant is a colonially breeding, monogamous species with hatching asynchrony and long and extensive biparental care. There are no apparent sex differences in the adult plumage, but males are on average 10% larger and 20% heavier than females (Koffijberg & Van Eerden 1995, Liordos & Goutner 2008). There is considerable inter-individual variation in breeding success (nul to six offspring) and timing of breeding that seems to be related to abundance of the preferred food items and weather conditions (Voslamber 1988, Stempniewicz et al. 2000, 2003, Minias et al. 2012). All these features make the Great Cormorant a suitable study species to quantify variation in offspring sex ratio in relation to timing of breeding and brood size. Considering the sex differences in body size we assumed that the smaller female chicks would be less vulnerable to poor environmental conditions, such as food shortage, than the larger male chicks. If so, we expected that offspring sex ratio should be female-biased under less favourable conditions. As availability of food resources seem to deteriorate with the progress of the breeding season (Stempniewicz et al. 2000, 2003), we expected female-biased sex ratio in later broods. For the same reasons, we expected that females hatched later in the breeding season would be more able to maintain normal growth than males. As facultative brood reduction is believed to be related to food availability for a given breeding attempt (Temme & Charnov 1987, Forbes & Ydenberg 1992), we also expected female-biased offspring sex ratios in larger broods, where such a proportion of the sexes should decrease intra-brood competition and probability of brood reduction.
Methods
We carried out the study in the Great Cormorant colony at the Jeziorsko reservoir (51°40′N, 18°40′E; central Poland) in 2010. From the laying period onwards, we monitored 50 nests (of 110). At the time of hatching, we selected randomly 30 nests for further study. In this sub-sample of 30 nests, four eggs were lost during the incubation period, probably as a result of predation (3.0%, n = 132 eggs). Of the 128 eggs that survived to hatching, 16 failed to hatch (12.5%). We were able to check the contents of about half of the unhatched eggs and only two eggs showed signs of embryo development. The hatching period in the 30 nests lasted 22 days, with the first chicks hatched on 12 April and the median date of hatching 17 April. From all hatchlings (112 from the 30 broods) we took blood samples for molecular sexing (c. 10 μL, preserved in 96% ethanol) and took biometric measurements (wing length to the nearest 1 mm, culmen length and tarsus length to the nearest 0.1 mm, body mass to the nearest 1 g) to establish the hatching positions within 2 days of hatching of the last chick in a given nest. We found only one dead chick between the time of hatching and sampling, and sampled the carcass for molecular sexing. We established the hatching position of the dead nestling based on the daily nest controls. At the time of blood sampling and measurement we tagged all chicks using flexible Velcro strips of different colours wrapped around the chick's leg. We enlarged these strips according to the chick's size during successive visits. At the age of 13 days we removed the Velcro strips and marked all chicks with individually numbered metal rings.
We established the hatching position from the biometric data following methods used for other waterbirds with hatching asynchrony, including various cormorants (Williams & Cooper 1983, Shaw 1985, Stockland & Amundsen 1988). All measurements of body size and body mass were reduced to the first principal component (PC1) of a principal component analysis (PCA) following the recommendation of Freeman and Jackson (1990). We standardized the variables to equal unit variances (Z-scores) prior to analysis. The PC1 extracted from the analysis accounted for 94.4% of the variability. We assigned hatching positions to each chick according to its size ranks based on the PC1 values. In our colony usually one chick hatched per day P. Minias pers. obs.), so size differences among the nestlings were evident.
We checked the broods every 3rd day until the oldest chick in a given nest reached the age of 22 days (± 1 day), at which time all chicks were measured and weighed (to the nearest 20 g) for the second time. We decided to stop visiting broods at that age, as chicks older than 25 days may jump out of the nest when humans approach (Platteeuw et al. 1995, P. Minias pers. obs.). We noted the number of chicks and the occurrence of brood reduction during each visit. We did not find any signs of predation; in all recorded cases of chick mortality, we found the carcass in the nest, indicating brood reduction via starvation or siblicide.
We performed molecular sexing on DNA extracted from the blood with the Blood Mini kit (A&A Biotechnology, Gdynia, Poland) after ethanol evaporation. We performed amplification of the CHD region with the primer pair 2550F and 2718R (Fridolfsson & Ellegren 1999) according to the protocol described in Griffiths et al. (1998), using a 50 °C annealing temperature for the PCR reaction. The difference in PCR product size (c. 200 bp) was clearly visible when separated on 2% agarose gel.
We expressed the hatchling sex ratio in the population as the percentage of males in the total number of offspring sampled (n = 112) and tested the deviation of the population sex ratio from parity using a G-test. We also calculated the egg sex ratio for 14 broods in which all eggs hatched successfully (n = 60).
Following the recommendations of Krackow and Tkadlec (2001), we used a generalized linear mixed model with binomial distributions and a logit link function to assess whether offspring sex was affected by hatching date, hatching position or brood size (independent variables). As data from siblings are non-independent we included brood identity as a random factor to avoid pseudoreplication (Hurlbert 1984). We used data from all broods (hatchling sex ratio) in this analysis. Additionally, for the broods where all eggs hatched (n = 14) we checked the relationship between hatching date and offspring sex ratio using a Spearman rank correlation.
To analyse nestling body mass at the age of 22 days in relation to sex, hatching date and hatching position (independent variables) we used a general linear mixed model, with brood identity entered as a random factor to avoid pseudoreplication. Brood size was not related to offspring sex ratio and therefore we did not explore further the factor of brood size. We included the interaction between sex and hatching date in the model to check for a sex-specific relationships. In the analysis, hatching position was treated as a continuous variable due to the low number of chicks from the last two hatching positions, which consequently could not form separate categories. Non-significant variables were excluded from the full models with backward stepwise methods.
Finally, we used logistic regression to check for the effect of different variables (brood sex ratio at hatching, the sex of the last chick in the brood, hatching date and brood size) if brood reduction occurred. For this purpose we codified the brood reduction binomially with ‘1’ for broods where it occurred and with ‘0’ where it did not occur. The sex ratio, treated as a covariate, was arcsin-transformed prior to the analysis (Zar 1996). We determined the significance of independent variables using Wald statistics (W).
Results
Overall, 56% of the 112 sexed chicks were male, and the hatching sex ratio did not differ from parity (G1,112 = 1.76, P = 0.19). In nests where all eggs hatched, the egg sex ratio was exactly 1 : 1 (n = 60 offspring from 14 nests). The mean number of hatchlings per nest was 3.73 (se = 0.78) young (n = 30) and three and four chicks were the most frequent brood sizes (43.3% each). The sex of nestlings was not affected by hatching position (F4,90 = 0.48, P = 0.75) or brood size (F2,90 = 0.66, P = 0.52) but it was significantly related to hatching date (F1,96 = 3.97, P = 0.049). There was a higher proportion of male chicks at the beginning of the breeding season which decreased as the season progressed (β = −0.088, se = 0.044; Fig. 1a). The seasonal decline in the proportion of males was also found for broods in which all eggs hatched (r = −0.62, n = 14, P = 0.018; Fig. 1a).
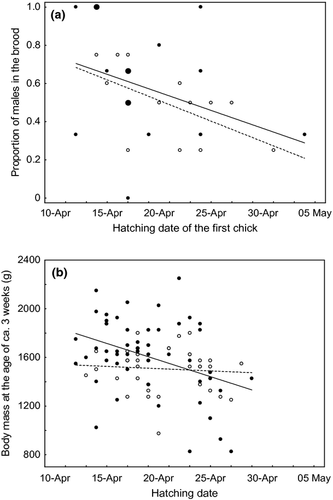
Different patterns for young males and females were found when analysing their body mass at the age of c. 3 weeks in relation to their hatching date. After controlling for brood identity (F26,60 = 2.10, P = 0.009) and hatching position (F1,60 = 42.79, P < 0.001) we found a significant effect of an interaction between sex and hatching date on chick body mass (F1,60 = 7.36, P = 0.009). The analyses performed separately for both sexes revealed that males had a significantly lower body mass if they hatched later in the season (F1,53 = 11.36, P = 0.003, β = −4.70, se = 8.29), whereas there was no such relationship for female chicks (F1,37 = 0.21, P = 0.65; Fig. 1b).
Brood reduction was recorded in nine of the 30 nests; seven nests lost the youngest chick, one nest lost the penultimately hatched chick, and one nest lost the two youngest chicks. The overall sex ratio of the lost chicks was 1 : 1 (n = 10). The occurrence of brood reduction was not affected by the brood sex ratio (W = 1.71, P = 0.19), the sex of the last chick in the brood (W = 0.26, P = 0.61) or hatching date (W = 0.20, P = 0.66) but it was significantly related to brood size (W = 5.64, P = 0.018). The probability of brood reduction increased with brood size (β = 2.61, se = 1.03), with a 13.6-fold increase in the probability of brood reduction with every additional chick in the brood.
Discussion
We observed a decreasing proportion of male hatchlings as the breeding season progressed, in both egg and hatchling sex ratio. The observed pattern could be a result of sex-ratio adjustment at the primary level. The mechanism of adjusting the primary sex ratio is largely unknown but it may be controlled through maternal body condition (Nager et al. 1999, Whittingham & Dunn 2000, Rutkowska & Badyaev 2008). If late breeding female Great Cormorants were in poor body condition, that could reduce their ability to produce sons. There is no evidence that early breeders were in better body condition than late breeders but in many species of colonial waterbirds, individuals starting to breed early in the season are older and more experienced, and thus they might also be in better body condition than late breeders (e.g. Hipfner 1997).
Assuming that the larger male offspring would be more vulnerable to poor environmental conditions (for references see Introduction), a decrease in the proportion of male Great Cormorant hatchlings, the larger sex, with progressing breeding season might be viewed as adaptive. We did not have data on the seasonal variation of environmental conditions such as food availability in our study area and through the breeding season, but such changes have been reported in other cormorant colonies. Early breeding cormorants took advantage of a greater availability of highly profitable prey items when their food demand peaked, whereas the maximum food requirements of late breeders coincided with the peak availability of prey that is less energy-rich and more difficult to forage (Stempniewicz & Grochowski 1997, Stempniewicz et al. 2003). Thus, if the environmental conditions deteriorate over the course of the breeding season, late-laying Great Cormorants may benefit from raising female-biased broods. This seems to be partly supported by our results, since the body mass of males, though not females, decreased with increasing hatching date.
The observed sex differences in the relationship between hatching date and offspring body mass are consistent with our expectations of the female nestlings coping better in poorer environments. Such differential environmental sensitivity was demonstrated for other sexually size-dimorphic species (e.g. Røskaft & Slagsvold 1985, Torres & Drummond 1997, Velando et al. 2002, Kalmbach et al. 2005). It could be that the males' higher vulnerability to unfavourable conditions results from their greater nutritional demands compared with females (Fiala & Congdon 1983, Teather 1987, Teather & Weatherhead 1988, Anderson et al. 1993, but see Torres & Drummond 1999).
We did not find any relationship between offspring sex ratio in Great Cormorant and the occurrence of brood reduction. Brood reduction seemed to be related to brood size, occurring more frequently in larger broods. This suggests that hatching asynchrony but not brood sex composition serves to decrease brood size when necessary (Lack 1954, Temme & Charnov 1987, Forbes & Ydenberg 1992).
Our results indicate that variation in brood sex ratio in relation to the timing of breeding in Great Cormorants might be adaptive. Offspring sex ratio can be of importance for the breeding success of Great Cormorant, particularly when considering offspring quality in relation to timing of breeding. Late breeders produced fewer males, which seemed to be more vulnerable than female offspring and thus may have increased their reproductive success by biasing their investment towards the sex that is more likely to be successful.
The fieldwork was performed by permission of the Bioethical Commission and Provincial Nature Protection Bureau of Łódź and the General Environmental Protection Directorate in Poland. The study was supported by grants from the University of Gdańsk (BW/L120-5-0422-0) and from the Ministry of Science and Higher Education (N N303 319940), as well as by the scholarship of the European Social Fund and the Polish National Budget in the D-RIM project of the Human Capital Programme. We are very grateful to Peter Senn for linguistic editing of the manuscript. We also thank Bartosz Lesner, Anna Piasecka and Radosław Włodarczyk for their help with the fieldwork, as well as Dariusz Jakubas, Zbigniew Wojciechowski and anonymous reviewers for their valuable comments on the first draft of the manuscript.




