UV reflectance as a cue in egg discrimination in two Prinia species exploited differently by brood parasites in Taiwan
Abstract
Birds are capable of seeing the ultraviolet light (UV) spectrum and as a consequence have evolved UV-reflective structures with signalling functions. Avian eggs also reflect in the UV spectrum but the importance of UV egg matching in egg rejection decisions has been equivocal. Here we conducted egg rejection experiments in the congeneric and sympatrically breeding Yellow-bellied Prinia Prinia flaviventris and Plain Prinia Prinia inornata in Taiwan to assess the role of UV as a cue in egg discrimination. Yellow-bellied Prinia is a host of Oriental Cuckoo Cuculus optatus, whereas Plain Prinia is not. We coated one prinia egg in the experimental clutches with a cream containing a UV-blocking agent, while the rest of the eggs were coated with cream only. We also experimentally parasitized prinias with non-mimetic model eggs with reduced UV reflectance. Yellow-bellied Prinia and Plain Prinia rejected their own UV-blocked eggs in 18.2 and 8.3% of the experiments, respectively, and the difference was not significant. However, Yellow-bellied Prinia rejected 100% of the non-mimetic eggs, whereas the Plain Prinia rejected only 5%. Hence, UV reflectance alone is a cue in egg discrimination, but the importance of reflectance outside the UV spectrum in these two prinia species is much more responsive to selection as a consequence of brood parasitism.
Cuckoo–host interactions are model systems for studying co-evolution (Rothstein & Robinson 1998). In such systems, hosts evolve anti-parasite adaptations to counter brood parasitism, which in turn selects for better cuckoo trickery (Rothstein & Robinson 1998, Davies 2000). The most common host defence against brood parasitism is the evolution of recognition and rejection of foreign egg (Davies 2000, Peer & Sealy 2004). Hosts of avian brood parasites use various egg morphology cues such as colour and luminance, spotting pattern and egg size/shape to distinguish the parasitic egg and some species rely on multiple cues (Rothstein 1982, Mason & Rothstein 1986, Marchetti 2000, Avilés et al. 2010, Spottiswoode & Stevens 2010, Antonov et al. 2011). Avian and human vision differ fundamentally in several aspects including the presence of ultraviolet-sensitive photoreceptors and oil droplets in the avian eye that are absent from the human eye (Goldsmith et al. 1984, Vorobyev et al. 1998). Birds perceive ultraviolet (UV) light and use it in food selection (Viitala et al. 1995, Church et al. 1998). Furthermore, patches of adult plumage, nestling skin and gapes reflect in the UV region and serve as signals in mate choice and offspring care (e.g. Andersson & Amundsen 1997, Siitari & Huhta 2002, Jourdie et al. 2004). As avian eggs also reflect in the UV region, hosts of brood parasites might use UV information as a cue to recognize foreign eggs (Cherry & Bennett 2001). This hypothesis has recently received research attention but the only two experimental tests yielded inconsistent results (Avilés et al. 2006, Honza & Polačiková 2008).
Here we experimentally assessed the importance of UV as a cue in egg rejection decisions by blocking the UV reflectance of single eggs within clutches of the Yellow-bellied Prinia Prinia flaviventris and Plain Prinia Prinia inornata on Taiwan. We also assessed egg discrimination in relation to non-mimetic model eggs with a very low UV reflectance. The two prinias breed sympatrically, but only Yellow-bellied Prinia has been engaged in co-evolutionary interactions with the only brood parasite breeding in Taiwan, the Oriental Cuckoo Cuculus optatus (Lin 2008, Yang et al. 2012). If UV alone is used as a cue in egg recognition, we predicted some rejection of UV-blocked eggs but no rejection of eggs with unaltered UV reflectance. However, if reflectance outside the UV range is a more important cue, rejection rates of non-mimetic and largely UV-blocked eggs in the two species should be related to the degree to which they have interacted with the local parasite.
Methods
This study was conducted at Shou-Feng, Hualien County in eastern Taiwan (23°51′33.0″N, 121°31′16.9″E) in 2010–2011 (April–July). Yellow-bellied Prinia and Plain Prinia are small passerines in the Cisticolidae found in southern Asia (MacKinnon & Phillips 1999) and breed sympatrically at high densities in the study area (L. Wang, Y.-C. Hsu & W. Liang unpubl. data). Both species build semi-open nests in Couch Grass Miscanthus floridulu but lay distinctly coloured eggs (Fig. 1). Yellow-bellied Prinia is parasitized by the Oriental Cuckoo, which lays highly mimetic eggs (L. Wang, Y.-C. Hsu & W. Liang unpubl. data; see also Yang et al. 2012), and also by the Plaintive Cuckoo Cacomantis merulinus (Well 1999). However, Plain Prinia has not yet been recorded as a cuckoo host (Lin 2008, Yang et al. 2012).
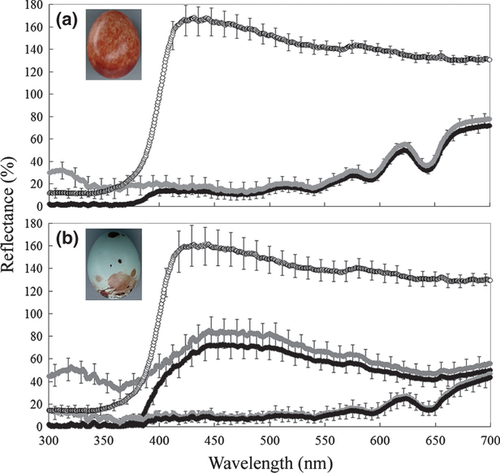
Experiments were performed when the hosts laid their last egg or within 2–3 days following clutch completion. In the model egg treatment, one host egg was exchanged with a white model egg, appearing completely non-mimetic to host eggs and having low UV reflectance (Figs 1 and 2). In the UV manipulation treatment, we coated one randomly selected host egg with a UV-blocking powder (ESK-UV638, Lisheng Development Plasticizing Co. Ltd., Taichung, Taiwan, www.lisheng117.com.tw) mixed with cream (CPC Corporation Taiwan, product DI 27875). All the remaining host eggs in the clutch were coated with cream to control for the effects of the cream application. UV blocking effectively set to zero the UV-reflectance of the treated eggs in the 300–370 nm wavelength interval but left reflectance shapes over the remaining wavelength range (400–700 nm) unaltered (Fig. 1). UV-blocking cream was applied only once on each experimental egg because its effects persisted for 6 days (see below). We also established a control group of nests that were visited at the same frequency as experimental nests and the eggs were handled but not manipulated in any other way.
Experimental nests were monitored on a daily basis for 6 days to record host response, which was classified as acceptance (foreign or UV-blocked egg incubated) or rejection (foreign or UV-blocked egg gone) (Moksnes et al. 1991). No desertions were recorded.
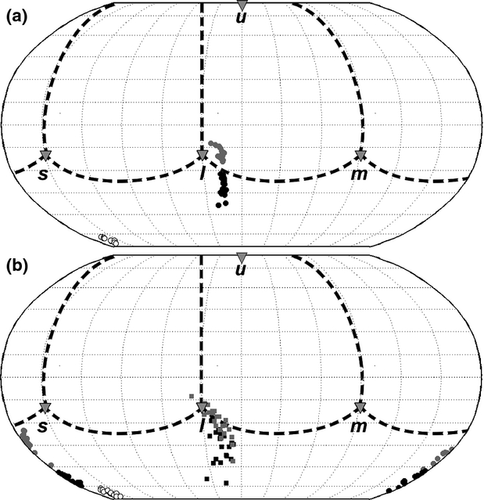
Reflectance spectra were processed through tetrahedral colour space (Goldsmith 1990) using the tetracolorspace software (Stoddard & Prum 2008). We used the average spectral sensitivity curves for UVS-type retinas from Endler and Mielke (2005). In a tetrahedron each spectrum is represented by a point, where the vertices correspond to exclusive stimulation of the UV, blue, green and red-sensitive cones in the avian eye. To visualize hue distributions independently of chroma, we mapped colours onto a unit sphere centred on the achromatic origin using a Robinson projection, in which the position of each point (egg in this case) is determined by the horizontal (RGB) and vertical (UV) components of hue.
Results
After 6 days of incubation, the RGB hue was not significantly different between UV-blocked and UV-unaltered eggs (Yellow-bellied Prinia: paired t20 = −1.26, P = 0.22; Plain Prinia spot colour: t22 = −0.53, P = 0.60; Plain Prinia ground colour: t22 = −0.90, P = 0.38, Fig. 2). However, UV blocking significantly reduced the UV hue of the treated eggs (Yellow-bellied Prinia: t20 = 14.2, P < 0.001; Plain Prinia spot colour: t22 = 4.0, P < 0.001; Plain Prinia ground colour: t22 = 13.4, P < 0.001; Fig. 2).
Yellow-bellied Prinias rejected all the non-mimetic model eggs (10/10), whereas Plain Prinias rejected only 5% (1/21) of them, a highly significant difference ( = 26.8, P < 0.001). Yellow-bellied Prinia and Plain Prinia rejected their own eggs treated with a UV-blocking agent in 18.2% (4/22) and 8.3% (2/24) of the experiments, respectively, and only UV-blocked eggs were rejected. No significant between-species difference in rejection rates in this treatment was found (Fisher's exact test, P = 0.405). We recorded no egg rejection in the control groups in either species (0/10 for each species).
= 26.8, P < 0.001). Yellow-bellied Prinia and Plain Prinia rejected their own eggs treated with a UV-blocking agent in 18.2% (4/22) and 8.3% (2/24) of the experiments, respectively, and only UV-blocked eggs were rejected. No significant between-species difference in rejection rates in this treatment was found (Fisher's exact test, P = 0.405). We recorded no egg rejection in the control groups in either species (0/10 for each species).
Discussion
The only two previous experimental studies investigating the influence of UV matching on egg rejection used either natural cuckoo eggs (Avilés et al. 2006) or foreign conspecific eggs (Honza & Polačiková 2008) and their results are inconsistent. Avilés et al. (2006) found that only seven of 43 (16.3%) experimental eggs and seven of 46 (15.2%) control eggs introduced were ejected, suggesting that most of the responses against experimental eggs were not due to UV-blocked eggs being detected as parasitic eggs by the Eurasian Magpie Pica pica. Honza and Polačiková (2008) demonstrated that both UV wavelengths and total brightness of experimental eggs within the 325–700 nm range significantly influenced host rejection behaviour in the Blackcap Sylvia atricapilla, thus supporting the significance of UV reflectance in the egg recognition processes of the host. Despite the relatively low frequency of rejections in the UV-treatment, our study shows for the first time that hosts may reject their own eggs when the UV reflectance spectrum is altered.
By manipulating host eggs, inherently consistent in egg traits, we controlled for all the other confounding egg characteristics likely to induce egg discrimination, thus revealing the specific effect of UV in egg rejection decisions. Altering the UV of a host's own eggs rather than cuckoo eggs (which are also bigger, and different in shape and shell texture) is a stronger approach to experimentally testing the hypothesis that UV reflectance alone can be a cue in triggering rejection responses.
There is to date no evidence that hosts reject their own eggs in the absence of brood parasitism, even when challenged by presentations of brood parasites at the nest (Davies & Brooke 1988, Røskaft et al. 2002, Čapek et al. 2010). Our study shows that manipulating UV reflectance per se elicits egg rejection responses in UV-sensitive hosts of brood parasites.
An important issue regarding the role of UV is posed by the results of egg rejection experiments in studies using model eggs that inherently have a low or close to zero UV reflectance (Cherry et al. 2007). Artificial eggs painted to mimic host eggs are largely accepted by some Common Cuckoo Cuculus canorus hosts possessing well-developed egg discrimination abilities (Davies & Brooke 1989). Therefore, these results imply that UV reflectance alone might not be a sufficient cue in host egg recognition. A wider range of visual cues might be used to reject alien eggs by hosts. Our study is the first to provide strong evidence in support of this hypothesis by testing two congeneric, sympatrically breeding passerines having a different history of co-evolutionary interactions with the local brood parasite. Although both prinia species responded slightly and similarly to gross manipulations of the UV spectrum, their responses to non-mimetic eggs having a very low UV reflectance were dramatically different. Yellow-bellied Prinia, a host of a cuckoo having evolved mimetic eggs, rejected all non-mimetic and largely non-UV-reflective eggs, whereas a very low rejection rate of such eggs was apparent in the Plain Prinia, which is not parasitized by the Oriental Cuckoo. The reasons for the differential utilization of these sympatric prinias by the Oriental Cuckoo are the subject of ongoing research.
We thank Rauri Bowie, Farah Ishtiaq, Brian Peer and an anonymous referee for helpful comments on this manuscript. Brian Peer kindly helped with improving the English text of this manuscript. This work was supported by the National Natural Science Foundation of China (No. 31071938 to W.L., B.G.S. and A.A., No. 31101646 to C.Y. and A.A., No. 31272328 to W.L., and 31260514 to C.Y.); a China Postdoctoral Science Foundation funded project (20110490967) to C.Y., and by the Program for New Century Excellent Talents in University (NCET-10-0111) to W.L. We thank Shun-Jen Cheng for assistance in the field.




