Potential drug–drug interactions between antiretroviral drugs and comedications, including dietary supplements, among people living with HIV: A clinical survey
Funding information: The work was supported by an unrestricted educational grant from GlaxoSmithKline.
Abstract
Objective
Age-related comorbidities, polypharmacy and thereby the risk of potential drug–drug interactions (PDDIs) among people living with HIV (PLWH) have increased over the years. We estimated the prevalence of comedications, including dietary supplements, and evaluated PDDIs among PLWH receiving antiretroviral therapy (ART) in Denmark in an outpatient setting.
Methods
Information on prescription medication, over-the-counter medication and dietary supplements was obtained from adult PLWH receiving ART attending two outpatient clinics in Denmark. The PDDIs were identified using the University of Liverpool's drug interaction database. Associations between PDDIs and relevant variables were compared using logistic regression models.
Results
A total of 337 PLWH receiving ART with a median age of 53 years (interquartile range: 45–61) were included; 77% were male and 96% had a HIV-RNA viral load < 50 copies/mL. Twenty-six per cent of participants received five or more comedications and 56% consumed dietary supplements. Co-administration of drugs requiring dose adjustment or monitoring was identified in the medication lists of 52% of participants, and 4.5% were on drugs that should not be co-administered. Male sex [odds ratio (OR) = 1.9, 95% confidence interval (CI): 1.0–3.4], being on a protease inhibitor (OR = 4.3, 95% CI: 1.9–9.7), receiving five or more comedications (OR = 3.3, 95% CI: 1.5–7.2), taking over-the-counter medications (OR = 1.9, 95% CI: 1.1–3.3) and dietary supplements (OR = 2.0, 95% CI: 1.2–3.3) were independent predictors of PDDIs.
Conclusion
Potential drug–drug interactions were common among our study population Our study confirms that polypharmacy and being on a protease inhibitor-based regimen increase the risk of PDDIs considerably and highlights the importance of questioning PLWH about dietary supplement intake.
INTRODUCTION
The life expectancy of people living with HIV (PLWH) has greatly improved as a result of antiretroviral therapy (ART), while the prevalence of age-related comorbidities among PLWH has increased over the years [1-3]. Further, PLWH seem to have a higher prevalence of comorbidities such as cardiovascular and renal disease compared with uninfected individuals [4]. Consequently, clinicians often face the challenge of polypharmacy, commonly defined as the concurrent administration of more than five medications, and the risk of drug–drug interactions (DDIs) when prescribing new medications to PLWH [5]. The acknowledgement of this challenge is reflected by the major revisions to the European AIDS Clinical Society's guidelines in 2019, which included a new panel focusing on DDIs [6].
The frequency of polypharmacy in PLWH aged > 50 years in the United States and Europe has been reported to be in the range 30–94% [5]. The risks associated with polypharmacy include reduced treatment adherence and adverse drug reactions, which may initiate a so-called prescribing cascade due to the adverse reactions being misinterpreted as a new disease, leading to the prescription of more drugs and increased risk of DDIs [5]. Studies on PLWH receiving ART show a prevalence of drug interactions that are contraindicated in the range 1–7% and interactions requiring dose modification ranging from 18% to 63% [7-16].
The mechanisms of DDIs between ART drugs and other medications can occur at the level of absorption with change in gastric pH or chelation; the level of metabolism with inhibition or induction of intestinal and hepatic cytochrome-P450 (CYP) enzymes, glucuronidation or drug transporters; and the level of excretion with the inhibition of renal drug transporters [5]. Drug interactions can cause toxicity and/or loss of therapeutic effect and thereby increased risk of development of HIV drug resistance.
The aims of the current study were to get an overview of the prevalence of non-ART comedications, including dietary supplements, and to evaluate potential DDIs (PDDIs) among PLWH in Denmark in an outpatient setting.
METHODS
Study population and design
This study was conducted at two Infectious disease outpatient clinics in Copenhagen, Denmark, during two time periods: September 2018 to January 2019 at Copenhagen University Hospital, Amager and Hvidovre; and October–November 2019 at Copenhagen University Hospital, Rigshospitalet. PLWH aged > 18 years and receiving ART were enrolled consecutively during certain days in the outpatient clinics by their infectious disease specialist. Twelve physicians in total were involved in the data collection.
Information on current medications was obtained by patient self-report and electronic medical prescription history. Prescription medication, over-the-counter medication, vitamins/minerals and herbal preparations were noted in a chart along with the last measured CD4 count and plasma HIV RNA level of the participant. The chart was handed over to an investigator physician, who screened the medication list for PDDIs using the University of Liverpool's drug interaction database by entering all information into the interaction checkers [17].
The medication audit did not include any additional investigation of the participant, and ethical approval was therefore not required. The collection of data was approved by the Regional Centre for Data Protection Reports (VD-2018-260).
DEFINITIONS
Potential drug–drug interaction
The Liverpool HIV Drug Interaction checker [17] categorizes the severity of an interaction by the use of coloured symbols: red means drugs should not be co-administered; orange that there is a potential interaction which may require dose adjustment or close monitoring; yellow that there is a potential interaction predicted to be of weak intensity; and green that no clinically significant interaction is expected. Finally, the quality of evidence behind this categorization is ranked as very low, low, moderate or high. In our study, PDDIs were defined as red and orange interactions in the Liverpool database, while yellow and green interactions were not considered a PDDI. It was not part of the assessment to determine whether the appropriate dose modification had been performed.
Antiretroviral therapy
Antiretroviral therapy regimens are grouped as either nonnucleoside reverse transcriptase inhibitor (NNRTI)-based, integrase strand transferase inhibitor (INSTI)-based or protease inhibitor (PI)-based, which is an NNRTI, INSTI or PI drug combined with a backbone of, usually, two NRTIs. Boosted PIs [i.e. atazanavir/cobistat (ATV/c) or darunavir/ritonavir (DRV/r)] and unboosted PIs were both classified under the PI-based regimen. Participants receiving both a PI and an INSTI or a boosted INSTI (i.e. EVG/c) were classified as a ‘boosted INSTI or PI + INSTI based’ regimen. Finally, participants receiving other combinations, i.e. an INSTI, NNRTI and PI or a C-C chemokine receptor type 5 (CCR5) inhibitor, were classified as ‘other’.
Comedications
Prescription medications were defined as drugs prescribed by a doctor and taken by the participant at the time of their clinic visit. Medications such as paracetamol and non-steroid anti-inflammatory drugs (NSAIDs) can be either prescribed by a doctor or bought as over-the-counter medicine in smaller packages. If taken daily in a fixed dose, these medications were classified as prescription medications within the therapeutic class of central nervous system drugs. If taken as needed, they were classified as over-the-counter medication. Vitamins, minerals and herbals were grouped independently as ‘dietary supplement’ from other over-the-counter medications to separate the possible association between these supplements and PDDIs.
Statistical methods
Categorical values are presented as numbers (percentages) and continuous variables as medians (interquartile range, IQR). Characteristics are described for the total population and according to PDDIs (yes/no). Associations between PDDIs and age, sex, CD4 cell count, HIV viral load, ART regimen, number of comedications, over-the-counter medicine (yes/no) and dietary supplements (yes/no) were compared using univariate and multivariate logistic regression models.
All analyses were performed using R software version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study population characteristics
The medical audits were performed on a total of 337 ART-treated PLWH (Table 1). The median age was 53 years (IQR: 45–61), median CD4 T-cell count was 636 cells/μL (IQR: 455–840) and viral suppression (HIV-RNA < 50 copies/mL) was noted in 96% (n = 317) of the population. Forty-two per cent (n = 142) received an NNRTI-based regimen and, of these, 29 participants received rilpivirine (RPV), while the remaining 113 received efavirenz (EFV, n = 77), nevirapine (NVP, n = 32) or etravirine (ETR, n = 4). Twentynine per cent (n = 98) received an INSTI-based regimen and 15% (n = 51) a PI-based regimen. Further, 9% (n = 30) were treated with both a PI and INSTI or a boosted INSTI and 5% (n = 16) were classified as treated with an ‘other’ regimen.
| Total (n = 337; 100%) | PDDI (n = 175; 52%) | No PDDI (n = 162; 48%) | |
|---|---|---|---|
| Age (years, median) | 53 | 55 | 52 |
| Interquartile range | 45–61 | 47–64 | 43–58 |
| Sex (%) | |||
| Male | 260 (77) | 141 (81) | 119 (73) |
| Female | 78 (23) | 34 (19) | 43 (27) |
| CD4 T-cell count (median) (cells/μL) | 636 | 590 | 660 |
| Interquartile range | 455–840 | 417–784 | 517–872 |
| HIV-RNA levels | |||
| < 50 copies/mL | 317 (95) | 165 (95) | 152 (96) |
| ≥ 50 copies/mL | 15 (5) | 8 (5) | 7 (4) |
| ART regimen (%) | |||
| NNRTI-based | 142 (42) | 64 (37) | 78 (48) |
| INSTI-based | 98 (29) | 46 (26) | 52 (32) |
| Boosted INSTI- or PI + INSTI-based | 30 (9) | 20 (11) | 10 (6) |
| PI-based | 51 (15) | 34 (19) | 17 (11) |
| Other | 16 (5) | 11 (6) | 6 (3) |
| Prescription comedications (%) | |||
| No drugs | 83 (25) | 23 (13) | 60 (37) |
| 1 drug | 55 (16) | 25 (14) | 30 (18) |
| 2–4 drugs | 111 (33) | 62 (35) | 49 (30) |
| ≥ 5 drugs | 88 (26) | 65 (37) | 23 (14) |
| Over-the-counter comedication (%) | |||
| Yes | 102 (30) | 64 (37) | 38 (24) |
| No | 235 (70) | 111 (63) | 124 (76) |
| Dietary supplements (%) | |||
| Yes | 188 (56) | 109 (62) | 79 (49) |
| No | 149 (44) | 66 (38) | 83 (51) |
- Abbreviations: ART, antiretroviral treatment; INSTI, integrase inhibitor; NNRTI, nonnucleotide reverse transcriptase inhibitor; PI, protease inhibitor.
Participants with a PDDI (54%) were older, had a lower CD4 count and more often received a PI-based ART regimen, over-the-counter medicine and dietary supplements than participants with no PDDI (Table 2).
| Odds ratio, unadjusted (95% CI) | p-value | Odds ratio, adjusted* (95% CI) | p-value | |
|---|---|---|---|---|
| Age | ||||
| < 50 years | 1 | 1 | ||
| ≥ 50 years | 1.6 (1.0–2.4) | 0.048 | 0.8 (0.5–1.4) | 0.42 |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.5 (0.9–2.5) | 0.12 | 1.9 (1.0–3.4) | 0.042 |
| CD4 T-cell count | ||||
| < 350 cells/μL | 1 | 1 | ||
| 350–500 cells/μL | 1.1 (0.5–2.5) | 0.88 | 1.4 (0.5–3.7) | 0.48 |
| ≥ 500 cells/μL | 0.5 (0.2–1.0) | 0.042 | 0.5 (0.3–1.2) | 0.13 |
| HIV-RNA levels | ||||
| ≤ 50 copies/mL | 1 | 1 | ||
| ≥ 50 copies/mL | 1.1 (0.4–3.0) | 0.92 | 1.1 (0.3–3.7) | 0.94 |
| ART regimen | ||||
| NNRTI-based | 1 | 1 | ||
| INSTI-based | 1.1 (0.6–1.8) | 0.95 | 1.2 (0.7–2.2) | 0.52 |
| Boosted INSTI- or PI + INSTI-based | 2.4 (1.1–5.6) | 0.80 | 2.4 (0.9–6.0) | 0.07 |
| PI-based | 2.4 (1.3–4.8) | 0.009 | 4.3 (1.9–9.7) | <0.001 |
| Other | 2.7 (0.9–8.1) | 0.08 | 2.9 (0.9–9.7) | 0.09 |
| Prescription comedications | ||||
| No drugs | 0.5 (0.2–0.9) | 0.034 | 0.5 (0.2–1.0) | 0.056 |
| 1 drug | 1 | 1 | ||
| 2–4 drugs | 1.5 (0.8–2.9) | 0.21 | 1.4 (0.7–2.9) | 0.32 |
| ≥ 5 drugs | 3.4 (1.7–6.9) | < 0.001 | 3.3 (1.5–7.2) | 0.004 |
| Over-the-counter comedication | ||||
| No | 1 | 1 | ||
| Yes | 1.9 (1.2–3.0) | 0.009 | 1.9 (1.1–3.3) | 0.003 |
| Dietary supplements | ||||
| No | 1 | 1 | ||
| Yes | 1.7 (1.1–2.7) | 0.013 | 2.0 (1.2–3.3) | 0.009 |
- Abbreviations: ART, antiretroviral treatment; INSTI, integrase inhibitor; NNRTI, nonnucleotide reverse transcriptase inhibitor; PI, protease inhibitor.
- a Odds ratios adjusted for all the variables listed in the table.
- Note: Bold values = all p-values <0.005 (as this was our statistical significance trehshold and to facilitate the readers.)
Prevalence of comedications and dietary supplements
Overall, 75% (n = 254) of the participants had one or more prescription comedications, with 26% (n = 88) receiving five or more different non-HIV prescription drugs (Table 1). The distribution of prescribed drugs according to therapeutic classes is shown in Figure 1. The most common prescribed drugs were classified within the cardiovascular (e.g. antihypertensive drugs, statins) and central nervous system (e.g. analgesics, benzodiazepines) and taken by 33% (n = 110) and 32% (n = 107) of the participants, respectively. The third most common group, taken by 29% (n = 96) of participants, was within the urogenital system, with drugs against erectile dysfunction constituting the majority.
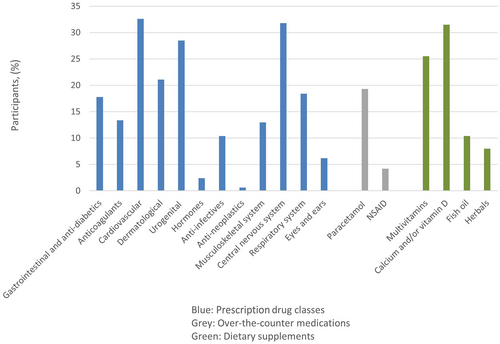
A total of 30% (n = 102) used over-the-counter medications, with paracetamol by far being the most common medication, taken as needed by 19% (n = 65) of participants. Other over-the-counter medications included NSAIDs (n = 14), laxatives (n = 13) and antihistamines (n = 5).
In addition, more than half of participants (56%) reported consumption of dietary supplements, most commonly calcium supplements with or without vitamin D, which was taken by 32% (n = 106) of participants, and multivitamins by 26% (n = 86) of participants. Eight per cent (n = 27) reported taking various herbal supplements, such as garlic tablets, ginger, algae supplements, ginseng and St John's Wort (n = 1).
Prevalence of potential red drug–drug interactions
Potential red drug–drug interactions classified as red were noted in 4.5% (n = 15) of participants. One participant had three red interactions in their medical audit, two participants had two red interactions, while the remaining 12 participants had one red interaction, all of which are listed in Table 3. Seven of the fifteen red interactions may lead to a decrease in the exposure of the antiretroviral drug – sometimes referred to as the antiretroviral drug being the victim drug (marked by blue shading in Table 3).
| Drug interaction | Antiretroviral drug | Comedication or antiretroviral drug | Effect (quality of evidence) | N |
|---|---|---|---|---|
| Red | PI (ATV/c or DRV/r/c) | Fluticasone or budesonide or mometasone (nasal spray) or mometasone (topical) | Risk of systemic corticosteroid effects (low) | 5 |
| NNRTI (RPV) | Pantoprazole | Significant decreases in rilpivirine plasma concentrations may occur (moderate) | 4 | |
| PI (ATV/c or DRV/r) | Apixaban | Concentrations of apixaban are expected to increase (very low) | 3 | |
| PI (ATV/c) | Omeprazole or pantoprazole | Significant decreases in atazanavir plasma concentrations may occur (very low) | 2 | |
| NNRTI (EFV) | Norelgestromin (patch) | Expected to reduce the contraceptive efficacy of norelgestromin (very low) | 1 | |
| PI (DRV/c) | NNRTI (NVP) | Expected to decrease DRV/c exposure, which may result in loss of therapeutic effect and development of resistance to darunavir (very low) | 1 | |
| INSTI (EVG/c) | Mometasone (topical) | Risk of systemic corticosteroid effects (very low) | 1 | |
| INSTI (EVG/c) | Budesonide (nasal spray) | Risk of systemic corticosteroid effects (moderate) | 1 | |
| Orange | INSTI (DTG/RAL/EVG) | Multivitamins | Simultaneous co-administration may decrease dolutegravir concentrations (very low) | 36 |
| NNRTI (EFV/NVP) | Sildenafil | May decrease concentrations of sildenafil (very low) | 27 | |
| NRTI (TDF) | Ibuprofen | Risk of nephrotoxicity (very low) | 24 | |
| NNRTI (EFV/NVP/ETR) | Benzodiazepine | Could potentially decrease benzodiazepine exposure (very low) | 17 | |
| PI (ATV/DRV/c/r) | Sildenafil | May increase sildenafil concentrations (low) | 14 | |
| PI (DRV/c/r) | NRTI (TDF/TAF) | Risk of renal impairment (very low) | 14 | |
| NNRTI (EFV/NVP) | Amlodipine | May decrease amlodipine exposure (very low) | 11 | |
| INSTI (DTG) | Calcium/magnesium/iron supplements | Simultaneous co-administration may decrease dolutegravir concentrations (moderate) | 11 | |
| NNRTI (EFV) | Betamethasone/mometasone (nasal) | May decrease concentrations of the steroid (very low) | 9 | |
| PI (ATV/DRV/c/r) | Benzodiazepine | Could potentially increase benzodiazepine exposure (very low) | 9 | |
| PI (DRV/c/r) | Atorvastatin/rosuvastatin | May increase plasma concentrations of the lipid-lowering agent (moderate) | 8 | |
| NRTI (TDF) | Valaciclovir | May increase concentrations of tenofovir or valaciclovir (very low) | 7 | |
| NNRTI (EFV) | Ibuprofen | May increase ibuprofen concentrations (very low) | 7 | |
| NNRTI (EFV/NVP) | Atorvastatin/simvastatin | May decrease concentrations of the lipid-lowering agent (low) | 7 | |
| NNRTI (EFV) | Clopidogrel | May decrease conversion of clopidogrel to its active metabolite (very low) | 5 | |
| INSTI (EVG/c) | Sildenafil | May increase concentrations of sildenafil (very low) | 5 | |
| PI (DRV/c/r) | Amlodipine | Expected to increase the plasma concentrations of amlodipine (very low) | 3 | |
| NNRTI (EFV) | Cannabis | May increase the effect of cannabis (very low) | 3 | |
| NNRTI (RPV) | Garlic | Concentrations of rilpivirine could be decreased (very low) | 2 | |
| PI (ATV) | Garlic | Concentrations of atazanavir could be decreased (very low) | 1 |
- Note: Drugs marked in blue are ‘victim drugs’ – the exposure of the antiretroviral drug can be increased or decreased by the comedication. Effects are based on the information in the Liverpool Drug Interaction database [17].
- Abbreviations: ATV, atazanavir; c, cobisistat; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; INSTI, integrase inhibitor; N, number of participants with this interaction; NNRTI, non-nucleotide reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; r, ritonavir; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Topical or nasal steroids were administered in five participants treated with a boosted PI (ATV/c or DRV/c or DRV/r) and two participants with EVG/c.
Three participants had a prescription of a boosted PI (ATV/c or DRV/r) and the factor Xa inhibitor apixaban.
Four participants had RPV prescribed alongside pantoprazole and two participants had a prescription for ATV/c and pantoprazole or omeprazole.
One woman had a prescription for EFV and a norelgestromin/ethinylestradiol patch as contraception.
One participant received DRV/c and NVP concomitantly.
Prevalence of potential orange drug–drug interactions
Fifty-two per cent (n = 174) had one or more orange interactions in their medical audit. More than half of these participants (n = 88) had multiple (two to six) orange interactions. The most common interactions as well as those that lead to the antiretroviral drug being the victim drug (n = 50) are presented in Table 3 - Overview of all red drug interactions and most common orange drug interactions among the study population.
The most common PDDI was noticed when an INSTI was coadministered with a multivitamin tablet or mineral supplement such as calcium, magnesium or iron.
Twenty-seven participants had a prescription for an NNRTI and sildenafil, an erectile dysfunction agent, while 14 participants had co-administration of a boosted PI and sildenafil and five participants had co-administration of EVG/c and sildenafil.
Seventeen participants were treated with an NNRTI and had a prescription for a benzodiazepine and nine participants were treated with a boosted PI and benzodiazepine. Seven participants were treated with an NNRTI and a lipid-lowering agent and 11 participants with an NNRTI and amlodipine (n = 11).
Another common potential DDI was registered when tenofovir disoproxil fumarate (TDF) was administered alongside ibuprofen (n = 24).
Fourteen participants were treated with a TDF or tenofovir alafenamide and boosted DRV.
Finally, three participants reported taking garlic supplements with RPV or ATV.
Factors associated with potential drug–drug interactions
In the univariate logistic regression analyses, several factors seemed to significantly increase the odds of PDDIs: age > 50 years, male sex, PI-based ART regime, increasing number of prescription medications, taking over-the-counter medication and taking dietary supplements (Table 2). In the multivariate analyses, after adjusting for all remaining variables, age was no longer associated with PDDIs. Males had an adjusted odds ratio (OR) of 1.9 (95% CI: 1.0–3.4) compared with females and being on a PI-based regime increased the odds of having a PDDI by 4.3 (95% CI: 1.9–9.7) compared with being on a NNRTI-based regime. Participants receiving five or more non-ART comedications had an adjusted OR of 3.3 (95% CI: 1.5–7.2) of PDDIs compared with those taking only one comedication. Finally, taking over-the-counter medications (OR = 1.9, 95% CI: 1.1–3.3) and dietary supplements (OR = 2.0, 95% CI: 1.2–3.3) increased the odds of PDDIs.
DISCUSSION
In our study population of 337 PLWH receiving ART in Denmark, PDDIs were found in more than half of the medical audits, which is within the range of the prevalence rates found in other similar studies [10-16]. The differences in the rates of interactions could be explained by differences in methodology such as identification of PDDIs by other software [11, 16]; by further evaluation of the identified PDDIs as clinically significant or not by assessing the ‘summary of product characteristics’ for each product [15]; by having HIV pharmacologists review them; or by excluding PDDIs when dose adjustments had already been made [10].
We found that being male was associated with increased odds of PDDI, even after adjustment for number of comedications, age, HIV status and ART regimen. This finding has no biologically plausible explanation but could be caused by the high number of males in our population taking medications against erectile dysfunction, which constituted a high portion of the orange DDIs identified.
As expected, the odds of PDDIs increased with an increasing number of comedications and with being on a PI-based ART regimen. These findings are in agreement with previous reports [8, 10, 12-16, 18, 19]. Protease inhibitors have numerous drug interactions because they are metabolized by the cytochrome P450 enzyme system and all are inhibitors of CYP3A4. Additionally, there might be a rise in plasma exposure of PI with increasing age [20], making it even more important to be alert when reviewing the lists of medication of elderly PLWH. The most common red interaction in our study was between a PI (ATV or DRV) and inhaled or topical glucocorticoid, which could be avoided by prescribing another glucocorticoid such as beclomethasone.
Further, taking one or more dietary supplements was associated with an OR of PDDI of 2.0 (95% CI: 1.2–3.3), indicating that supplements could be another area to look at when evaluating medications. Participants might not automatically report dietary supplements, and mineral supplements and multivitamins can decrease the concentration of INSTIs, which makes it important to identify whether supplements are being taken. The simple advice to separate the administration of an INSTI and multivitamin preparations by 2–6 h (depending on the INSTI concerned) can make a central difference. Likewise, garlic has the potential to reduce the concentration of the administered ART. Three of the 15 participants in our study with a HIV-RNA viral load > 50 copies/mL had PDDIs that could possibly decrease the concentration of one of the prescribed antiretroviral drugs. Two of these participants received an INSTI and a dietary supplement, while the final participant received DRV/c and NVP. However, whether the detectable viral loads and the drug interactions in these participants have a causal link is not possible to determine. The treating physicians were made aware of the PDDIs.
A limitation to our study is that a potential DDI is not equal to an actual or clinically significant drug interaction. First, many of the PDDIs identified in our medical audits are based on quality of evidence classified as ‘very low’ in the Liverpool interaction database, i.e. animal or in vitro studies or single case reports. Further, we did not explore whether the correct dose adjustments had already been made in case an orange interaction was identified, which is why the number of actual or clinically significant DDIs may be lower than the potential DDIs. Also, to evaluate whether a PDDI is, in fact, clinically significant, a follow-up with each patient would be required to assess possible toxicities or lack of efficacy of the drugs in question, which was outside the scope of this study. Finally, we did not specifically ask the physicians to perform a medicine review at the visit where the participants in the study were included. Some prescriptions may therefore not be relevant, although we expect this number to be low as it is part of official guidelines to perform a medicine review annually [21].
It is a strength of our study that we performed medical audits from two different centres and 12 different infectious disease specialists, thereby reducing the effect of prescribing habits. Further, the prospective collection of data and the specific information on dietary supplement intake should hopefully give an accurate and complete documentation of the drug regimens of the patients included in our study.
For the clinician, it can be overwhelming to keep track of all drug interactions with the ART of their patients. Further, non-ART medications may be prescribed by the infectious disease specialist, other hospital doctors or the general practitioner, which can make it challenging to stay up to date with a patient's full list of medications. Nevertheless, this and other studies point to the importance of being alert when taking care of PLWH receiving a PI or complex ART regimen and/or when PLWH have five or more comedications, including dietary supplements. Further, our study suggests that clinicians should pay special attention when a person receives comedications such as glucocorticoids, anticoagulants, proton pump inhibitors, multivitamins, ibuprofen and benzodiazepines. We propose that clinicians could manage PDDIs by taking a full medication history and being vigilant with regard to any new medications in the medication list; by applying methods to reduce polypharmacy and inappropriate prescribing [22]; and by using available software tools such as the Liverpool interaction database or, if available, consulting a pharmacologist.
CONCLUSION
Potential drug–drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs. Our study confirms that polypharmacy and being on a PI-based regimen increase the risk of PDDIs considerably. Further, our study highlights the importance of questioning PLWH about dietary supplement intake.
CONFLICT OF INTEREST
OK reports personal fees, research grant and non-financial support from Gilead, and personal fees from ViiV, Merck and Janssen. TB reports grants from Novo Nordisk Foundation, Simonsen Foundation, Kai Foundation, Erik and Susanne Olesen's Charitable Fund and Lundbeck Foundation, grants and personal fees from GSK and Pfizer, and personal fees from Boehringer Ingelheim, Merck, Astra Zeneca and Janssen, outside the submitted work. AML reports speakers’ honorarium/travel grants from Gilead, speakers’ honorarium/travel grants from GSK, speakers’ honorarium from Pfizer and advisory board activity from Gilead, GSK and Pfizer. NW has been a clinical investigator, member of an advisory board or lecturer for Abbvie, Merck, Gilead and GSK and has received unrestricted grants for research or scientific meetings from Abbvie, Gilead, GSK and the Novo Nordisk Foundation. All are unrelated to this manuscript. The remaining authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
MT and TB designed the study. KPD, JG, A-BEH, OK, A-ML, BØL, MVR, LR, NW and TB collected the data. MT drafted the manuscript. All authors discussed the results, commented on and critically revised the manuscript, and approved the final version.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the corresponding author.




