In vitro assessment of the potential for dolutegravir to affect hepatic clearance of levonorgestrel
These authors contributed equally to this work.
This study was presented, in part, at the 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis and Other Antiviral Drugs. Noordwijk, The Netherlands. 14–16 May 2019 (abstract number 49).
Funding information
This study was funded by the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant Number: 1R01HD085887 (Scarsi)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abstract
Objectives
The World Health Organization recommends that all countries adopt dolutegravir-based antiretroviral therapy as the preferred regimen for all individuals living with HIV. Levonorgestrel is a commonly used hormonal contraceptive, which undergoes drug–drug interactions with some antiretrovirals, but the potential interaction between dolutegravir and levonorgestrel has not been examined. We aimed to evaluate cytochrome P450 (CYP)-mediated levonorgestrel metabolism and quantify the effects of dolutegravir on levonorgestrel apparent intrinsic clearance (CLint.app.) and CYP gene expression.
Methods
In vitro CYP-mediated CLint.app. of levonorgestrel was quantified using a recombinant human CYP (rhCYP) enzyme system. A primary human hepatocyte model of drug metabolism was used to assess the effects of dolutegravir on (1) levonorgestrel CLint.app., using liquid chromatography-tandem mass spectrometry, and (2) the expression of specific CYP enzymes, using quantitative real-time polymerase chain reaction.
Results
Levonorgestrel clearance was mediated by multiple rhCYPs, including rhCYP3A4. Under control conditions, levonorgestrel CLint.app. was 22.4 ± 5.0 μL/min/106 hepatocytes. Incubation with 43.1 nM of unbound dolutegravir elevated levonorgestrel CLint.app. to 31.4 ± 7.8 µL/min/106 hepatocytes (P = 0.168), while 142.23 nM increased levonorgestrel CLint.app. to 37.0 ± 2.9 µL/min/106 hepatocytes (P = 0.012). Unbound dolutegravir ≥ 431 nM induced expression of CYP3A4 (≥ two-fold) in a dose-dependent manner, while 1.44 μM of unbound dolutegravir induced CYP2B6 expression 2.2 ± 0.3-fold (P = 0.0004).
Conclusions
In summary, this in vitro study suggests that dolutegravir has the potential to increase hepatic clearance of levonorgestrel by inducing both CYP3A and non-CYP3A enzymes. The observed in vitro dolutegravir–levonorgestrel drug–drug interaction should be further examined in clinical studies.
INTRODUCTION
The second-generation HIV integrase inhibitor dolutegravir, has a favourable safety profile and a low potential for drug–drug interactions (DDIs) [1]. Nevertheless, a slightly increased risk of neural tube defects occurring in the developing foetuses of women who had received dolutegravir at the time of conception, or during early pregnancy, was reported [2, 3]. The World Health Organization guidelines recommend the use of dolutegravir as first-line antiretroviral therapy (ART) in all people living with HIV, with the caveat that effective contraception should also be offered to individuals of childbearing potential, and that they should be fully informed of the potentially increased risk of neural tube defects [4].
Drug–drug interactions are known to occur between some antiretrovirals and hormonal contraceptives [5, 6]. For example, we have previously demonstrated a significant DDI between the HIV nonnucleoside reverse transcriptase inhibitor efavirenz and the levonorgestrel contraceptive implant [7]. One study has been conducted with dolutegravir and the ethinyl oestradiol/norgestimate oral contraceptive and found no evidence of DDIs [8]. Two recent studies that evaluated the combination of dolutegravir and another progestin-releasing contraceptive implant – etonogestrel – found a minimal effect in etonogestrel exposure in women receiving dolutegravir [9, 10].
There is limited mechanistic information on the pathways involved in the absorption, distribution, metabolism and excretion of steroid hormone contraceptives [11]. For example, oxidative metabolism of levonorgestrel is purported to be mediated by CYP3A4 [12, 13], but other mechanisms are also thought to play a role [14]. Levonorgestrel can be administered orally (with high bioavailability) or via subdermal implants; the volume of distribution is estimated to be between 160 and 260 L [15].
The aims of this study were to obtain in vitro data to support a better understanding of the interaction between dolutegravir and levonorgestrel by: (1) evaluating the contribution of specific key human cytochrome P450 (CYP) enzymes in levonorgestrel metabolism; (2) quantifying the effect of dolutegravir on the apparent intrinsic clearance (CLint.app.) of levonorgestrel in a primary human hepatocyte-based in vitro model of drug metabolism; (3) quantifying the potential for dolutegravir to cause DDIs through the expression of key CYP enzymes.
METHODS
A description of the chemicals, reagents, stock solutions and primary hepatocytes used in this study is provided in the supplementary section.
Recombinant human cytochrome P450 enzyme studies and culture of cryopreserved primary human hepatocytes
The incubation method for conducting recombinant human cytochrome P450 (rhCYP) enzyme studies was adapted from Youdim et al. [16]. Cryopreserved primary human hepatocytes were cultured as described previously [17]. Following incubation for 60 min, Williams’ Medium E (WME) was removed from the cell culture plates and levonorgestrel was extracted and quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described in the supplementary methods section.
Concentrations of drugs used in this study
Primary cryopreserved hepatocytes were incubated with a range of concentrations of dolutegravir, which spanned the unbound concentration range reported in human plasma in vivo (10.3–240 ng/mL; 24.6–57.2 nM) [18]. The concentration of levonorgestrel used in hepatocyte experiments (1 nM), was selected as it was within the range of reported plasma concentrations of levonorgestrel released from Jadelle® contraceptive subdermal implants between 1 month (1.39 nM; 435 pg/mL) and 3 years (0.90 nM; 280 pg/mL) after implant insertion [19].
The concentration of levonorgestrel used in rhCYP studies (5 µM), which is several-fold higher than would be encountered in vivo [19], was selected to ensure that substrate concentration was not a limiting factor in rhCYP reactions. This concentration was also several-fold lower than the mean observed Km of progesterone metabolism by CYP3A4, CYP2C19 and CYP2C9 reported in analogous liver microsome studies (80, 37 and 46 µM, respectively) [20]. The degree of protein binding of dolutegravir in WME incubation medium was determined by a rapid equilibrium dialysis method and is described in the supplementary section [17].
RNA extraction, complementary DNA synthesis and quantitative real-time PCR
Total RNA was extracted from hepatocytes using the Omega BioTek E-Z 96 Total RNA kit (VWR, East Grinstead, UK). RNA quality and quantity were assessed by spectrophotometry using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). First-strand synthesis was carried out using TaqMan® Reverse Transcription Reagents (Applied Biosystems, Waltham, MA, USA). Fold-changes in gene expression were determined as described by Pfaffl [21]. Further details are described in the supplementary methods.
Calculation of the apparent intrinsic clearance of levonorgestrel by a single recombinant human CYP
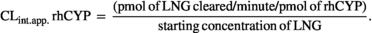 (1)
(1)Calculation of the total intrinsic clearance of levonorgestrel by recombinant human CYP
 (2)
(2)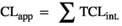 (3)
(3)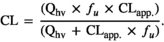 (4)
(4)Calculation of the CLint.app. of levonorgestrel by primary human hepatocytes
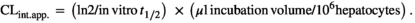 (5)
(5)Results were expressed as the arithmetic mean (± SD) (μL/min/106 hepatocytes) of a total of three donors per condition tested. Three biological replicates were quantified for each condition, tested using cells obtained from three separate donors (HU1574, HU1587 and HU1832; Table 1).
| Donor | Sex | Race | Age (years) | Medications | Drug use |
|---|---|---|---|---|---|
| HU1574 | Male | Caucasian | 70 | Atorvastatin, 80 mg q.d.; lisinopril, 5 mg q.d.; aspirin, 81 mg q.d.; Tamsulosin, 4 mg q.d. | None reported |
| HU1587 | Female | Caucasian | 43 | Vitamin D oral; multivitamin oral; calcium + vitamin D + vitamin K | None reported |
| HU1832 | Female | Caucasian | 53 | None reported | Alcohol and tobacco use |
Note
- q.d., once a day.
Statistical analysis
Statistical significance was assessed by independent-sample t-test with equal variances assumed using IBM® SPSS® Statistics (v.25; IBM Corporation, Armonk, NY, USA), in which P-values ≤ 0.05 were considered significant.
RESULTS
Relative contribution of specific recombinant human cytochrome P450 enzymes to levonorgestrel apparent intrinsic clearance in vitro
Given that progesterone has been reported to be metabolized by CYP3A4, CYP3A5, CYP2C9 and CYP2C19 [20, 28, 29], and that levonorgestrel is a progestin-type synthetic steroid hormone contraceptive with a chemical structure similar to that of progesterone, an assessment of potential CYP2C9- and CYP2C19-mediated metabolism of levonorgestrel was warranted, and was tested using a recombinant enzyme system involving the separate incubation of levonorgestrel with baculosomes expressing either rhCYP3A4, or rhCYP3A5, or rhCYP2C9, or rhCYP2C19 following pre-incubation with, or without, selective chemical inhibitors of each respective rhCYP.
Following incubation of levonorgestrel (5 µM) for 60 min, levonorgestrel CLint.app. mediated by rhCYP3A4 was 0.182 ± 0.036 µL/min/pmol. Similarly, levonorgestrel CLint.app. rhCYP3A5 was 0.178 ± 0.027 µL/min/pmol following incubation with rhCYP3A5 [30]. Incubation of levonorgestrel with rhCYP2C9 resulted in a levonorgestrel CLint.app. rhCYP2C9 of 0.062 ± 0.010 µL/min/pmol [16, 31] while levonorgestrel CLint.app. rhCYP2C19 was 0.046 ± 0.005 µL/min/pmol. CYP1A2 and CYP2B6 have important roles in drug metabolism [32] and have also been implicated in steroid hormone metabolism, including the metabolism of testosterone [33] (CYP2B6), oestradiol and oestrone [34] (CYP2B6), and a minor role for CYP1A2 in mediating progesterone metabolism [28]. It was therefore of interest to determine the potential role of rhCYP1A2 and rhCYP2B6 in levonorgestrel metabolism. Levonorgestrel CLint.app. rhCYP1A2 was 0.028 ± 0.012 µL/min/pmol [35]. Levonorgestrel CLint.app. rhCYP2B6 resulted in a levonorgestrel clearance rate of 0.052 ± 0.004 µL/min/pmol [36].
Next, the in vitro CLint.app. rhCYP clearance rates obtained for CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP3A4 and CYP3A5 were used to estimate the total intrinsic clearance of levonorgestrel that would be achieved by each respective single CYP in the adult female liver as a whole (TCLH) (Eq. 2). The largest TCLH observed was that of CYP3A4, with a TCLH of 52.5 ± 10.4 L/h (Figure 1). The TCLH values of CYP3A5 and CYP2C9 were 9.4 ± 1.4 and 11.6 ± 1.9 L/h, respectively (Figure 1). By comparison, the relative contributions of CYP1A2, CYP2B6 and CYP2C19 to levonorgestrel TCLH were 3.4 ± 1.5, 2.6 ± 0.2 and 1.6 ± 0.2 L/h, respectively (Figure 1).
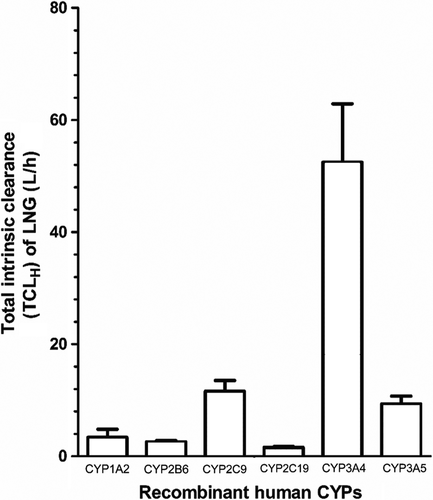
The total apparent clearance (CLapp) of levonorgestrel by the hepatic CYPs measured was 80.97 ± 15.53 L/h, and the total systemic plasma clearance of levonorgestrel was 2.38 ± 0.46 L/h.
Effects of dolutegravir on the apparent intrinsic clearance of levonorgestrel
The use of cryopreserved human hepatocytes is a recommended method for assessing metabolism-based DDIs in vitro [37]. As such, the effect of dolutegravir on levonorgestrel CLint.app. by cryopreserved primary human hepatocytes obtained from three different donors (HU1574, HU1587 and HU1832) was quantified. Under control conditions, where hepatocytes were incubated with 1 nM levonorgestrel alone, levonorgestrel CLint.app. was 22.4 ± 5.0 µL/min/106 cells. Rifampin was used as a positive control to induce levonorgestrel CLint.app. by hepatocytes. Incubation with 10 µM rifampin has previously been shown to optimally induce CYP3A4 enzymatic activity in hepatocytes in vitro [38, 39], including achieving the maximal CLint.app. of CYP3A4 substrates in this model [17]. As expected, incubation with 10 µM rifampin for 72 h increased levonorgestrel CLint.app. by 109%, to 46.7 ± 6.9 µL/min/106 cells (P = 0.008; Figure 2).
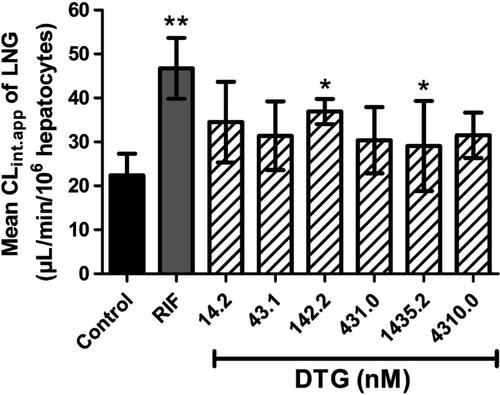
The median unbound dolutegravir (DTGunbound) reported in human plasma is 24.6–57.2 nM [18]. We selected concentrations of DTGunbound to span this range when assessing the effects of dolutegravir on levonorgestrel CLint.app.. Quantification of the amount of protein-binding of dolutegravir within our hepatocyte-based model of drug metabolism showed that the unbound fraction (fu) of dolutegravir in WME incubation medium was 0.43 ± 0.09 (n = 9). A levonorgestrel CLint.app. of 34.5 ± 9.2 µL/min/106 cells (P = 0.114; Figure 2) was observed following 72 h incubation with 0.033 µM dolutegravir (DTGunbound = 14.2 nM). A trend towards elevated levonorgestrel CLint.app. was apparent at all concentrations of dolutegravir tested, with 0.1 µM dolutegravir (DTGunbound = 43.1 nM), resulting in a levonorgestrel CLint.app. of 31.4 ± 7.8 µL/min/106cells (P = 0.168; Figure 2). Incubation of hepatocytes with 0.33 µM dolutegravir (DTGunbound = 142.23 nM) and 3.33 µM dolutegravir (DTGunbound = 1.44 µM) for 72 h significantly increased levonorgestrel CLint.app. to 37.0 ± 2.9 µL/min/106cells (P = 0.012),= and 32.8 ± 4.2 µL/min/106cells (P = 0.050), respectively (Figure 2).
Analysis of the effects of dolutegravir on the expression of CYP1A2, CYP2B6 and CYP3A4 in human hepatocytes
United States Food and Drug Administration guidelines recommend evaluation of the potential of an investigational drug to induce CYP1A2, CYP2B6 and CYP3A4 [37]. To evaluate the potential of dolutegravir to induce CYP expression, an in vitro gene expression assay in human hepatocytes was used to assess the effects of incubating a range of concentrations of dolutegravir (0.00033–10 μM; DTGunbound, 0.142–4310 nM) on the expression of CYP1A2, CYP2B6 and CYP3A4. Also, hepatocytes were incubated separately with phenobarbital (1 mM) – a positive control for induction of the expression of CYP1A2 [40] and CYP2B6 [41] – as well as rifampin (10 µM), which served as a positive control for the induction of the expression of CYP2B6 [41], and CYP3A4 [38, 42].
Although incubation with the CYP1A2 inducer phenobarbital (1 mM) induced CYP1A2 expression by 2.7 ± 0.6-fold (P = 0.043; Figure 3a), dolutegravir did not induce the expression of CYP1A2 (by ≥ two-fold above vehicle control) at any of the concentrations tested (Figure 3a). By contrast, incubation with a total dolutegravir concentration of 3.33 µM (DTGunbound = 1.44 µM) and 10 μM (DTGunbound = 4.310 µM) induced the expression of CYP2B6 by 2.2 ± 0.3-fold and 2.3 ± 0.7-fold, respectively (Figure 3b). Under the same conditions, phenobarbital (1 mM) induced CYP2B6 expression by 2.6 ± 1.1-fold (Figure 3b), while rifampin (10 µM) induced CYP2B6 expression by 2.0 ± 0.5-fold (Figure 3b).
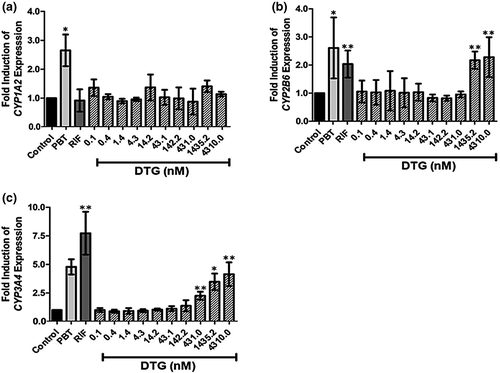
Incubation with rifampin (10 µM) induced a 7.7 ± 1.9-fold increase in CYP3A4 expression, while incubation with phenobarbital (1 mM) elevated CYP3A4 expression levels 4.8 ± 0.7-fold (Figure 3c). Incubation with DTGunbound = 431 nM (total dolutegravir concentration of 1 μM), significantly induced expression of CYP3A4 by 2.3 ± 0.4-fold (Figure 3c). Dolutegravir-induced expression of CYP3A4 increased in a dose-dependent manner, with 3.33 μM dolutegravir (DTGunbound = 1.44 μM) resulting in a 3.5 ± 0.7-fold induction of CYP3A4 expression, and 10 μM dolutegravir (DTGunbound 4.31 μM) eliciting a 4.1 ± 1.0-fold induction (Figure 3c).
DISCUSSION
The need to assess the likelihood of DDIs occurring between antiretrovirals and hormonal contraceptives has recently been highlighted [43]. However, the mechanistic potential for DDIs between dolutegravir and levonorgestrel had not been previously examined. Furthermore, a paucity of information existed regarding the metabolic pathways mediating levonorgestrel metabolism. Here, it was found that rhCYP3A4 was the predominant CYP mediating apparent clearance of levonorgestrel (Figure 1), a finding that supports the established view that CYP3A4 is an important mediator of levonorgestrel metabolism [12, 13]. Interestingly, secondary to CYP3A4, both CYP2C9 and CYP3A5 mediated apparent clearance of levonorgestrel to a similar degree (Figure 1). Meanwhile, CYP1A2, CYP2B6 and CYP2C19 each had relatively minor roles as mediators of levonorgestrel clearance (Figure 1).
It was recently concluded that CYP3A may not represent the predominant pathway by which levonorgestrel is metabolized [11]. A recently published study examining the effects of rifampin on the pharmacokinetics of various progestin-based oral contraceptives showed that exogenous progestins exhibited differential vulnerability to rifampin-mediated CYP3A4 induction [14], and that levonorgestrel was among the least vulnerable progestins to rifampin-induced CYP3A4 activity. The present study supports this observation, showing a predominant, but not exclusive, role for CYP3A4 in mediating levonorgestrel metabolism [14]. CYP3A4 is the predominant CYP involved in progesterone metabolism [20, 28, 29], and our findings reveal that the CYPs involved in metabolism of levonorgestrel share some similarity with CYP-mediated progesterone metabolism pathways, where CYP1A2 [28], CYP2C9 [20, 28] and CYP2C19 [20, 28] each has a contributory role, secondary to CYP3A4. It remains possible, therefore, that other CYPs may also contribute to levonorgestrel metabolism. Indeed, a role for CYP3A7 – the predominant foetal isoform of CYP3A enzymes – as an important mediator of etonogestrel metabolism, was recently described [44]. Levonorgestrel implants are contraindicated in women receiving antiepileptic drugs due to the potential for DDIs that may result in contraceptive failure [45]. Notwithstanding the CYP3A induction potential of the antiepileptic drugs phenytoin and oxcarbazepine, phenytoin–levonorgestrel and oxcarbazepine-levonorgestrel DDIs may be mediated by non-CYP3A enzymes [46, 47]. Indeed, this further underlines the recently reviewed general need to better define the pharmacokinetics of mixed-mechanism DDIs [48]. While administering CYP3A inducers can decrease systemic levonorgestrel exposure by > 40% in vivo [11], it has been highlighted that the CYP3A inducers used when conducting these studies also have the potential to induce the expression of other enzymes, including phase II enzymes, together with multiple phase I enzymes, and drug transporters [11]. Although assessing the relative contribution of phase II enzymes and drug transporters in levonorgestrel clearance was beyond the scope of the current study, this should be addressed in future in vitro studies in order to better evaluate the DDI vulnerability of levonorgestrel.
The total systemic plasma clearance of levonorgestrel was estimated to be 2.38 ± 0.46 L/h. The total clearance of levonorgestrel by various routes of administration in adult females ranges between 3.9 and 4.2 L/h [15, 49]. The reasons for these differential values may be explained by the following: (1) the potential contribution of CYPs other than rhCYP1A2, rhCYP2B6, rhCYP2C9, rhCYP2C19, rhCYP3A4 and rhCYP3A5 were not quantified in the present study; (2) the potential contribution of phase II enzymes and drug transporters were not considered when estimating levonorgestrel total systemic plasma clearance using rhCYP data herein; (3) the potential contribution of extrahepatic metabolism of levonorgestrel was not considered; (4) the likely effects of interindividual variability in CYP expression and other pharmacokinetic parameters were not considered; (5) in vitro rhCYP systems are known to frequently underestimate drug clearance and may differ from the individuals included in the reported studies; and (6) the integration of protein binding in our calculation could differ from the clinical studies used for the comparison [50, 51].
The potential for dolutegravir to cause a DDI was measured by quantifying the effects of dolutegravir on the expression of CYP1A2, CYP2B6 and CYP3A4 [52]. At unbound dolutegravir concentrations ≥ 7.5-fold higher than have been reported in plasma, dolutegravir induced the expression of CYP2B6 and CYP3A4, but did not induce increased expression of CYP1A2 (Figure 3). The observed effects of dolutegravir on gene expression contradict a previous study [53], which suggested that expression of neither CYP2B6 nor CYP3A4 was affected at total dolutegravir concentrations up to and including 40 µM [53]. The reasons for these differences are not known, but may be due to inter-donor variation in the hepatocytes, or to differences in the duration of incubation with dolutegravir with hepatocytes (48 h [53] vs. 72 h in the present study). Indeed, while incubation of hepatocytes with test compounds for either 48 or 72 h are both recommended for in vitro assessment of the induction potential of compounds [52], consideration should be given to the fact that chronically administered drugs, including dolutegravir, will be administered in vivo for prolonged periods of time. Furthermore, studies using prototypic inducers of CYP expression have shown that temporal differences can lead to differential expression of key drug-metabolizing CYPs [41, 54, 55]. Nevertheless, at concentrations spanning the range of unbound concentrations of dolutegravir reported in human plasma (10.3–240 ng/mL; 24.6–57.2 nM [18] ) dolutegravir had no effect on the expression of CYP2B6 or CYP3A4 in the present study (Figure 3). CYP2C9 is characterized by lower (two- to three-fold), but correlated, inducibility compared with CYP3A4, and, consequently, it is likely that at relevant dolutegravir concentrations CYP2C9 does not play a significant role in this interaction [56].
In vitro data was used to describe the combination of dolutegravir and levonorgestrel implants by quantifying the effects of dolutegravir on levonorgestrel CLint.app.. Using an in vitro hepatocyte-based model of drug metabolism [17], the incubation with rifampin increased levonorgestrel clearance by 109%, and this experimental effect is comparable to clinical observation, where levonorgestrel exposure was decreased by 58% [14]. Incubation with dolutegravir at concentrations spanning the range of median DTGunbound in plasma (10.3–240 ng/mL; 24.6–57.2 nM) [18] resulted in a trend towards elevated levonorgestrel CLint.app. (Figure 2). Therefore, while these observations show that a DDI exists between dolutegravir and levonorgestrel in vitro, it remains unclear whether a dolutegravir–levonorgestrel DDI may occur with the DTGunbound plasma concentrations encountered in vivo.
In summary, this study reveals novel CYP-mediated mechanisms of levonorgestrel metabolism, supporting the notion that levonorgestrel undergoes metabolism by CYP enzymes additional to CYP3A, and shows that incubation with dolutegravir can significantly elevate levonorgestrel CLint.app. and expression of CYP2B6 and CYP3A4, albeit at concentrations greater than the clinical therapeutic range of dolutegravir in humans, raising the possibility that clinical dolutegravir–levonorgestrel DDIs may occur. Future studies should aim to further elucidate the mechanisms mediating levonorgestrel metabolism in vitro, and clinical studies should aim to evaluate the effects of dolutegravir on levonorgestrel exposure in humans to ensure their safe and effective combination for females living with HIV.
AUTHOR CONTRIBUTIONS
OR, MS, AO, KKS and HK participated in designing the research. OR and HK conducted experiments. OR, HK and MS performed the data analysis. OR, HK, AO, ML, KKS and MS contributed to the writing of the manuscript.




