Protease inhibitors to treat hepatitis C in the Swiss HIV Cohort Study: high efficacy but low treatment uptake†
Abstract
Objectives
Direct-acting antiviral agents (DAAs) have become the standard of care for the treatment of chronic hepatitis C virus (HCV) infection. We aimed to assess treatment uptake and efficacy in routine clinical settings among HIV/HCV coinfected patients after the introduction of the first generation DAAs.
Methods
Data on all Swiss HIV Cohort Study (SHCS) participants starting HCV protease inhibitor (PI) treatment between September 2011 and August 2013 were collected prospectively. The uptake and efficacy of HCV therapy were compared with those in the time period before the availability of PIs.
Results
Upon approval of PI treatment in Switzerland in September 2011, 516 SHCS participants had chronic HCV genotype 1 infection. Of these, 57 (11%) started HCV treatment during the following 2 years with either telaprevir, faldaprevir or boceprevir. Twenty-seven (47%) patients were treatment-naïve, nine (16%) were patients with relapse and 21 (37%) were partial or null responders. Twenty-nine (57%) had advanced fibrosis and 15 (29%) had cirrhosis. End-of-treatment virological response was 84% in treatment-naïve patients, 88% in patients with relapse and 62% in previous nonresponders. Sustained virological response was 78%, 86% and 40% in treatment-naïve patients, patients with relapse and nonresponders, respectively. Treatment uptake was similar before (3.8 per 100 patient-years) and after (6.1 per 100 patient-years) the introduction of PIs, while treatment efficacy increased considerably after the introduction of PIs.
Conclusions
The introduction of PI-based HCV treatment in HIV/HCV-coinfected patients improved virological response rates, while treatment uptake remained low. Therefore, the introduction of PIs into the clinical routine was beneficial at the individual level, but had only a modest effect on the burden of HCV infection at the population level.
Introduction
Chronic hepatitis C virus (HCV) infection is a major cause of morbidity and mortality in HIV-infected patients 1. In the Swiss HIV Cohort Study (SHCS), hepatic decompensation and hepatocellular carcinoma were among the most common causes of death in recent years 2. Before the approval of direct-acting antiviral agents (DAAs), the effectiveness of HCV treatment in routine clinical settings was very limited as a result of both poor treatment uptake and efficacy. In the SHCS, only 12.5% of HIV/HCV-coinfected patients started treatment with pegylated interferon (PEG) and ribavirin (RBV) during an observation period of 4 years 3. Contraindications of interferon-based therapy, fear of side effects and reluctance of patients and physicians to start HCV treatment were frequent barriers to treatment 4. Until recently, many patients and their physicians chose to wait for more efficient HCV treatment options, including DAAs. Fortunately, there have been major recent breakthroughs in the treatment of HCV infection with the approval of numerous DAAs. The addition of the first-generation protease inhibitors (PIs) boceprevir and telaprevir to PEG/RBV increased significantly sustained virological response rates (SVRs) in HCV genotype 1-infected patients 5, 6. Recent studies demonstrate similar response rates in HIV/HCV-coinfected compared with HCV-monoinfected patients 7-15. However, at the population level, DAAs will only have a significant benefit in terms of the burden of HCV disease if both treatment efficacy and uptake increase considerably. In HCV-monoinfected patients, the approval of boceprevir and telaprevir had little impact on treatment uptake 16. The effect of DAAs on treatment uptake and efficacy in HIV-infected populations is largely unknown.
In this study, we took advantage of the long-term prospective evaluation of HIV/HCV-coinfected patients in a nationwide representative cohort to evaluate the impact of DAAs in routine clinical practice. The SHCS provides an ideal platform for studying changes in treatment uptake because all HIV/HCV-coinfected patients are followed prospectively irrespective of treatment eligibility or indication. Furthermore, treatment safety and efficacy could also be assessed in difficult-to-treat patients with multiple comorbidities, substance abuse or psychiatric conditions who are typically excluded from clinical trials. The overarching aim of this study was to evaluate treatment uptake and efficacy of DAAs in routine clinical practice in order to inform patients, clinicians and public health authorities regarding how HCV treatment strategies could be improved at both the individual and the population levels.
Methods
Study population
HCV treatment uptake and efficacy were assessed in the framework of the SHCS (www.shcs.ch) which prospectively has enrolled HIV-infected adults in Switzerland since 1988. This nationwide cohort includes at least 45% of the cumulative number of HIV infections declared to the Swiss public health authorities, 69% of all patients living with AIDS, and 75% of patients receiving antiretroviral therapy (ART) in Switzerland 17. Representativity has remained stable over the years as a result of continuous open enrolment by both hospital centres and private care physicians. Detailed clinical and laboratory data are recorded at study entry and every 6 months thereafter and include information on HCV serology, HCV RNA, transaminases, HCV treatment and liver-related events (decompensation, variceal bleeding or hepatic encephalopathy, hepatocellular carcinoma, and liver-related death), as well as data on liver histology and transient elastography.
Assessment of HCV treatments
Because the routine 6-monthly follow-up visits are insufficient to capture the key events during HCV treatment, we implemented a case-report form in 2011, specifically designed to retrieve all pertinent information during HCV therapy. This information is collected prospectively for all patients who start HCV treatment and includes detailed information on PEG/RBV and DAA dosage, reports on major side effects, and self-reported adherence. Furthermore, HCV viral loads are recorded at baseline, at weeks 1, 2, 4, 8, 12, 24, 48, 60 and 72 of treatment, and at treatment stop or treatment failure. Missing data were obtained by chart review at the study centres.
Treatment safety and efficacy were assessed in all patients with chronic HCV genotype 1 infection who started DAA treatment from approval of the first HCV PIs in September 2011 to September 2013. All DAA treatments in the SHCS were analysed, including those prescribed within clinical trial protocols. Patients with acute HCV infection were excluded. Faldaprevir was prescribed exclusively in the frame of an international phase III trial (ClinicalTrials.gov identifier NCT01399619). Thirteen of the patients also received a lead-in with silibinin for 2 weeks according to a separate study protocol 18. Otherwise, the treatment was at the discretion of the physician. Data on treatment uptake and response rates for the time period 2009 to 2011 were retrieved from the SHCS database. All patients signed an informed consent form including a section for genetic testing. The study was approved by the local ethical committees and the SHCS scientific board.
Definitions of virological response rates
Treatment responses were defined according to the European Guidelines for the Study of the Liver (www.easl.eu). Rapid virological response (RVR) was defined as undetectable HCV RNA at week 4 of DAA treatment. For those treated with a PEG/RBV lead-in, RVR was assessed at week 8 of therapy, i.e. 4 weeks after starting DAA. Early virological response (EVR) was defined as undetectable HCV RNA after 12 weeks of treatment. End-of-treatment virological responses (EOTR) were assessed at the time of the last drug intake, and sustained virological responses were assessed at 12 weeks (SVR12) and 24 weeks (SVR24) after treatment discontinuation. Virological response rates were only considered if the corresponding time-point (e.g. end-of-treatment responses 48 weeks after starting therapy) lay within the study period (i.e. before closure of the study database in March 2014). This avoided systematic biases towards poorer outcomes, as those who fail treatment or discontinue therapy prematurely are more likely to experience an endpoint within the study period.
Laboratory measurements
Quantitative HCV RNA measurements were performed for all patients with the COBAS® TaqMan® HCV Test v2.0 on the COBAS AmpliPrep/TaqMan48 system (Roche Diagnostics International AG, Rotkreuz, Switzerland) according to the manufacturer's protocol, with a lower limit of detection of 15 IU/mL. For interleukin-28B (IL-28B) genotyping, the DNA was extracted using the QIAcube with the QIAamp DNA Mini kit (Qiagen, Hilden, Germany) and genotypes were determined using TaqMan® Genotyping Master Mix (Life Technologies, Carlsbad, CA, USA). Patients who failed treatment were analysed individually for the presence of HCV resistance-associated genetic polymorphisms before and after treatment. The HCV genome was sequenced using next-generation sequencing (NGS) methods 19 in two patients and by population sequencing in four patients.
Assessment of liver fibrosis
Where available, liver fibrosis stage was derived from liver biopsy and expressed as a Metavir score. Alternatively, liver fibrosis was determined using transient elastography (Fibroscan®; Echosens S.A.S.U., Paris, France). According to previous studies in HIV/HCV-coinfected patients, we used a cut-off value of ≥ 9.5 kPa for Metavir ≥ F3 20 and ≥ 12.4 kPa for cirrhosis 21, 22.
Statistical analysis
All calculations were performed using stata 12.1 statistical software (Stata Corp, College Station, TX, USA) or prism 6 (GraphPad Software, Inc., La Jolla, USA). Frequency comparisons were performed using χ2 tests. For two-group comparisons, the Mann−Whitney U-test was used. All testing was two-tailed and P-values < 0.05 were considered to indicate statistical significance.
Results
Baseline parameters
Fifty-seven patients with chronic HCV genotype 1 infection started treatment in the study period between 1 September 2011 and 31 August 2013 with a combination of PEG/RBV and an HCV PI. Thirty-seven patients were treated with telaprevir, eight with boceprevir and 12 with faldaprevir. Among the patients treated with telaprevir, 32 (86%) received the drugs three times daily and five (14%) received them twice daily. Six patients treated with PEG/RBV alone were not included in the outcome analyses. The baseline characteristics are summarized in Table 1. Among the treated patients, 29 (57%) had advanced liver fibrosis (≥ Metavir F3), and 15 (29%) had cirrhosis. Most patients were infected with HCV subtype 1a (73.3%). The large majority of treated patients had well-controlled HIV infection and suppressed HIV RNA. Faldaprevir-based treatment was restricted to noncirrhotic treatment-naïve patients or patients with previous relapse as per study protocol.
| Treated (n = 57) | All treated (n = 57) | Not treated (n = 453) | P# | |||
|---|---|---|---|---|---|---|
| Telaprevir (n = 37) | Boceprevir (n = 8) | Faldaprevir (n = 12) | ||||
| Demographics | ||||||
| Age [median (IQR)] | 49 (44–51) | 46.5 (44.5–52.5) | 41 (35–55) | 48 (42.5–52) | 47 (41–51) | 0.309 |
| Gender, male [n/total (%)] | 31/37 (84) | 6/8 (75) | 9/12 (82) | 46/57 (81) | 312/453 (69) | 0.066 |
| Ethnicity [n/total (%)] | ||||||
| White | 31/37 (84) | 7/8 (88) | 11/11 (100) | 49/56 (88) | 425/453 (94) | 0.078 |
| Nonwhite | 6/37 (16) | 1/8 (12) | 0/11 (0) | 7/56 (13) | 28/453 (6) | |
| Mode of HIV transmission [n/total (%)]a | ||||||
| IDU | 21/37 (57) | 7/8 (88) | 4/11 (36) | 32/56 (57) | 312/453 (69) | 0.007 |
| MSM | 5/37 (14) | 1/8 (12) | 4/11 (36) | 10/56 (18) | 43/453 (9) | |
| Heterosexual | 8/37 (22) | 0/8 (0) | 1/11 (9) | 9/56 (16) | 86/453 (19) | |
| Other | 3/37 (6) | 0/8 (0) | 2/11 (19) | 5/56 (9) | 12/453 (3) | |
| IL-28B, CC [n/total (%)] | 12/31 (39) | 3/7 (43) | 4/11 (36) | 19/49 (39) | NA | |
| Alcohol intake > 50 g/day [n/total (%)] | 1/36 (3) | 0/7 (0) | 0/11 (0) | 1/54 (2) | 32/162 (20) | 0.002 |
| Illicit drug use [n/total (%)] | 3/37 (8) | 1/8 (13) | 1/12 (8) | 5/57 (9) | 94/453 (21) | 0.031 |
| Depression [n/total (%)] | 8/36 (22) | 1/8 (13) | 1/11 (9) | 10/55 (18) | 105/438 (24) | 0.338 |
| HIV treatment | ||||||
| CD4 count at baseline (cells/μL) [median (IQR)] | 569 (444–786) | 444 (383–616) | 528 (288–654) | 533 (430–740) | 539 (380–765) | 0.876 |
| Controlled HIV RNA < 20 copies/mL [n/total (%)] | 34/37 (92) | 7/8 (88) | 9/11 (82) | 50/56 (89) | 331/453 (73) | 0.008 |
| Patient on ART [n/total (%)] | 37/37 (100) | 7/8 (88) | 12/12 (100) | 56/57 (98) | 387/421 (92) | 0.086 |
| Adherence to ART [n/total (%)] | ||||||
| Never missed | 34/35 (97) | 7/8 (88) | 9/11 (82) | 50/54 (93) | 308/399 (77) | 0.009 |
| ≤ 1 missed/month | 35/35 (100) | 8/8 (100) | 11/11 (100) | 54/54 (100) | 366/399 (92) | 0.028 |
| Hepatitis C | ||||||
| HCV genotype [n/total (%)] | ||||||
| GT 1A | 27/37 (73) | 6/8 (75) | 11/12 (92) | 44/57 (77) | 330/453 (73) | 0.484 |
| GT 1B-E | 10/37 (27) | 2/8 (25) | 1/12 (8) | 13/57 (23) | 123/453 (27) | |
| HCV viral load (log10 copies/mL) [median (IQR)] | 5.9 (4.3–6.3) | 6.3 (5.9–6.7) | 6.3 (6.2–6.4) | 6.0 (4.5–6.4) | 6.2 (5.7–6.6) | 0.034 |
| Liver fibrosis stage [n/total (%)]b | ||||||
| ≥ F3 | 23/33 (70) | 5/7 (71) | 1/11 (9) | 29/51 (57) | 47/217 (22) | <0.001 |
| Cirrhosis | 12/33 (36) | 3/7 (43) | 0/11 (0) | 15/51 (29) | 31/217 (14) | 0.010 |
| Treatment history [n/total (%)] | ||||||
| Treatment naïve | 14/36 (39) | 3/8 (37.5) | 9/12 (75) | 26/56 (46) | 352/453 (78) | <0.001 |
| Previous nonresponsec | 19/36 (53) | 2/8 (25) | 0/12 (0) | 21/56 (38) | 101/453 (22)d | <0.001 |
| Relapse | 3/36 (8) | 3/8 (37.5) | 3/12 (25) | 9/56 (16) | ||
- a Suspected mode of HIV transmission; injecting drug use does not differentiate between former and persistent habits.
- b Most recent examination: Fibroscan® stiffness ≥ 9.5 kPa translates to Metavir ≥ F3 20; Fibroscan® stiffness ≥ 12.4 kPa translates to cirrhosis 21, 22.
- c Previous nonresponse: either previous virological breakthroughs, null response, or partial response.
- d Not differentiated between previous nonresponse and relapse in control group of patients who were not treated.
- For the comparison ‘all treated’ vs. ‘not treated’.
- ART, antiretroviral therapy; IDU, injecting drug use; IQR, interquartile range; IL, interleukin; MSM, men who have sex with men. NA, none available.
We next assessed factors associated with not starting HCV therapy during follow-up. Uncontrolled alcohol intake and illicit drug use were more common in those who did not start therapy (alcohol consumption > 50 g/day, 20% compared with 2% in treated patients; P = 0.002; illicit drug use, 21% compared with 9%, respectively; P = 0.031). The proportion of patients receiving methadone or buprenorphine substitution was higher in untreated patients (40% compared with 15% in treated patients; P < 0.001). Advanced liver fibrosis was less common in untreated compared with treated patients (22% compared with 57%, respectively; P < 0.001). There were no significant differences in depressive disorders at baseline (24.0% in untreated and 18.2% in treated patients; P = 0.3). The proportion of treatment-naïve patients was higher in untreated patients (78% versus 46% in treated patients; P < 0.001).
Cascade of care
We assessed overall treatment uptake and efficacy among 1836 SHCS patients with positive HCV serology (Fig. 1). Of these, 1102 (60%) had detectable HCV RNA at the beginning of the study period in September 2011. Among 516 patients with chronic HCV genotype 1 infection, 57 started treatment with an HCV PI plus PEG/RBV and additionally six with PEG/RBV alone. Overall, 12% of patients with HCV genotype 1 infection started therapy and 7% were cured.
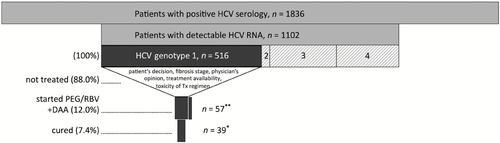
Cascade of care between 1 September 2011 and 31 August 2013. *Extrapolated from available data (n = 35); **57 patients were treated with direct-acting antiviral agents (DAAs); in addition, six patients were treated with pegylated interferon and ribavirin (PEG/RBV) without DAAs. HCV, hepatitis C virus. Tx, therapy.
Treatment response
RVRs were 78%, 89% and 71% in treatment-naïve patients, patients with relapse and previous nonresponders, respectively. At the end of treatment, 84% of treatment-naïve patients, 88% of patients with previous relapse and 62% of previous nonresponders had an undetectable HCV RNA. Sustained virological responses (SVR12) were 78% for treatment-naïve patients, 86% in patients with previous relapse and 40% in patients with a history of previous nonresponse (Fig. S1). Detailed clinical, laboratory and virological characteristics of patients with virological failures are summarized in Supplementary Table S1.
Toxicity of treatment and adverse events
Haematological toxicity was frequent. Adverse events were classified in accordance with the criteria published by the Common Terminology Criteria for Adverse Events (CTCAE) manual Version 4.0 23. Any kind of grade 3 cytopaenia was present in 42 patients (74%): nine (16%) patients experienced at least grade 3 anaemia, and three (5%) grade 4 anaemia; neutropenia of at least grade 3 was observed in 37 patients (65%) and grade 4 in eight patients (14%); thrombocytopenia of at least grade 3 was present in 14 patients (25%), with two patients (4%) having grade 4 thrombocytopenia. In 24 patients (42%), the dose of interferon-alpha had to be reduced at any time during treatment. In 25 patients (44%), the starting dose of ribavirin had to be reduced. Erythropoietin was given in 19 patients (33%), 10 patients (18%) received at least one erythrocyte concentrate, and 13 patients (23%) were treated with granulocyte-colony stimulating factor during HCV treatment. One patient switched from telaprevir to boceprevir because of a maculopapular rash. Changes in ART to avoid significant drug−drug interactions were made in 59% of patients before starting telaprevir, in 25% before starting boceprevir and in 33% before starting faldaprevir. All patients maintained control of HIV replication, with no HIV virological failures during follow-up. Four patients stopped treatment early because of depressions; there were no treatment interruptions because of severe somatic toxicity. No deaths occurred in the treated population throughout the observation period.
Among 453 patients not treated for HCV during the study period, 24 (5%) patients died. Seven (1.5%) deaths were liver-related.
Uptake and efficacy before and after the introduction of DAAs in Switzerland
Overall, treatment incidence slightly increased from 3.8 [95% confidence interval (95% CI) 2.8–5.2] per 100 patient-years before to 6.1 (95% CI 4.9–7.5) per 100 patient-years after the introduction of DAAs (Fig. 2). There was an increase in treatment incidence among treatment-experienced patients (from 2.8 to 9.4 per 100 patient-years). Treatment incidence remained below 5 per 100 patient-years in treatment-naïve patients. Compared with the era before the introduction of HCV DAAs, treatment efficacy increased considerably (Fig. 2) in both treatment-naïve and -experienced patients. Treatment uptake was assessed in those with available information on liver fibrosis by treatment period. In the period 2009–2011, treatment was started in 5.9% of patients with Metavir stage ≤ F2 and in 16.5% of those with ≥ F3. In the period 2011–2013, the respective proportions were 13% for ≤ F2 and 38% for those with advanced fibrosis or cirrhosis.
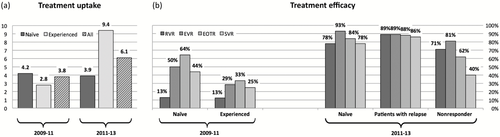
Treatment (a) uptake and (b) efficacy before and after approval of direct-acting antiviral agents (DAAs) in Switzerland. Treatment uptake is expressed in treatments per 100 patient-years, and efficacy as the proportion of patients with a respective virological response. RVR, rapid virological response (week 4); EVR, early virological response (week 12); EOTR, end-of-treatment response; SVR, sustained virological response (12 weeks after end of treatment). A nonresponder was defined as having previous virological breakthroughs, being a null responder or being a partial responder.
Discussion
We investigated treatment uptake and efficacy in a nationwide representative cohort of HIV/HCV-coinfected patients in order to inform patients, clinicians and public health authorities regarding how to improve HCV treatment strategies in light of the recent breakthroughs in HCV therapy.
Treatment efficacy
In this routine clinical setting, treatment efficacy was comparable to previous observations in the registration trials in HCV-monoinfected patients. In our study, 78% of treatment-naïve patients achieved an SVR, compared with 75% in the telaprevir-based ADVANCE trial (A new Direction in HCV Care: A Study of Treatment-Native Hepatitis C Patients With Telaprevir) 24 and 65% in the boceprevir-based SPRINT-2 trial (Serine Protease Inhibitor Therapy 2) 5. Virological response rates were very good in patients with previous relapse, with 86% reaching SVR, in line with the results from the registration trials using telaprevir 25 or boceprevir 26. SVRs were considerably lower in previous nonresponders, with an overall SVR of 40% in our study compared with 59% in those with previous partial response and 29% in those with previous null response in the REALIZE study (Re-treatment of Patients With Telaprevir-based Regimen to Optimize Outcomes) 25, and 52% in patients with prior nonresponse in the RESPOND-2 trial (Retreatment with HCV Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol 2) 26. Similar results were reported among HIV/HCV-infected treatment-naïve patients treated with telaprevir 8 or boceprevir 15, with SVRs of 74% and 63%, respectively. Neukam and colleagues observed an SVR of 69% among HIV/HCV-coinfected patients treated with either boceprevir- or telaprevir-based regimens in a real-life setting 10. Even higher response rates were observed by Cotte et al., with an SVR24 of 80% in treatment-experienced HIV/HCV-coinfected patients treated with telaprevir 11. Further studies among HIV/HCV-coinfected patients reported SVRs between 57% and 60% with telaprevir-based regimens 7, 12-14. Poizot-Martin et al. reported an SVR12 of 53% in treatment-experienced HIV/HCV-coinfected patients treated with boceprevir 9.
As expected, drug resistance mutations were frequent in those with virological failure (Table S1). Virological failures were not associated with low adherence. Haematological adverse events and dose modifications were frequent; however, there were no deaths in the treated population. Overall, the efficacy of DAAs in our HIV/HCV-coinfected patients followed in routine clinical practice was similar to observations in registration trials and in other routine clinical settings. This finding underscores that HCV treatment protocols that emerged from selected patient populations in registration trials are applicable to routine clinical settings and result in similar response rates. Our results also demonstrate that, in the DAA era, response rates in HIV/HCV-coinfected patients are similar to those in HCV-monoinfected patients.
Treatment uptake
While treatment efficacy increased considerably after the introduction of first-generation DAAs, treatment uptake remained below 10 per 100 patient-years. However, we noted an increase in treatment uptake among treatment-experienced patients (Fig. 2). Treatment-experienced patients are typically those with more advanced liver disease at high risk of liver-related events where eradication of HCV with PEG/RBV has been previously attempted without success. This is also reflected in the overrepresentation of treatment-experienced patients in those who received DAA therapy (Table 1). The emerging era of interferon-free HCV therapy led to the decision to defer treatment in most patients with less advanced liver disease, according to current treatment recommendations (www.eacs.org). The treatment incidence in our cohort was similar to a recent observation in Europe in the pre-DAA era, with an incidence of between 3.4 and 5.9 per 100 patient-years 27. In two studies within the SHCS, less than one-third of the HIV/HCV-coinfected patients were eligible for treatment with interferon/ribavirin, and only 12% started therapy 3, 4. Similar rates of noneligibility were found in a large registry cohort of US veterans in 2000–2005 28. A recent meta-analysis assessed the treatment cascade for chronic HCV infection in the USA and estimated that 37% of diagnosed patients received treatment and 21% were cured 29. Persons who inject drugs (PWID) are at particular risk for not being treated, and increased efforts should be made to ensure better options for these patients 30-32. In our study, alcohol abuse and illicit drug use were important barriers to starting treatment. A recent review estimates that, across Europe, only 0.5 to 5.2% of HCV-infected patients were treated in 2013 33. Thus, the treatment uptake in our cohort is above the average outside specialized referral centres. Interferon-free regimens are expected to considerably increase hepatitis C treatment uptake and efficacy. Wedemeyer and colleagues predicted that a treatment rate of approximately 10% using highly effective DAA treatments could achieve a > 90% reduction in total HCV infections by 2030 34. Therefore, in the SHCS, a relatively modest increase in the treatment rate could already have a substantial benefit on the future disease burden. In contrast to the PIs assessed in this study, the next DAA generation provides new interferon-free treatment options for non-1 genotypes. As the majority of HCV infections are caused by non-1 genotypes (Fig. 1), this will be a major advantage for improving treatment uptake and efficacy in HIV-infected patients.
Strengths and limitations
A major strength of our study is that treatment efficacy and uptake were assessed in a nationwide representative cohort of HIV/HCV-coinfected patients and the results and conclusions are therefore applicable to many similar routine clinical settings. The standardized close clinical and laboratory monitoring allowed a detailed assessment of treatment efficacy and safety in routine clinical practice. Furthermore, the long-term prospective standardized assessment of HCV treatments in the SHCS allowed for comparisons of HCV treatments in different time periods. An important limitation is that the heterogeneity in treatment protocols and the relatively small number of treated patients preclude comparisons of treatment efficacy and safety between treatment regimens. However, this study did not aim to compare different DAAs, but rather to assess the overall effectiveness of DAAs in routine clinical practice. Another limitation is that this study investigated first-generation DAAs, which will soon be replaced by more effective and tolerable drugs. However, given the huge costs involved in making next-generation DAAs available 35, 36, first-generation DAAs might still be used in many countries and thus the results reported here should provide important information for similar routine clinical settings world-wide.
Implications for future treatment options
The results of our study need to be seen in the context of the emerging second DAA wave with SVR > 90% 37-40. DAAs significantly improve treatment responses at the individual level. However, as demonstrated in our study, even 100% cure rates would only have a modest effect on the burden of hepatitis C at the population level if treatment uptake does not increase. The shortened treatment duration and the excellent safety and tolerability profiles of the next-generation DAAs raise the hope that more patients will benefit from HCV treatment in the near future.
Contributions to authorship
AR, JF, KJM, SH and VS had the idea of and initiated the study; SH and VS performed the analyses and drafted the manuscript together with AR and JF. KJM and JB performed and interpreted the HCV genotypic resistance test. All co-authors were involved in data collection and amended and commented on the manuscript and approved the final version.
Acknowledgements
We thank all patients participating in the Swiss HIV Cohort Study as well as all clinical staff at the different sites that have contributed to this study and the SHCS. This study has been financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant #148522) and by SHCS project #688. The data are gathered by the Five Swiss University Hospitals, two Cantonal Hospitals, 15 affiliated hospitals and 36 private physicians (listed at http://www.shcs.ch/31-health-care-providers).
Conflict of interest: AR has received honoraria for serving on advisory boards, unrestricted grants, and travel grants from Janssen-Cilag, MSD, Gilead Sciences, Abbvie, Bristol-Myers Squibb, and Boehringer Ingelheim; all remuneration went to his home institution. JF has received honoraria for serving on advisory boards, unrestricted grants, and travel grants from Janssen-Cilag, MSD, Gilead Sciences, Abbvie, Bristol-Myers Squibb, ViiV and Boehringer Ingelheim; all remunerations went to his home institutions. SH has received honoraria for serving on advisory boards and travel grants from Bristol-Myers Squibb, Gilead Sciences, and Janssen-Cilag. VS has received travel grants from Gilead Sciences. All remunerations went to their respective home institutions. JA has received honoraria for serving on advisory boards from Janssen-Cilag, Gilead and MSD; all remunerations went to the institution. KJM has received travel grants from Roche Diagnostics, Tibotec, Bristol-Myers Squibb, and Abbott; and travel grants and honoraria from Gilead Sciences. The University of Zurich has received research grants from Gilead Sciences, Roche Diagnostics, and Merck Sharp & Dohme for studies in which KJM has served as the principal investigator and advisory board honoraria from Gilead Sciences. No commercial sponsor had any involvement in the design or conduct of this study, namely the collection, management, analysis or interpretation of the data, or the preparation, decision to submit, review or approval of the manuscript. HFG has been an adviser and/or consultant to the following companies: GlaxoSmithKline, Abbott, Gilead, Novartis, Boehringer Ingelheim, Roche, Tibotec, Pfizer and Bristol-Myers Squibb, and has received unrestricted research and educational grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, and Merck Sharp & Dohme (all money went to the institution). All other authors declare that they have no competing interests.
Appendix
The members of the Swiss HIV Cohort Study are: V. Aubert, J Barth, M Battegay, E Bernasconi, J Böni, HC Bucher, C Burton-Jeangros, A Calmy, M Cavassini, M Egger, L Elzi, J Fehr, J Fellay, H Furrer (Chairman of the Clinical and Laboratory Committee), CA Fux, M Gorgievski, H Günthard (President of the SHCS), D Haerry (deputy of ‘Positive Council’), B Hasse, HH Hirsch, I Hösli, C Kahlert, L Kaiser, O Keiser, T Klimkait, R Kouyos, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, K Metzner, N Müller, D Nadal, G Pantaleo, A Rauch (Chairman of the Scientific Board), S Regenass, M Rickenbach (Head of Data Center), C Rudin (Chairman of the Mother & Child Substudy), F Schöni-Affolter, P Schmid, D Schultze, J Schüpbach, R Speck, P Tarr, A Telenti, A Trkola, P Vernazza, R Weber, and S Yerly.




