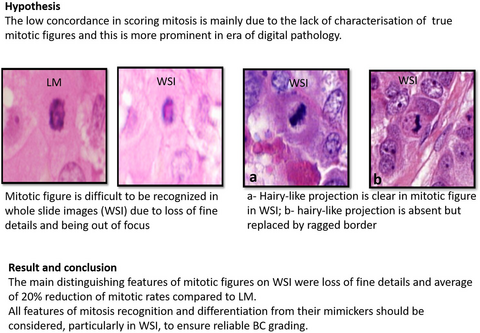
Visual assessment of mitotic figures in breast cancer: a comparative study between light microscopy and whole slide images
Abstract
Background and aims
Visual assessment of mitotic figures in breast cancer (BC) remains a challenge. This is expected to be more pronounced in the digital pathology era. This study aims to refine the criteria of mitotic figure recognition, particularly in whole slide images (WSI).
Method and results
Haematoxylin and eosin (H&E)-stained BC sections (n = 506) were examined using light microscopy (LM) and WSI. A set of features for identifying mitosis in WSI and to distinguish true figures from mimickers was developed. Changes in the mitotic count between the two platforms was explored. Morphological features of mitoses were recorded separately, including absence of nuclear membrane, chromatin hairy-like projections, shape, cytoplasmic features, mitotic cell size and relationship to surrounding cells. Each mitotic phase has its own mimickers. Fifty-eight per cent of mitoses showed absent hairy-like projection in WSI; however, 89% retained their ragged nuclear border, which distinguished them from mimickers including apoptotic cells, lymphocytes and dark elongated hyperchromatic structures. Mitosis in WSI showed loss of fine details, and there was a 20% average reduction rate of mitotic counts when compared to the same area on LM. Using refined mitosis recognition criteria in WSI resulted in a twofold improvement of interobserver concordance. However, when compared to LM, 19% of cases were underscored in WSIs.
Conclusions
All morphological features of mitosis should be considered to enable recognition and differentiation from their mimickers, particularly in WSI, to ensure reliable BC grading. Refining mitotic cut-offs per specific area when using WSI, based on the degree of reduction and association with outcome, is warranted.
Graphical Abstract
Introduction
Cellular proliferation is a hallmark of cancer, and is a key component in the Nottingham grading system (NGS) of breast cancer (BC) that reflects tumour behaviour and prognosis.1-3 Although various methods have been reported to assess the proliferation rate in BC, the most widely accepted in clinical practice is the evaluation of mitotic activity by visual counting of mitoses in haematoxylin and eosin (H&E)-stained tumour tissue sections using glass slides and light microscopy (LM).4, 5 However, the identification of mitotic figures in BC relies upon a constellation of morphological features that overlap with other structures (mimickers). This causes difficulties in identifying mitotic figures and distinguishing them from their mimickers, as demonstrated by low concordance rates reported among pathologists.6, 7
Using digitalised whole slide images (WSI) in clinical pathology reporting undoubtedly has many advantages. However, as a newly introduced technology, adaptation of current practice guidelines that are exclusively based upon conventional LM is a mandatory step prior to moving to a fully digitalised system,8, 9 especially in areas which already show high levels of discordance among pathologists, such as mitotic scoring. It is likely that eyeballing assessment of mitoses in WSI will remain routine practice until validated and robust automated methods are available in the clinical setting. Visual assessment of mitoses in WSI should not follow methodologies derived from LM until comparative studies demonstrate their effectiveness or modifications are recommended to ensure non-inferiority in WSI. These include defining mitoses and mitotic count cut-offs for scoring per an established surface size. Not all mitoses seen using LM can be identified on digital screens within the same area, due to variability of the tissue plane which cannot be fully captured during scanning due to limitations of the technique.5
In this study, we aimed to refine morphological features of mitoses to enable identification in WSI compared to LM and to provide more details on distinguishing them from mimickers. In addition, the pattern of changes in mitotic counts in the same area was assessed between the two platforms.
Material and methods
STUDY COHORT
A total of 506 surgically excised primary BC specimens were included in this study. All samples were fixed and processed in a standardised method according to existing protocols, as previously published.3, 10 Freshly cut, 4-m-thick, full-face sections were stained with H&E and the slides were histologically reviewed to select the representative tumour section. In this study, 40 BC cases characterised by very high mitoses were initially selected to study the morphological features of mitosis in detail. Then the remaining cases were selected to represent the various BC histological types and grades to provide different mitotic ranges. Of the cohort cases, 55% were grade 3 while grades 1 and 2 tumours represented 15 and 30%, respectively. No special type (NST) tumours comprised 75%, while invasive lobular carcinoma and other histological types were 10 and 15%, respectively.
H&E-stained slides were digitally scanned at ×40 magnification using a high-throughput scanner (Panoramic 250 Flash III; 3D Histech, Budapest, Hungary), and the WSIs were viewed with CaseViewer software (version 2.2.0.85; 3D-Histech) using a high-resolution screen (27″, 2560 × 1440 pixels). Digital images were assessed for quality of scanning by examining a single/several ×10/×20/×40 fields and slides with obvious scanning errors, for example, missing tissue, large areas out of focus, hazy stripes or darker background, were rescanned. For comparative analysis, the same section for each case was examined simultaneously using LM (Eclipse Ni-U; Nikon Instruments Inc, Tokyo, Japan) with a × 400 and field diameter of 0.63 mm.
COMPARING MORPHOLOGY OF MITOSES BETWEEN LM AND WSI
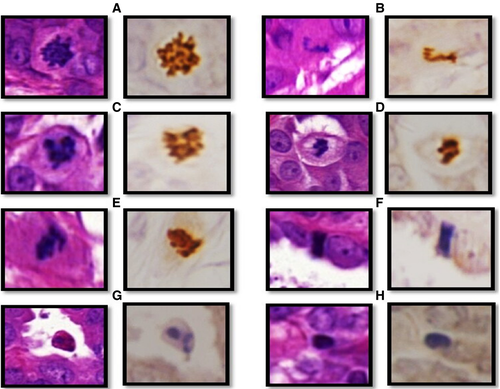
LM at ×400 magnification was used to identify mitoses. Features of mitosis and mimickers, as previously published,6, 11-13 were applied to identify mitoses. To confirm mitosis and to differentiate between true mitosis and mimickers in challenging cases, phosphohistone H3 (PHH3) immunohistochemical (IHC) staining was used, as previously described.14 PHH3-positive structures with some features of mitosis were considered mitoses, while PHH3-negative structures were counted as mimickers (Figure 1). In some figures it was difficult to confirm their nature, even by PHH3, and these figures were labelled as indeterminate (doubtful) structures.
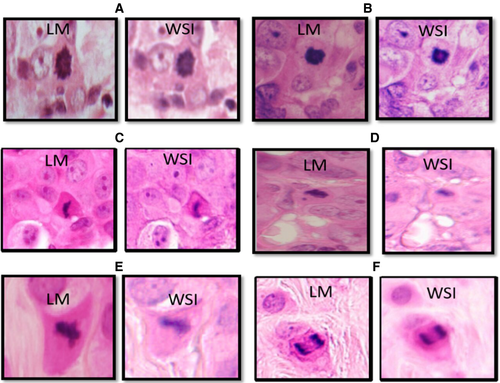
Each figure identified in LM was compared with its corresponding image in WSI and the similarities/differences between WSI and LM were recorded. Based on the morphological differences of mitoses between LM and WSIs, eight morphological features were identified to define mitoses in WSI and to distinguish them from mimickers. These features included: 1, nuclear membrane (absent/present/indiscernible); 2, hairy-like projection (fine nuclear extensions) (present/absent); 3, nuclear outline (regular/irregular, which was defined as a ragged border) and was different from the presence of hairy-like projections; 4, shape of the chromatin threads (symmetrical/asymmetrical); 5, homogeneity of chromatin distribution (homogeneous/heterogeneous); 6, cytoplasmic features (colour, granularity, ratio to the nucleus); 7, relative mitotic cell size compared to nearby neoplastic cells; and 8, relation to surrounding cells (compressed between two cells/stretched in the periphery of cell sheets or nests/no specific relationship).
To validate the recorded morphological features and to investigate subjectivity in their interpretation, three pathologists with experience in breast pathology were asked to identify and record the aforementioned morphological features for 300 mitoses extracted from WSIs. The concordance rate for identifying each feature was calculated.
VALIDATION OF THE REFINED CRITERIA FOR MITOSIS IDENTIFICATION
Based on the aforementioned steps, eight morphological criteria were identified to define mitotic figures and to distinguish them from other imitators; these were labelled as refined criteria. Moreover, to determine the level of interobserver agreement in recognising true mitotic forms using the refined mitotic criteria in WSIs, 20 regions of interest (ROIs) defined as areas of highest mitotic activity (hotspots) measuring 5 × 5 mm2 were annotated. Six pathologists were asked to count all true mitoses in these ROIs by applying the existing criteria and then recount them using the refined criteria.
COMPARISON OF MITOTIC COUNTS BETWEEN LM AND WSI
To evaluate differences between mitotic counts in LM and WSIs, if any, mitoses were counted within the same area using LM and WSI in 341 cases. The mitotic count was evaluated in hot-spots over an area of 3 mm2, which is almost equivalent to 10 high-power fields (HPF) of the microscope used in this study (with a field diameter of 0.63). The refined criteria were applied to identify mitoses. The absolute counts of mitoses in both LM and WSIs were also converted into three scores (scores 1, 2 and 3) using the existing cut-off points according to the College of American Pathologists for an area of 3 mm2, where 0–11 mitoses is equivalent to score 1, 12–22 is score 2 and > 22 mitoses/3 mm2 is score 3.10, 15
To further characterise mitoses and to identify the reasons behind mitotic reduction rate in WSIs, the prevalence and features of each mitotic phase (prophase/metaphase/anaphase/telophase) together with the type of mitosis (typical/atypical) were also evaluated. Supporting information, Figure S1 illustrates various mitotic phases, including the typical and atypical forms with their description.
STATISTICAL ANALYSIS
All statistical analyses were performed using SPSS version 26 (SPSS Inc., Chicago, IL, USA). The degree of interobserver agreement in detection of mitosis within the selected ROIs was assessed using interclass correlation coefficient (ICC) and the kappa test for continuous and categorical data, respectively; < 0 is interpreted as poor, 0–0.2 as slight, 0.2–0.4 fair, 0.4–0.6 moderate, 0.6–0.8 good and > 0.8 almost perfect. A χ2 test was carried out to correlate between variables. The sensitivity and specificity of mitosis identification in WSI was also calculated using the mitosis identified in the conventional LM (with or without PHH3) as ground truth. Sensitivity was calculated using the formula: true-positive (TP) mitosis in WSIs/[TP mitoses in WSI + false-negative (FN) mitosis that could not be identified in WSIs], while specificity was calculated as true-negative (TN) mitosis in WSIs/TN mitosis in WSIs + false-positive (FP) that was detected in WSIs. The difference rate between mitotic counts in WSIs and LM was assessed using the correlation coefficient test. For all tests, P < 0.05 (two-tailed) was considered statistically significant.
ETHICAL APPROVAL
Informed consent was obtained from all individuals prior to surgery to use their tissue materials for research. This study was approved by the Yorkshire and the Humber–Leeds East Research Ethics Committee (REC reference: 19/YH/0293) under the IRAS Project ID: 266925. Informed consent was obtained from all individuals prior to surgery to use their tissue materials in research. All samples were properly coded and anonymised. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Release of data was also pseudo-anonymised, as per the UK Human Tissue Act regulations. This article does not contain any studies with animals performed by any of the authors.
Results
COMPARING MORPHOLOGY OF MITOSES BETWEEN LM AND WSI
- 58% of mitoses showed absent hairy-like projection in WSI. In contrast, none of the mimickers showed hairy-like projections. Irregular nuclear outline (ragged border) was a valuable distinguishing feature to differentiate mitoses from their mimickers as 89% of mitoses showed this feature, while most mimickers showed regular nuclear outlines;
- Although nuclear membrane was absent in all mitoses (100%) this was also difficult to appreciate in all mitotic mimickers with hyperchromatic dense nuclei, including apoptotic bodies and elongated hyperchromatic nuclei. However, nuclear membrane was easily identified in all lymphocytes;
- Although mitotic cells showed granular or clumped chromatin, the staining pattern was homogeneous in most cases (91%) (Figure 2);
- Almost all mitoses (99%) showed abundant cytoplasm while most of the elongated hyperchromatic structures (80%) and all lymphocytes showed absent or only a thin rim of cytoplasm. Deep eosinophilic cytoplasm was seen in 84% of apoptotic bodies, while only 8% of mitoses showed this cytoplasmic makeup; 81% of mitoses showed slightly eosinophilic cytoplasm, whereas 7% showed clear cytoplasm. In addition, cytoplasmic granularity was observed in 31% of mitoses and this was completely absent in mimickers;
- Cell size was a distinguishing feature between mitotic and apoptotic cells with 67% of mitoses being relatively similar in size to nearby neoplastic cells, whereas 70% of apoptotic cells were of smaller size. Lymphocytes were clearly smaller than neoplastic cells; however, 92% of elongated hyperchromatic structures were relatively similar in size to nearby neoplastic cells and mitotic cells;
- Fragmentation and cytoplasmic retraction (represented by clear space around apoptotic cells) were unique features of apoptosis, with 25 and 30% of apoptotic bodies showed fragmentation and cytoplasmic retraction, respectively, while no mitoses presented these features; and
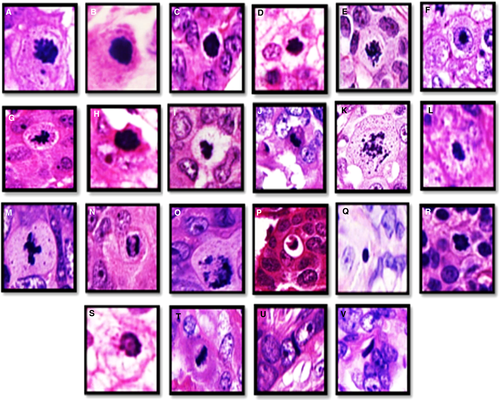
- 87% of elongated hyperchromatic structures showed a specific relation to surrounding neoplastic cells (either compressed between two cells or stretched at the periphery of cell aggregates and sheets). Mitoses and other mimickers did not show such specific relationships with nearby cells. Supporting information, Figures S3–S5, show how to differentiate between mitoses and different mimickers in WSI.
| Recorded criteria |
Mitosis (n = 964, 74%) |
Apoptosis (n = 121, 9%) |
Hyperchromatic nuclei (n = 178, 14%) |
Lymphocytes (n = 17, 1%) |
Indeterminate Structures (n = 20, 2%) |
P-value |
|---|---|---|---|---|---|---|
| Nuclear membrane | ||||||
| Absent | 964 (100) | 121 (100) | 0(0) | 0 (0) | 0 (0) | < 0.0001 |
| Present | 0 (0) | 0 (0) | 4 (2) | 17 (100) | 0 (0) | |
| Indiscernible | 0(0) | 0 (0) | 174 (98) | 0(0) | 20 (100) | |
| Hairy-like projection | ||||||
| Absent | 555 (58) | 121 (100) | 178 (100) | 17 (100) | 19 (95) | < 0.0001 |
| Present | 409 (42) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | |
| Outline or border of the hyperchromatic nuclei | ||||||
| Regular | 109 (11) | 113 (93) | 171 (96) | 17 (100) | 6 (30) | < 0.0001 |
| Irregular | 855 (89) | 8 (7) | 7 (4) | 0 (0) | 14 (70) | |
| Nuclear symmetry | ||||||
| Symmetrical | 738 (77) | 91 (76) | 130 (73) | 17 (100) | 8 (40) | < 0.0001 |
| Asymmetrical | 226 (23) | 30 (24) | 48 (27) | 0 (0) | 12(60) | |
| Homogeneity of chromatin | < 0.0001 | |||||
| Homogenous | 877 (91) | 77 (64) | 173 (97) | 15 (88) | 17 (85) | |
| Heterogeneous | 87 (9) | 44 (36) | 5 (3) | 2 (12) | 3 (15) | |
| Cytoplasmic features | ||||||
| Slightly eosinophilic | 784 (81) | 11 (9) | 27 (15) | 0 (0) | 15 (75) | < 0.0001 |
| Deeply eosinophilic | 81 (8) | 101 (84) | 5 (3) | 0 (0) | 3 (15) | |
| Clear | 64 (7) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | |
| Basophilic | 22 (3) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | |
| Rim or not seen | 13 (1) | 9 (7) | 143 (80) | 17 (100) | 2 (10) | |
| Relative cell size compared to nearby cells | ||||||
| Similar | 734 (67) | 33 (27) | 163 (92) | 6 (35) | 18 (90) | < 0.0001 |
| Increased | 210 (21) | 3 (3) | 13 (7) | 0 (0) | 2 (10) | |
| Decreased | 20 (2) | 85 (70) | 2 (1) | 11 (65) | 0 (0) | |
| Relation to surrounding cells | ||||||
| No specific relation | 964 (100) | 121(100) | 23 (13) | 17 (100) | 20 (100) | < 0.0001 |
| Stretched at the periphery | 0 (0) | 0 (0) | 95 (53) | 0 (0) | 0 (0) | |
| Compressed between 2 cells | 0 (0) | 0 (0) | 60 (34) | 0 (0) | 0 (0) |
- Significant P-values are shown in bold type.
- WSI, whole slide images.
The concordance rate in identifying the various morphological features of mitosis in WSI among three pathologists showed the highest agreement in recognising the following features: absence of nuclear membrane, ragged nuclear border and slightly eosinophilic cytoplasm, similarly sized to nearby neoplastic cells (Table 2).
| Recorded criteria | Interobserver concordance | |||
|---|---|---|---|---|
| First observer (%) | Second observer (%) | Third observer (%) | Concordance rate | |
| Nuclear membrane | ||||
| Absent | 100 | 100 | 100 | 1.0 |
| Present | 0 | 0 | 0 | 1.0 |
| Hairy-like projection | ||||
| Absent | 58 | 70 | 60 | 0.6 |
| Present | 42 | 30 | 40 | 0.4 |
| Nuclear outline | ||||
| Regular | 10 | 2 | 1 | 0.4 |
| Irregular | 90 | 98 | 99 | 0.9 |
| Nuclear symmetry | ||||
| Symmetrical | 80 | 82 | 90 | 0.8 |
| Asymmetrical | 20 | 18 | 10 | 0.2 |
| Homogeneity of chromatin | ||||
| Homogeneous | 93 | 95 | 95 | 0.9 |
| Heterogeneous | 7 | 5 | 5 | 0.6 |
| Cytoplasmic features | ||||
| Slightly eosinophilic | 82 | 85 | 75 | 0.8 |
| Deeply eosinophilic | 8 | 9 | 10 | 0.9 |
| Clear | 7 | 6 | 12 | 0.8 |
| Basophilic | 2 | 0 | 3 | 0.3 |
| Rim or not seen | 1 | 0 | 0 | 0.3 |
| Relative cell size compared to nearby neoplastic cells | ||||
| Similar | 77 | 86 | 70 | 0.7 |
| Increased | 20 | 14 | 28 | 0.3 |
| Decreased | 3 | 0 | 2 | 0.2 |
| Relation to surrounding cells | ||||
| No specific relation | 100 | 100 | 100 | 1.0 |
| Specific relation* | 0 | 0 | 0 | 1.0 |
- * Specific relation was defined as either compressed between two cells or stretched along nests/sheets of neoplastic cells. The level of agreement is considered perfect if the concordance rate from 0.8 to 1, moderate from 0.5 to 0.8, weak from 0.2 to 0.5, and very weak when less than 0.2.
DEFINING AND VALIDATING THE REFINED CRITERIA FOR IDENTIFYING MITOSES
Based on these results, a set of refined criteria to identify mitoses was produced (Table 1 and Table 3). Although the presence of abundant eosinophilic cytoplasm with irregular nuclear outline favours mitosis, the presence of deep eosinophilic cytoplasm by itself could not reliably exclude mitosis, as it was observed in 8% of the identified mitotic figures. The interobserver concordance in identifying and counting true mitoses in 20 selected ROIs was of kappa 0.8 when using the refined criteria compared to only 0.4 when using the existing criteria. There was improvement in recognition of mitoses compared to applying the existing criteria in the same cases.
| Existing criteria | Refined criteria |
|---|---|
|
|
|
- WSI, whole slide images.
COMPARISON OF MITOTIC COUNTS BETWEEN LM AND WSIS
As some of the features of mitosis were lost in WSI, we decided to evaluate the impact of losing some mitoses in WSIs compared to LM as the gold standard in 341 cases (a total of 5807 mitoses).
The sensitivity of mitosis detection in WSIs was 87%, while the specificity was 94%. Although there was a strong linear positive correlation between mitotic count in LM and WSIs (r = 0.95, P < 0.0001), there was a systematic underestimation of mitoses in WSIs compared to LM by approximately 20% of all mitotic figures (y = 0.8) (Figure 4). The mean mitotic count in the 341 cases as assessed by LM was 17 mitoses/3 mm2 (range = 0–130), compared to a mean count of 13 mitoses/3 mm2 (range = 0–102) when the same areas were assessed, case by case, in WSI.
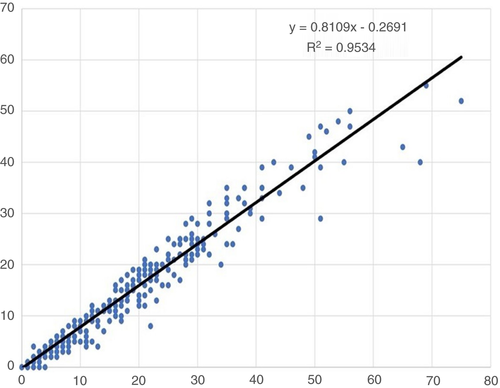
Using categorical scores for mitotic counts, WSI assessment showed a tendency to underestimate mitotic scores when compared to LM; 37% of cases with score 1 in WSI were assigned a mitotic score of 2 on LM, while 27% of cases scored as 2 in WSI were assigned mitotic score 3 with LM (Table 4). Overall, a total number of 64 (18.8%) cases were underscored in WSIs when compared to LM. None of the WSI-based mitotic scores was higher than their corresponding scores in LM.
| Mitotic score on light microscopy | Mitotic score on whole scanned images (WSI) | P-value | ||
|---|---|---|---|---|
|
1 (n, %) |
2 (n, %) |
3 (n, %) |
||
| 1 | 144 (100) | 0 (0) | 0 (0) | < 0.0001 |
| 2 | 37 (37) | 63 (63) | 0 (0) | |
| 3 | 0 (0) | 27 (27) | 70 (73) | |
- WSI, whole slide images; LM, light microscopy.
There was no significant association between reduction rate and specific tumour type. However, this depended upon mitotic count, as 10% of cases with scanty mitoses showed a high reduction rate (between 30 and 100%). One case with only two mitoses identified in the selected ROI in LM which could not be seen in WSI accounted for the 100% reduction rate observed in this study. There was no impact upon categorical mitotic score in these cases, as they were all scored as 1 in both platforms.
In this study, 14% of mitoses could not be recognised with confidence in WSIs due to some degree of haziness and loss of image focus. To further investigate the reasons behind underestimating mitotic counts in WSI and the impact of mitotic shape (mitotic phases, atypical mitoses) on our ability to recognise and distinguish them from mimickers, a total number of 9068 mitoses were examined from the whole slide (n = 125) to assess the frequency of mitotic phases and shapes. We found that 76% of the observed mitoses were typical mitoses, while 24% were atypical forms. The most common phase of mitosis was metaphase (48%), which could overlap morphologically with dark elongated hyperchromatic nuclei, followed by prophase (36%), which overlaps with apoptotic bodies and lymphocytes. Anaphase and telophase (2%) were the least common phases, but were the easiest to identify as mitoses in WSIs. When comparing the prevalence of different mitotic phases, we found that prophase structures in WSIs showed 18% reduction when compared with their respective counts in LM, while metaphase showed 8% reduction. Atypical mitoses showed 9% reduction and both anaphase and telophase showed an approximately 2% reduction from their corresponding counts in LM. Chromatin asymmetry was observed in 91% of atypical mitoses. Most typical mitoses (89%) were relatively similar in size to nearby neoplastic cells, while 60% of atypical mitoses showed a relative increase in size. Supporting information, Table S1 shows the morphological features of mitosis recorded by phase.
Discussion
In the current study, we aimed to refine the features of mitotic figures identification in WSI and examine the impact on mitotic counts. Mitotic count has been shown to be one of the independent prognostic factors in BC, and is a well-established component of the Nottingham histological grade.2, 3
The presence of certain morphological features, such as clearly visible hairy-like projections of chromatin, are helpful in identifying the mitoses in LM, which has the advantage of fine-tuning adjustment. However, these features cannot be identified easily in all mitoses and this issue is likely to be more pronounced in WSIs. Previous studies indicated that concordance rates in detecting mitoses ranged between 30 and 60% when using LM.16, 17
Although we have demonstrated that interobserver agreement for Nottingham grade using LM is not inferior to that reported using WSI in a previous study, a tendency towards a lower mitotic count in WSI was observed.18, 19
The guidelines and diagnostic criteria for mitoses that are well established and currently in use are derived from LM practice, and whether they can be applied as such to WSIs or need further adjustments remains to be investigated before using WSI in routine practice. The lack of defined criteria for assessing mitoses digitally may lead to inconsistent reporting, which would have clinical implications.4, 20
Artificial intelligence (AI) will be the future technology to help BC diagnosis and prognosis. One of the aims in this study was to provide preliminary data that can be used as evidence-based data to train AI algorithms. Our findings can also be applied to and facilitate BC grading through accurate mitotic counts in WSI using eyeballing assessment in digitalised pathology laboratories that have access to WSI facilities but do not implement AI tools.
We began this study with an evaluation of 2000 mitotic figures to assess the morphological features of mitosis in detail. Subsequently, a total number of 9068 mitoses were examined from the WSIs (n = 125) to assess the prevalence of mitotic phases, and finally 5807 mitoses were evaluated to assesses rate of reduction from 341 cases. Therefore, a total of 16 875 figures were examined in this study.
This study is the first, to our knowledge, to refine mitosis detection criteria in WSIs. We are proposing a set of refined morphological criteria for identifying mitoses and differentiating them from mimickers in WSIs. Although hairy-like projections were considered the cornerstone feature in identifying mitoses in LM6, 21 these projections are not obvious in approximately 60% of mitotic figures in WSIs. In previous studies, assessing mitoses in WSI depended upon existing criteria to define mitosis in LM, which include absent nuclear membrane and clear hairy-like projections at the outline of the nucleus.4, 21 However, the lack of fine-tuning facility in whole slide scanners makes identifying these fine details a challenging task. As a result, numerous mitoses may go undetected in WSI, leading to potentially significant underscoring.4 Therefore, redefining mitotic morphology based on direct head-to-head comparison with LM is likely to improve our ability to identify mitoses in WSIs by eyeballing.
Refined criteria can also increase the reliability of AI-based algorithms for mitosis detection by improving the quality of the training sets. Our refined criteria, when used in constellation, could improve overall BC grading and interobserver agreement. Table 3 shows the comparison between existing morphological criteria for mitosis identification and our refined criteria. In our study, a total of 14% of mitoses were not accurately recognised in WSI when compared to LM in the same areas. Consequently, underestimation of BC grade could occur if the existing LM-based mitosis cut-off values are used due to decreased sensitivity of mitosis counting in WSI. Therefore, modified cut-off values that consider the existing LM-based cut-offs, the difference between WSI and LM mitoses counts and the WSI area size to be used in the assessment of the grade mitoses scores should be developed to accurately grade BC in WSI. Ideally, testing these cut-off values using large cohorts with long-term outcome data should be carried out to validate their use in routine practice.
Several mitotic phases, particularly anaphase and telophase, were more clearly detected in WSI as the two chromatin poles were easily appreciated, despite out-of-focus images and no fine capability. Mitotic phases which are difficult to detect on LM (such as prophase) are less likely to be picked up in WSI.22
The challenges in differentiating true mitoses from other mimickers such as apoptotic bodies, lymphocytes or other dark hyperchromatic dense tumour cells are increasing with the use of one focal plane scanners. We assessed the full morphological features of a large number of hyperchromatic structures to investigate the differences between mitoses and mimickers. The majority of mitoses showed absent hairy-like projections, but were still identifiable as true mitoses due to an irregular nuclear outline (ragged border); this appearance may reflect the condensed nuclear material and the absence of nuclear membrane. A ragged border was evident in most mitoses in WSI, while most mimickers showed regular nuclear outlines.
Cytoplasmic features of mitotic cells were not studied in detail in previous studies. It was observed that almost all mitoses showed abundant cytoplasm, while its absence favours mimickers. We have categorised the various shades of eosinophilic cytoplasm as slightly eosinophilic (which represented the cytoplasm of most of mitoses) and deeply eosinophilic (which was compatible with most apoptoses and which was seen in only few mitoses). In addition, some mitoses showed clear cytoplasm, and few had basophilic or amphophilic cytoplasm. However, these mitoses usually show other characteristic features, including absent nuclear membrane, hairy-like projections and/or ragged nuclear borders, which helps identifying them. Cytoplasmic granularity is a unique feature observed in mitoses only, and could result from the fragmentation of the Golgi apparatus or from ribosomal activity during mitosis.23
Candidate figures lacking one or more criteria of the aforementioned criteria should be examined with caution before being categorised as mitoses. Although some studies recommend excluding challenging figures when using WSIs such as prophase, which can be easily confused with other mimickers,11, 24 and telophase, which may be counted as two mitoses,19 we found this to be impractical and may affect mitotic score and hence overall grading. By rigorously applying the refined criteria proposed in our study prophase identification may become less difficult, and we demonstrated this by improvement of interobserver concordance.
We observed a systematic reduction in mitosis count in WSI compared to LM with an overall rate of 20% when evaluating mitoses within a specified area. Notably, 10% of cases in which mitotic counts were around the cut-off showed down-scoring in WSI. In our study, we have assessed mitosis using a high-resolution screen (2560 × 1440 pixels). Therefore, we believe that the lower accuracy identifying mitoses is mainly due to the limitations in whole slide images caused by missing the fine-tuning capability (z-axis) in WSI; some mitoses become in-focus and easily identifiable and some become out-of-focus, and can either be missed or not identified as mitotic figures. Z-stacking or multilayer scanning is a valuable method to overcome this issue, but requires a considerably larger-sized file and extended scanning times.19 As a result, a refined cut-off for scoring mitoses in BC is warranted for adopting WSI.
In conclusion, with the introduction of digital platforms, pathologists reporting BC should be aware both of advantages and limitations, particularly when detailed assessment is required, such as counting mitoses when scoring BC. With refined criteria for mitosis identification, recognition of true mitoses in WSI can be improved. Although there is a tendency to underscore mitotic counts in WSIs, this may be addressed by adjusting mitotic scores for BC grade.
Acknowledgements
A.L. and A.I. are supported by and funded by the Egyptian Ministry of Higher Education and Scientific Research. We thank the Japanese Breast Cancer Society for supporting A.K. We thank Karim El dib for his contribution in validation of the results. The authors are part of the PathLAKE digital pathology consortium. These new Centres are supported by a £50 m investment from the Data to Early Diagnosis and Precision Medicine strand of the government’s Industrial Strategy Challenge Fund, managed and delivered by UK Research and Innovation (UKRI).
Conflicts of interest
All the authors declare that they have no conflicts of interest.
Open Research
Data Availability Statement
The authors confirm that the data used in this work are available upon reasonable request.