Histopathological and molecular profiling of lung adenocarcinoma skin metastases reveals specific features
Corresponding Author
Fabien Forest
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
University Hospital of Saint Etienne, Saint Etienne, France
Corneal Graft Biology, Engineering and Imaging Laboratory, Federative Institute of Research in Sciences and Health Engineering, Jean Monnet University, Saint-Etienne, France
Address for correspondence: Dr F Forest, CHU de Saint Etienne. Hôpital Nord, Service d’Anatomie et Cytologie Pathologiques, Avenue Albert Raimond, 42055, Saint Etienne CEDEX 2, France. e-mail: [email protected]
Search for more papers by this authorDavid Laville
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorCyril Habougit
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorVanessa Da Cruz
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorFrançois Casteillo
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorViolaine Yvorel
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorSandrine Bard-Sorel
Technopole Pathologie, Saint-Étienne, France
Search for more papers by this authorWilliam Godard
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Institut de Pathologie du Forez, Saint-Étienne, France
Search for more papers by this authorTiphanie Picot
University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorOlivier Tiffet
Department of Thoracic Surgery, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorJean-Luc Perrot
Department of Dermatology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorMichel Péoc'h
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorCorresponding Author
Fabien Forest
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
University Hospital of Saint Etienne, Saint Etienne, France
Corneal Graft Biology, Engineering and Imaging Laboratory, Federative Institute of Research in Sciences and Health Engineering, Jean Monnet University, Saint-Etienne, France
Address for correspondence: Dr F Forest, CHU de Saint Etienne. Hôpital Nord, Service d’Anatomie et Cytologie Pathologiques, Avenue Albert Raimond, 42055, Saint Etienne CEDEX 2, France. e-mail: [email protected]
Search for more papers by this authorDavid Laville
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorCyril Habougit
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorVanessa Da Cruz
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorFrançois Casteillo
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorViolaine Yvorel
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorSandrine Bard-Sorel
Technopole Pathologie, Saint-Étienne, France
Search for more papers by this authorWilliam Godard
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Institut de Pathologie du Forez, Saint-Étienne, France
Search for more papers by this authorTiphanie Picot
University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorOlivier Tiffet
Department of Thoracic Surgery, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorJean-Luc Perrot
Department of Dermatology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorMichel Péoc'h
Department of Pathology, University Hospital of Saint Etienne, Saint Etienne, France
Search for more papers by this authorAbstract
Aims
Little is known regarding the histopathological and molecular features of lung adenocarcinoma skin metastases. Our study is the largest, to our knowledge, to comprehensively explore these to date.
Methods and results
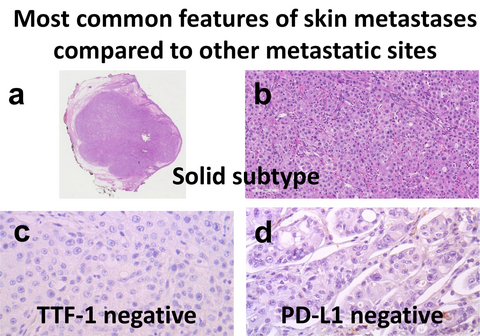
We performed a retrospective cohort study analysing 42 lung adenocarcinoma skin metastasis samples obtained from a database of 2659 lung adenocarcinomas collected between 2010 and 2020. EGFR exon 19 deletion was detected in one patient and KRAS mutations were detected in 12 (33.3%) patients. The programmed cell death ligand 1 (PD-L1) tumour proportion score was <1% in 27 patients, ≥1% and <50% in eight patients, ≥50% in six patients and not assessable in one patient. We showed that the predominant histopathological subtype is different from that at other metastatic sites (P = 0.024). Thyroid transcription factor I (TTF-1) was more often negative in skin metastases compared to other sites (P < 0.001). The EGFR mutation rate tended to be lower for skin metastases compared to other sites (P = 0.079). Skin metastases were associated with a high rate of PD-L1-negative cases (P = 0.022).
Conclusion
Our work shows that the skin metastases of lung adenocarcinoma have a specific histopathological profile.
Graphical Abstract
Conflicts of interest
None declared.
Supporting Information
| Filename | Description |
|---|---|
| his14463-sup-0001-TableS1.docxWord document, 13.7 KB | Table S1. Methods used for molecular testing for each gene stratified between skin metastases and other metastatic sites. |
| his14463-sup-0002-TableS2.docxWord document, 15.8 KB | Table S2. Immunohistochemical profile for TTF-1 on primary tumor, on another metastatic site and on skin metastases. Molecular profile was added for each case. |
| his14463-sup-0003-TableS3.docxWord document, 21.3 KB | Table S3. Clinicopathological data between the reference cohorts. The main difference between the two cohorts resides in the localization of samples. The histopathological cohort is a cohort of surgically resected metastatic samples whereas the molecular cohort is an unselected cohort of all consecutive patients who had a metastatic lung adenocarcinoma with a molecular analysis. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018; 68; 394–424.
- 2Walters S, Maringe C, Coleman MP et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 2013; 68; 551–564.
- 3Barlesi F, Mazieres J, Merlio JP et al. Routine molecular profileing of patients with advanced non-small-cel lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup. Lancet 2016; 387; 1415–1426.
- 4Ferrara MG, Di Noia V, D’Argento E et al. Oncogene-addicted non-small-cell lung cancer: treatment opportunities and future perspectives. Cancers 2020; 12; 1196.
- 5Shaukat I, Kern JJ, Höti N et al. Detection of RAS and RAS-associated alterations in primary lung adenocarcinomas. A correlation between molecular findings and tumor characteristics. Hum. Pathol. 2019; 84; 18–25.
- 6Forest F, Stachowicz M-L, Casteillo F et al. EGFR, KRAS, BRAF and HER2 testing in metastatic lung adenocarcinoma: value of testing on samples with poor specimen adequacy and analysis of discrepancies. Exp. Mol. Pathol. 2017; 103; 306–310.
- 7Yu HA, Sima CS, Shen R et al. Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J. Thorac. Oncol. 2015; 10; 431–437.
- 8Wang H, Agulnik J, Kasymjanova G et al. The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma. Lung Cancer 2019; 132; 36–38.
- 9Miyagi J, Kinjo T, Tsuhako K et al. Extremely high Langerhans cell infiltration contributes to the favourable prognosis of HPV-infected squamous cell carcinoma and adenocarcinoma of the lung. Histopathology 2001; 38; 355–367.
- 10Zilionis R, Engblom C, Pfirschke C et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019; 50; 1317–1334.e10.
- 11Moreira AL, Ocampo PSS, Xia Y et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020; 15; 1599–1610.
- 12Mansuet-Lupo A, Bobbio A, Blons H et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest 2014; 146; 633–643.
- 13Casteillo F, Guy J-B, Dal-Col P et al. Pathologic subtypes of lung adenocarcinoma brain metastasis is a strong predictor of survival after resection. Am. J. Surg. Pathol. 2018; 42; 1701–1707.
- 14Marcoval J, Penín RM, Llatjós R et al. Cutaneous metastasis from lung cancer: retrospective analysis of 30 patients. Australas. J. Dermatol. 2012; 53; 288–290.
- 15Travis WD, Brambilla E, Nicholson AG et al. WHO Classification of tumours of the lung, pleura, thymus and heart. Lyon: IARC, 2015.
- 16Rokutan-Kurata M, Yoshizawa A, Nakajima N et al. Discohesive growth pattern (Disco-p) as an unfavorable prognostic factor in lung adenocarcinoma: an analysis of 1062 Japanese patients with resected lung adenocarcinoma. Mod. Pathol. 2020; 33; 1722–1731.
- 17Travis WD, Brambilla E, Noguchi M et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma—an executive summary. Proc. Am. Thorac. Soc. 2011; 8; 381–385.
- 18Da Cruz V, Yvorel V, Casteillo F et al. Histopathological subtyping is a prognostic factor in stage IV lung adenocarcinoma. Lung Cancer 2020; 147; 77–82.
- 19Bourhis A, Remoué A, Le Flahec G et al. Avoiding non-contributive molecular results in cancer samples: proposal of a score-based approach for sample choice. Pathology 2019; 51; 524–528.
- 20 R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018.
- 21Therneau TA. Package for survival analysis in S. R package version 3.2-11; 2012. Available at: https://CRAN.R-project.org/package=survival
- 22Alcaraz I, Cerroni L, Rütten A et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am. J. Dermatopathol. 2012; 34; 347–393.
- 23Hu SCS, Chen GS, Wu CS et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J. Am. Acad. Dermatol. 2009; 60; 379–387.
- 24Takahashi Y, Eguchi T, Lu S et al. Preponderance of high-grade histologic subtype in autologous metastases in lung adenocarcinoma. Am. J. Respir. Crit. Care Med. 2018; 197; 816–818.
- 25Pelosi G, Scarpa A, Forest F et al. The impact of immunohistochemistry on the classification of lung tumors. Exp. Rev. Respir. Med. 2016; 10; 1105–1121.
- 26Casteillo F, Fournel P, Da Cruz V et al. TTF-1-positive metastatic endometrioid carcinoma. Appl. Immunohistochem. Mol. Morphol. 2017; 00; 1.
- 27Schilsky JB, Ni A, Ahn L et al. Prognostic impact of TTF-1 expression in patients with stage IV lung adenocarcinomas. Lung Cancer 2017; 108; 205–211.
- 28Zito Marino F, Rossi G, Montella M et al. Heterogeneity of PD-L1 expression in lung mixed adenocarcinomas and adenosquamous carcinomas. Am. J. Surg. Pathol. 2020; 44; 378–386.
- 29Gagné A, Enlow W, Pigeon M-A et al. Comprehensive assessment of PD-L1 staining heterogeneity in pulmonary adenocarcinomas using tissue microarrays: impact of the architecture pattern and the number of cores. Am. J. Surg. Pathol. 2018; 42; 687–694.
- 30Ng Kee Kwong F, Laggner U, McKinney O et al. Expression of PD-L1 correlates with pleomorphic morphology and histological patterns of non-small-cell lung carcinomas. Histopathology 2018; 72; 1024–1032.
- 31Forest F, Casteillo F, Da Cruz V et al. Heterogeneity of PD-L1 expression in lung adenocarcinoma metastasis is related to histopathological subtypes. Lung Cancer 2021; 155; 1–9.
- 32Kabashima K, Honda T, Ginhoux F et al. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019; 19; 19–30.
- 33Bulman A, Neagu M, Constantin C. Immunomics in skin cancer—improvement in diagnosis, prognosis and therapy monitoring. Curr. Proteomics 2013; 10; 202–217.
- 34Schulman JM, Pauli ML, Neuhaus IM et al. The distribution of cutaneous metastases correlates with local immunologic milieu. J. Am. Acad. Dermatol. 2016; 74; 470–476.
- 35Reck M, Rodríguez-Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016; 375; 1823–1833.
- 36Gandhi L, Rodríguez-Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 2018; 378; 2078–2292.
- 37Frederickson AM, Arndorfer S, Zhang I et al. Pembrolizumab plus chemotherapy for first-line treatment of metastatic nonsquamous non-small-cell lung cancer: a network meta-analysis. Immunotherapy 2019; 11; 407–428.
- 38Bai R, Lv Z, Xu D et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020; 8; 34.
- 39Hong L, Negrao MV, Dibaj SS et al. Programmed death-ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in NSCLC. J. Thorac. Oncol. 2020; 15; 1449–1459.
- 40Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018; 378; 113–125.
- 41Jordan EJ, Kim HR, Arcila ME et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017; 7; 596–609.
- 42Cadranel J, Mauguen A, Faller M et al. Impact of systematic EGFR and KRAS mutation evaluation on progression-free survival and overall survival in patients with advanced non-small-cell lung cancer treated by erlotinib in a French prospective cohort (ERMETIC project-Part 2). J. Thorac. Oncol. 2012; 7; 1490–1502.