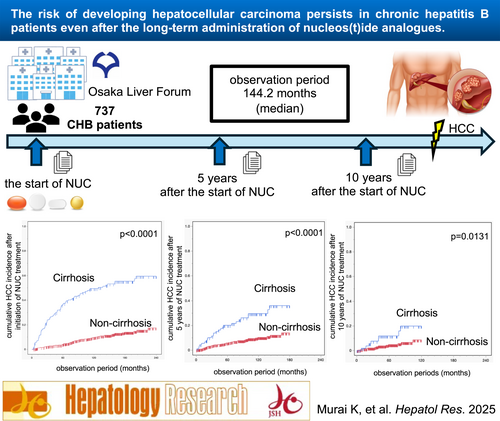
The Risk of Developing Hepatocellular Carcinoma Persists in Chronic Hepatitis B Patients Even After the Long-Term Administration of Nucleos(t)ide Analogs
Funding: This study was funded by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP24fk0310512 (H.H., T.Tat. and T.Tak.) and JP25fk0310534 (K.Mu., H.H. and T.K.).
ABSTRACT
Background and Aim
Nucleos(t)ide analogs (NUCs) are used in the treatment of chronic hepatitis B (CHB). It is still uncertain whether the risk of incident hepatocellular carcinoma (HCC) continues beyond 5 or 10 years after the initiation of NUC treatment. We aimed to elucidate this in CHB patients receiving long-term NUC treatment.
Methods
This was a multicenter, observational study that included patients who began NUC treatment between July 2000 and March 2019; patients were retrospectively enrolled up to March 2019 and followed up until August 2024.
Results
Among 737 CHB patients (156 with cirrhosis) who started NUC treatment, 147 developed HCC during a median follow-up period of 144.2 months. The 5-, 10-, and 15-year cumulative HCC rates were 11.2%, 18.4%, and 23.1%, respectively. Independent risk factors for subsequent HCC occurrence included older age, male sex, cirrhosis, low platelet count, low ALT levels, and high γ-GTP levels at NUC initiation. After 5 years, the risk factors were cirrhosis, high γ-GTP levels, and high AFP levels, whereas after 10 years, they were cirrhosis and diabetes. Landmark analysis revealed that the 5-year cumulative HCC incidence was 8.1% at 5 years and 5.8% at 10 years after NUC initiation. In noncirrhotic patients, cumulative HCC incidence at 5 years was 5.8%, whereas 5-year cumulative incidences starting at 5 and 10 years were 4.1% and 4.8%, respectively, remaining stable over time.
Conclusion
The risk of HCC persists long after NUC initiation. In noncirrhotic patients, the risk remains stable more than 10 years after NUC treatment initiation.
Graphical Abstract
Abbreviations
-
- ALD
-
- alcohol-associated liver disease
-
- BMI
-
- body mass index
-
- CHB
-
- chronic hepatitis B
-
- CI
-
- confidence interval
-
- ETV
-
- entecavir
-
- GDF15
-
- growth differentiation factor 15
-
- HBcrAg
-
- hepatitis B virus core-related antigen
-
- HBe antigen
-
- hepatitis B e antigen
-
- HBs antigen
-
- hepatitis B surface antigen
-
- HBsAgGi
-
- hepatitis B surface antigen glycan isomer
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- HR
-
- hazard ratio
-
- IFN
-
- interferon
-
- LAM
-
- lamivudine
-
- MASLD
-
- metabolic dysfunction-associated steatotic liver disease
-
- NUC
-
- nucleos(t)ide analog
-
- OLF
-
- Osaka Liver Forum
-
- SGLT2
-
- sodium-glucose cotransporter 2
-
- SNP
-
- single-nucleotide polymorphism
-
- SVR
-
- sustained virological response
-
- TAF
-
- tenofovir alafenamide
-
- TDF
-
- tenofovir disoproxil fumarate
1 Introduction
Hepatocellular carcinoma (HCC) is currently the third most common cause of cancer-related death globally [1], and chronic hepatitis B virus (HBV) infection is recognized as a significant risk factor. HBV infection poses a significant global public health challenge. Approximately 296 million individuals worldwide are estimated to be hepatitis B surface antigen (HBs antigen)-positive, with 820,000 deaths occurring each year due to HBV-related liver cirrhosis and hepatocellular carcinoma [2].
Currently, the anti-HBV therapies available for clinical use are interferon (IFN) and nucleos(t)ide analogs (NUCs) [3, 4]. Treatment with NUCs lowers serum HBV DNA titers and reduces the risk of HCC [5-8]. Even in patients who achieve viral suppression after several years of NUC therapy, the risk of HCC persists [5, 8]. The proportion of patients with HBV infection as the etiology of HCC has not decreased worldwide [9-11].
Currently, many chronic hepatitis B patients receive long-term NUC treatment. However, it is not clear whether the risk of HCC changes after prolonged treatment with NUCs, that is, treatment lasting for more than 5 or 10 years in chronic hepatitis B (CHB) patients. Evaluating the risk of HCC in patients receiving long-term NUC treatment and the effect of long-term NUC treatment on HCC is important for HCC surveillance in CHB patients receiving NUC treatment. In the present study, we collected data from CHB patients who received initial treatment with NUCs between July 2000 and March 2019 at multiple centers. Here, we identified the risk factors linked to the development of incident HCC in CHB patients receiving long-term NUC treatment and the suppressive effect of prolonged NUC therapy on the occurrence of incident HCC.
2 Methods
2.1 Study Design
The present study was a multicenter, observational investigation. Patients who received their first dose of NUCs between July 2000 and March 2019 at The University of Osaka Hospital and 18 affiliated facilities of the Osaka Liver Forum (OLF) (Supporting Information S3: Table S1) were retrospectively included, with follow-up extending until August 2024. Patients with a history of HCC, patients with HBV DNA titers below the level of detection at the start of NUC treatment, patients receiving concomitant IFN at the start of NUC treatment, and patients with other liver diseases (hepatitis C virus (HCV) or human immunodeficiency virus (HIV) infection, autoimmune diseases) or liver transplant were excluded. We assessed the incidence rate of HCC during NUC therapy. Patients who discontinued NUC treatment, who were transferred to another hospital, or who died were censored. Cirrhosis was diagnosed on the basis of liver biopsy, imaging, or portal hypertension, as reported in previous studies [6]. Diabetes mellitus was determined by treatment for diabetes. To examine the incidence of HCC more than 5 or 10 years after NUC treatment initiation, a landmark analysis was conducted using the 5-year or 10-year mark as the starting point.
2.2 HBV DNA
HBV DNA was measured through the COBAS Amplicor HBV Monitor test (detection range 2.6 to 7.6 log copies/mL), the COBAS TaqMan HBV test v2.0 (detection range 1.8 to 8.8 log copies/mL), or the COBAS 6800/8800 system HBV test (detection range 1.0 to 9.0 log IU/mL), depending on the timing and facility. In accordance with the manufacturer's guidelines and the recommendations of the Japan Society of Hepatology, HBV DNA values were converted from log copies/mL to log IU/mL [12].
2.3 Statistical Analysis
Statistical analyses were conducted through JMP version 17.1.0 (SAS Institute, Cary, USA) and EZR software [13]. Categorical data were analyzed through the chi-square test. The Mann–Whitney U test was used for continuous or ordinal data. The Kaplan–Meier method was used to estimate the cumulative incidence rates of HCC, and log-rank tests were used to evaluate the differences between cirrhotic and noncirrhotic patients. The Cox proportional hazards model was used to identify the independent factors associated with the occurrence of incident HCC. As factors for the multivariate analysis, we selected significant factors from the univariate analysis, excluding those with 30% or more missing values, unless otherwise specified. The risks are presented as hazard ratios (HRs) along with 95% confidence intervals (CIs). The Schoenfeld residual test was used to verify the proportional hazards model. A p-value < 0.05 was considered significant.
2.4 Ethics Approval
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki, the Ethical Guidelines for Clinical Studies from the Japanese Ministry of Health, Labour and Welfare, and all relevant laws in Japan. Approval for this study was obtained from the Ethics Review Committee of The University of Osaka Hospital (12,380), and the study protocol was approved by each participating institution. This research was conducted as an observational study without the use of human biological samples. As a result, written informed consent was not necessary from individual patients for the use of their clinical records, in accordance with the ethical guidelines provided by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare, Japan. The complete details of this study were made publicly available, and patients were given the option to opt out by accessing the study information on the institutions' websites.
3 Results
3.1 Characteristics in the Present Study
A total of 737 patients who began NUC treatment between July 2000 and March 2019 were included in this study. At the time of NUC treatment initiation, 175 patients were treated with lamivudine (LAM), 516 with entecavir (ETV), 23 with tenofovir disoproxil fumarate (TDF), and 23 with tenofovir alafenamide (TAF). The median age of the patients was 53 years, with 59.2% being male. Among them, 45.5% were positive for hepatitis B e antigen (HBe antigen), and 11.3% had diabetes mellitus. Cirrhosis was present in 156 patients (21.2%). The median observation period was 144.2 months. At the initiation of NUC treatment, the group of patients with cirrhosis was older; had higher proportions of males, patients with diabetes mellitus, and LAM administration; had significantly lower platelet counts; had lower ALT, albumin, and PT levels; and had higher γ-GTP, total bilirubin, and AFP levels than did the group of patients without cirrhosis (Table 1). γ-GTP levels did not correlate with body mass index (BMI), whereas diabetes mellitus and alcohol intake were associated with high γ-GTP levels (Supporting Information S2: Figure S1A–C).
| Total | Noncirrhosis | Cirrhosis | p-value | Missing values | |
|---|---|---|---|---|---|
| Number of patients | 737 | 581 | 156 | ||
| Age (median, range) | 53 (15–82) | 51 (15–82) | 57 (32–81) | < 0.0001 | 0 |
| Sex (male, female) | 436/301 (59.2%, 40.8%) | 332/249 (57.1%, 42.9%) | 104/52 (66.7%, 33.3%) | 0.0317 | 0 |
| BMI (kg/m2) (< 23, 23 ≤) | 268/258 (51.0%, 49.0%) | 220/197 (52.8%, 47.2%) | 48/61 (44.0%, 56.0%) | 0.1049 | 211 |
| Average daily alcohol intake (g) (< 20, 20 ≤) | 251/165 (60.3%, 39.7%) | 202/137 (59.6%, 40.4%) | 49/28 (63.6%, 36.4%) | 0.5120 | 321 |
| Diabetes mellitus (+, −) | 83/654 (11.3%, 88.7%) | 51/530 (8.8%, 91.2%) | 32/124 (20.5%, 79.5%) | < 0.0001 | 0 |
| NUC at the start of NUC treatment (LAM, ETV, TDF/TAF) | 175/516/46 (23.8%, 70.0%, 6.2%) | 126/414/41 (21.7%, 71.3%, 7.0%) | 49/102/5 (31.4%, 65.4%, 3.2%) | 0.0148 | 0 |
| HBs antigen (IU/mL) (< 250, 250 ≤) | 49/327 (13.0%, 87.0%) | 45/265 (14.5%, 85.5%) | 4/62 (6.1%, 93.9%) | 0.0639 | 361 |
| HBe antigen (+, −) | 324/388 (45.5%, 54.5%) | 250/310 (44.6%, 55.4%) | 74/78 (48.7%, 51.3%) | 0.3749 | 25 |
| HBV DNA (log IU/mL) (median, range) | 6.0 (1.4–8.4) | 6.0 (1.4–8.4) | 6.2 (1.5–8.3) | 0.6483 | 0 |
| HBcrAg (log U/mL) (< 3.0, 3.0 ≤) | 13/59 (18.1%. 81.9%) | 12/49 (19.7%, 80.3%) | 1/10 (9.1%, 90.9%) | 0.4010 | 665 |
| Platelet (× 104/μL) (median, range) | 15.7 (2.8–54.0) | 16.9 (3.9–54.0) | 9.5 (2.8–35.7) | < 0.0001 | 7 |
| AST (IU/L) (median, range) | 57 (15–1533) | 54 (15–1533) | 60 (25–659) | 0.1771 | 173 |
| ALT (IU/L) (median, range) | 70 (7–2986) | 76 (8–2986) | 60 (7–689) | 0.0009 | 1 |
| γ-GTP (IU/L) (median, range) | 47 (9–1001) | 43 (9–1001) | 60 (12–720) | 0.0001 | 55 |
| Total bilirubin (mg/dL) (median, range) | 0.8 (0.3–28.5) | 0.8 (0.3–10.1) | 1.0 (0.4–28.5) | < 0.0001 | 8 |
| Albumin (g/dL) (median, range) | 4.0 (1.4–5.1) | 4.1 (1.4–5.1) | 3.6 (1.6–4.7) | < 0.0001 | 19 |
| PT (%) (median, range) | 83 (30–138) | 86 (30–138) | 69 (40–107) | < 0.0001 | 132 |
| AFP (ng/mL) (median, range) | 6 (1–2370) | 5 (1–1509) | 14 (1–2370) | < 0.0001 | 142 |
| Creatinine (mg/dL) (median, range) | 0.70 (0.057–8.26) | 0.70 (0.09–8.26) | 0.72 (0.057–3.10) | 0.6872 | 103 |
| Observation period (months) | 144.2 (1.6–292.3) | 154.3 (1.6–292.3) | 80.4 (3.1–285.7) | < 0.0001 | 0 |
3.2 Cumulative Incidence of HCC After the Initiation of NUC Treatment and Risk Factors for Incident HCC
Among the 737 patients, 147 developed HCC; 63 patients were positive and 81 were negative for HBe antigen, and 81 patients had cirrhosis, whereas 66 did not. The cumulative incidence of HCC after the initiation of NUC was 11.2% at 5 years, 18.4% at 10 years, and 23.1% at 15 years (Figure 1A). The Cox proportional hazards model revealed that older age, male sex, the presence of cirrhosis, a low platelet count, a low ALT level, and a high γ-GTP level at the time of NUC treatment initiation were independent risk factors for incident HCC (Table 2). The HBs antigen was identified as a risk factor for incident HCC in the univariate analysis; however, it was excluded from the multivariate analysis because 49.0% of the data were missing (Table 2). The type of NUC at the start of NUC treatment was not identified as a risk factor for incident HCC in either the univariate analysis (Table 2) or the multivariate analysis (Supporting Information S3: Table S2). When patients were analyzed separately by HBe antigen status, the Cox proportional hazards model identified older age and cirrhosis as independent risk factors for incident HCC in HBe antigen-positive patients, whereas older age, male sex, low platelet count, and high γ-GTP levels were independent risk factors in HBe antigen-negative patients (Supporting Information S3: Tables S3–S6).
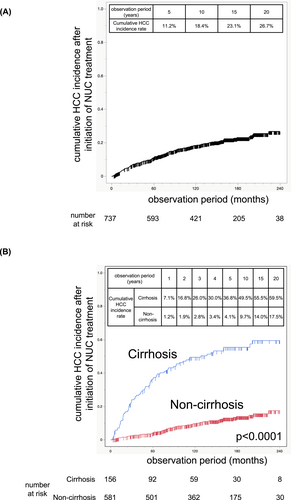
Cumulative incidence of HCC after initiation of NUC treatment. Cumulative incidence of HCC after the initiation of NUC treatment in the total cohort (A). Cumulative incidence of HCC after the initiation of NUC treatment in cirrhotic patients and noncirrhotic patients (B).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| Age | years old | per 1-year increase | 1.06 | 1.04–1.07 | < 0.0001 | 1.05 | 1.03–1.07 | < 0.0001 |
| Sex | Male/female | 1.54 | 1.08–2.18 | 0.0134 | 1.69 | 1.11–2.57 | 0.0116 | |
| Liver fibrosis | Cirrhosis/noncirrhosis | 6.13 | 4.43–8.50 | < 0.0001 | 2.63 | 1.61–4.28 | < 0.0001 | |
| BMI | kg/m2 | ≥ 23/< 23 | 1.07 | 0.73–1.57 | 0.7197 | |||
| Average daily alcohol intake | g | ≥ 20/< 20 | 1.10 | 0.70–1.71 | 0.6817 | |||
| Diabetes mellitus | Presence/absence | 2.19 | 1.47–3.25 | 0.0003 | 1.01 | 0.61–1.66 | 0.9838 | |
| NUC at the start of NUC treatment | LAM, ETV/TDF, TAF | 6.65 | 0.93–47.7 | 0.0593 | ||||
| HBs antigen | IU/mL | ≥ 250/< 250 | 9.67 | 1.34–69.7 | 0.0006 | |||
| HBe antigen | Positive/negative | 0.85 | 0.61–1.19 | 0.3455 | ||||
| HBV DNA | Log IU/mL | ≥ 3.0/< 3.0 | 1.68 | 0.62–4.53 | 0.2672 | |||
| HBcrAg | Log U/mL | ≥ 3.0/< 3.0 | 0.48 | 0.09–2.49 | 0.3848 | |||
| Platelet | × 104/μL | per 1 × 104/μL increase | 0.86 | 0.83–0.89 | < 0.0001 | 0.94 | 0.89–0.99 | 0.0135 |
| AST | U/L | per 1 U/L increase | 0.999 | 0.998–1.001 | 0.4719 | |||
| ALT | U/L | per 1 U/L increase | 0.99902 | 0.99782–0.99999 | 0.0467 | 0.99852 | 0.99679–0.99992 | 0.0361 |
| γ-GTP | U/L | per 1 U/L increase | 1.002 | 1.001–1.003 | 0.0008 | 1.003 | 1.002–1.005 | 0.0005 |
| Total bilirubin | mg/dL | per 1 mg/dL increase | 1.11 | 1.04–1.16 | 0.0047 | 1.09 | 0.94–1.26 | 0.2433 |
| Albumin | g/dL | per 1 g/dL increase | 0.43 | 0.33–0.55 | < 0.0001 | 0.84 | 0.57–1.27 | 0.4115 |
| PT | % | per 1% increase | 0.97 | 0.96–0.98 | < 0.0001 | 1.00 | 0.99–1.02 | 0.8059 |
| AFP | ng/mL | per 1 ng/mL increase | 1.001 | 1.000–1.002 | 0.0470 | 1.00 | 0.99–1.02 | 0.3875 |
| Creatinine | mg/dL | per 1 mg/dL increase | 1.11 | 0.61–1.51 | 0.6549 | |||
3.3 Cumulative HCC Incidence Stratified by the Presence or Absence of Cirrhosis and Risk Factors for HCC Development at 5 or 10 Years After NUC Initiation
The cumulative HCC incidence in cirrhotic patients was 7.1% at 1 year, 16.8% at 2 years, 26.0% at 3 years, 30.0% at 4 years, 36.8% at 5 years, 49.5% at 10 years, and 55.5% at 15 years, which was significantly greater than the cumulative HCC incidence in noncirrhotic patients (1.2% at 1 year, 1.9% at 2 years, 2.8% at 3 years, 3.4% at 4 years, 4.1% at 5 years, 9.7% at 10 years, and 14.0% at 15 years) (Figure 1B). When the risk factors for subsequent incident HCC at 5 or 10 years after NUC treatment initiation were examined, a Cox proportional hazards model revealed that older age, the presence of cirrhosis at baseline, high γ-GTP levels at 5 years after NUC treatment, and high AFP levels at 5 years after NUC treatment were independent risk factors for incident HCC after 5 years of NUC treatment and that the presence of cirrhosis and diabetes at baseline were independent risk factors for incident HCC after 10 years of NUC treatment (Tables 3 and 4). Among patients with normal ALT levels at 5 years after NUC treatment, older age, the presence of cirrhosis, and high γ-GTP levels at 5 years after NUC treatment were independent risk factors for incident HCC after 5 years of NUC treatment (Supporting Information S3: Tables S7 and S8).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| Age | years old | per 1-year increase | 1.05 | 1.03–1.08 | < 0.0001 | 1.06 | 1.03–1.09 | < 0.0001 |
| Sex | Male/female | 1.28 | 0.78–2.10 | 0.3261 | ||||
| Liver fibrosis | Cirrhosis/noncirrhosis | 3.35 | 2.05–5.49 | < 0.0001 | 2.54 | 1.31–4.96 | 0.0077 | |
| BMI | kg/m2 | ≥ 23/< 23 | 0.68 | 0.39–1.19 | 0.1779 | |||
| Average daily alcohol intake | g | ≥ 20/< 20 | 1.26 | 0.69–2.29 | 0.4519 | |||
| Diabetes mellitus | Presence/absence | 2.60 | 1.49–4.56 | 0.0023 | 1.58 | 0.80–3.13 | 0.2088 | |
| HBs antigen* | IU/mL | ≥ 250/< 250 | 1.03 | 0.42–2.51 | 0.9529 | |||
| HBe antigen* | Positive/negative | 0.75 | 0.37–1.52 | 0.4126 | ||||
| HBV DNA* | Log IU/mL | ≥ 3.0/< 3.0 | 0.23 | 0.03–1.68 | 0.0616 | |||
| HBcrAg* | Log U/mL | ≥ 3.0/< 3.0 | 0.75 | 0.14–4.09 | 0.7410 | |||
| Platelet* | × 104/μL | per 1 × 104/μL increase | 0.91 | 0.86–0.95 | < 0.0001 | 0.96 | 0.91–1.03 | 0.2399 |
| AST* | U/L | per 1 U/L increase | 1.006 | 0.996–1.012 | 0.2097 | |||
| ALT* | U/L | per 1 U/L increase | 1.000 | 0.991–1.004 | 0.9495 | |||
| γ-GTP* | U/L | per 1 U/L increase | 1.008 | 1.005–1.010 | < 0.0001 | 1.011 | 1.007–1.014 | < 0.0001 |
| Total bilirubin* | mg/dL | per 1 mg/dL increase | 1.03 | 0.55–1.72 | 0.9074 | |||
| Albumin* | g/dL | per 1 g/dL increase | 1.08 | 0.52–2.33 | 0.8392 | |||
| PT* | % | per 1% increase | 0.99 | 0.97–1.02 | 0.5134 | |||
| AFP* | ng/mL | per 1 ng/mL increase | 1.014 | 1.007–1.022 | < 0.0001 | 1.02 | 1.01–1.03 | 0.0006 |
| Creatinine* | mg/dL | per 1 mg/dL increase | 1.39 | 0.85–1.81 | 0.1529 | |||
- * At 5 years after the initiation of NUC treatment.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| Age | years old | per 1-year increase | 1.04 | 1.00–1.08 | 0.0327 | 1.035 | 0.995–1.076 | 0.0796 |
| Sex | Male/female | 1.80 | 0.75–4.30 | 0.1729 | ||||
| Liver fibrosis | Cirrhosis/noncirrhosis | 2.78 | 1.20–6.45 | 0.0273 | 2.63 | 1.13–6.10 | 0.0357 | |
| BMI | kg/m2 | ≥ 23/< 23 | 1.15 | 0.47–2.83 | 0.7630 | |||
| Average daily alcohol intake | g | ≥ 20/< 20 | 0.94 | 0.28–3.12 | 0.9128 | |||
| Diabetes mellitus | Presence/absence | 3.52 | 1.47–8.43 | 0.0114 | 3.06 | 1.27–7.38 | 0.0234 | |
| HBs antigen* | IU/mL | ≥ 250/< 250 | 0.63 | 0.23–1.75 | 0.3820 | |||
| HBe antigen* | Positive/negative | 0.94 | 0.12–7.53 | 0.9535 | ||||
| HBV DNA* | Log IU/mL | ≥ 3.0/< 3.0 | 0.00 | 0– | 0.9993 | |||
| HBcrAg* | Log U/mL | ≥ 3.0/< 3.0 | 0.67 | 0.12–3.75 | 0.6526 | |||
| Platelet* | × 104/μL | per 1 × 104/μL increase | 0.95 | 0.86–1.04 | 0.2885 | |||
| AST* | U/L | per 1 U/L increase | 1.01 | 0.97–1.04 | 0.4803 | |||
| ALT* | U/L | per 1 U/L increase | 0.99 | 0.94–1.01 | 0.6100 | |||
| γ-GTP* | U/L | per 1 U/L increase | 1.006 | 0.996–1.013 | 0.1893 | |||
| Total bilirubin* | mg/dL | per 1 mg/dL increase | 1.89 | 0.66–4.13 | 0.2081 | |||
| Albumin* | g/dL | per 1 g/dL increase | 1.25 | 0.38–6.53 | 0.7497 | |||
| PT* | % | per 1% increase | 0.98 | 0.95–1.02 | 0.4085 | |||
| AFP* | ng/mL | per 1 ng/mL increase | 0.99 | 0.82–1.02 | 0.6644 | |||
| Creatinine* | mg/dL | per 1 mg/dL increase | 0.53 | 0.02–1.40 | 0.4786 | |||
- * At 10 years after the initiation of NUC treatment.
3.4 Cumulative Incidence of HCC After 5 or 10 Years of NUC Treatment
To examine the risk of incident HCC more than 5 or 10 years after NUC treatment initiation, we conducted a landmark analysis using the 5-year or 10-year mark as the starting point. After 5 years of NUC treatment, among the 737 patients, 593 patients, including 92 cirrhotic patients, continued NUC treatment without developing HCC (Supporting Information S3: Table S9). After 10 years of NUC treatment, among the 737 patients, 421 patients, including 59 cirrhotic patients, continued NUC treatment without developing HCC (Supporting Information S3: Table S10). Landmark analysis revealed that the 5-year and 10-year cumulative incidence rates of HCC starting 5 years after NUC treatment initiation were 8.1% and 13.4%, respectively (Figure 2A). The 5-year cumulative incidence of HCC starting 10 years after NUC treatment initiation was 5.8% (Figure 2B).
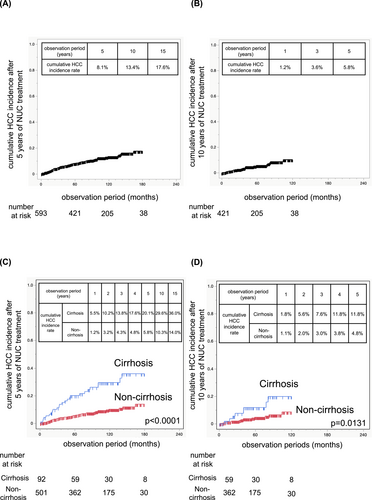
Cumulative incidence of HCC after 5 years and 10 years of NUC treatment. Cumulative incidence of HCC after 5 years of NUC treatment in the total cohort (A). Cumulative incidence of HCC after 10 years of NUC treatment in the total cohort (B). Cumulative HCC incidence after 5 years of NUC treatment in cirrhotic patients and noncirrhotic patients (C). Cumulative HCC incidence after 10 years of NUC treatment in cirrhotic patients and noncirrhotic patients (D).
3.5 The HCC Incidence Rate in Cirrhotic Patients Remains Higher Than That in Noncirrhotic Patients After Long-Term NUC Treatment
When cirrhotic and noncirrhotic patients were analyzed separately, the cumulative incidence of HCC starting 5 or 10 years after NUC treatment initiation was significantly greater in cirrhotic patients than in noncirrhotic patients (Figure 2C, Figure 2D). In cirrhotic patients, the 1-, 2-, 3-, 4-, and 5-year cumulative HCC incidence rates starting 5 years after NUC treatment initiation were 5.5%, 10.2%, 13.8%, 17.6%, and 20.1%, respectively (Figure 2C). Starting 10 years after NUC treatment initiation, the rates were 1.8%, 5.6%, 7.6%, 11.8%, and 11.8%, respectively (Figure 2D). These rates were lower than those reported during the first 5 years after NUC treatment initiation: 7.1% at 1 year, 16.8% at 2 years, 26.0% at 3 years, 30.0% at 4 years, and 36.8% at 5 years (Figure 1B). The complementary log–log plots for cirrhotic patients initially increased but appeared to level off over time (Supporting Information S2: Figure S2). When the baseline characteristics of cirrhotic patients at the start of NUC treatment were compared, those who developed HCC within 5 years had a higher proportion of patients with a low BMI and lower platelet counts and albumin levels than did those who developed HCC more than 5 years after NUC treatment initiation (Table 5).
| Within 5 years | More than 5 years | p-value | |
|---|---|---|---|
| Number of patients | 56 | 25 | |
| Age (median, range) | 60 (37–80) | 59 (43–69) | 0.4610 |
| Sex (male, female) | 40/16 (71.4%, 28.6%) | 16/9 (64.0%, 36.0%) | 0.5038 |
| BMI (kg/m2) (< 23, 23 ≤) | 14/25 (35.9%, 64.1%) | 12/6 (66.7%, 33.3%) | 0.0302 |
| Average daily alcohol intake (g) (< 20, 20 ≤) | 20/5 (80.0%, 20.0%) | 8/7 (53.3%, 46.7%) | 0.0748 |
| Diabetes mellitus (+, −) | 13/43 (23.2%, 76.8%) | 8/17 (32.0%, 68.0%) | 0.4046 |
| HBs antigen (IU/mL) (< 250, 250 ≤) | 0/21 (0%, 100%) | 0/12 (0%, 100%) | — |
| HBe antigen (+, −) | 25/30 (45.5%, 54.5%) | 15/9 (62.5%, 37.5%) | 0.1634 |
| HBV DNA (log IU/mL) (< 3.0, 3.0 ≤) | 2/54 (3.6%, 96.4%) | 0/25 (0%, 100%) | 0.3387 |
| HBcrAg (log U/mL) (< 3.0, 3.0 ≤) | 1/2 (33.3%, 66.7%) | 0/1 (0%, 100%) | 0.5050 |
| Platelet (× 104/μL) (median, range) | 8.2 (3.4–16.9) | 11.4 (5.4–20.5) | 0.0041 |
| AST (IU/L) (median, range) | 60 (25–211) | 55 (29–436) | 0.3571 |
| ALT (IU/L) (median, range) | 58 (20–555) | 51 (7–689) | 0.7242 |
| γ-GTP (IU/L) (median, range) | 60 (19–491) | 65 (19–336) | 0.6907 |
| Total bilirubin (mg/dL) (median, range) | 1.1 (0.5–28.5) | 0.9 (0.4–3.9) | 0.1679 |
| Albumin (g/dL) (median, range) | 3.4 (1.6–4.6) | 3.8 (2.3–4.7) | 0.0270 |
| PT (%) (median, range) | 65 (42–98) | 77 (49–96) | 0.0699 |
| AFP (ng/mL) (median, range) | 15 (2–2370) | 13 (3–205) | 0.6187 |
| Creatinine (mg/dL) (median, range) | 0.73 (0.50–1.30) | 0.71 (0.057–3.10) | 0.4064 |
3.6 The HCC Incidence Rate in Noncirrhotic Patients Remained at the Same Level After Long-Term NUC Treatment
In noncirrhotic patients, the 1-, 2-, 3-, 4-, and 5-year cumulative HCC incidence rates starting 5 years after NUC treatment initiation were 1.2%, 3.2%, 4.3%, 4.8%, and 5.8%, respectively (Figure 2C). Similarly, starting 10 years after NUC treatment initiation, the rates were 1.1%, 2.0%, 3.0%, 3.8%, and 4.8%, respectively (Figure 2D). These rates were comparable to those reported during the first 5 years after NUC treatment initiation: 1.2% at 1 year, 1.9% at 2 years, 2.8% at 3 years, 3.4% at 4 years, and 4.1% at 5 years (Figure 1B). In noncirrhotic patients, the HCC incidence rate remained at the same level after long-term NUC treatment. The complementary log–log plots for noncirrhotic patients revealed a linear increase (Supporting Information S2: Figure S2). When the baseline characteristics of noncirrhotic patients at the start of NUC treatment were compared, those who developed HCC within 5 years had higher γ-GTP levels than did those who developed HCC more than 5 years after NUC treatment initiation. In noncirrhotic patients, there were no differences in BMI, platelet count, or albumin level between those who developed HCC within 5 years and those who developed HCC more than 5 years after NUC treatment initiation (Table 6). Among noncirrhotic patients, those who developed HCC were older, had lower platelet and albumin levels, and had higher γ-GTP and AFP levels at NUC initiation (Supporting Information S3: Table S11). Among noncirrhotic patients treated with NUC for 5 years, those who developed HCC thereafter were older; had a higher diabetes incidence at NUC initiation; had lower platelet counts; and had higher AST, γ-GTP, and AFP levels at 5 years post-NUC initiation (Supporting Information S3: Table S12).
| Within 5 years | More than 5 years | p-value | |
|---|---|---|---|
| Number of patients | 23 | 43 | |
| Age (median, range) | 59 (41–64) | 56 (36–73) | 0.4187 |
| Sex (male, female) | 17/6 (73.9%, 26.1%) | 28/15 (65.1%, 34.9%) | 0.4647 |
| BMI (kg/m2) (< 23, 23 ≤) | 7/8 (46.7%, 53.3%) | 20/15 (57.1%, 42.9%) | 0.4958 |
| Average daily alcohol intake (g) (< 20, 20 ≤) | 3/9 (25.0%, 75.0%) | 17/12 (58.6%, 41.4%) | 0.2585 |
| Diabetes mellitus (+, −) | 2/21 (8.7%, 91.3%) | 8/35 (18.6%, 81.4%) | 0.2847 |
| HBs antigen (IU/mL) (< 250, 250 ≤) | 0/6 (0%, 100%) | 1/23 (4.2%, 95.8%) | 0.6111 |
| HBe antigen (+, −) | 6/17 (26.1%, 73.9%) | 17/25 (40.5%, 59.5%) | 0.2640 |
| HBV DNA (log IU/mL) (< 3.0, 3.0 ≤) | 1/22 (4.4%, 95.6%) | 1/42 (2.3%, 97.7%) | 0.6479 |
| HBcrAg (log U/mL) (< 3.0, 3.0 ≤) | 0/0 | 1/2 (33.3%, 66.7%) | — |
| Platelet (× 104/μL) (median, range) | 14.2 (8.2–22.6) | 14.4 (6.3–25.8) | 0.8823 |
| AST (IU/L) (median, range) | 58 (37–468) | 58 (26–1533) | 0.7313 |
| ALT (IU/L) (median, range) | 55 (27–617) | 63 (26–1288) | 0.8664 |
| γ-GTP (IU/L) (median, range) | 77 (36–987) | 65 (16–315) | 0.0168 |
| Total bilirubin (mg/dL) (median, range) | 0.9 (0.4–1.8) | 0.8 (0.4–5.8) | 0.4558 |
| Albumin (g/dL) (median, range) | 4.0 (3.3–4.4) | 3.9 (3.2–4.8) | 0.8184 |
| PT (%) (median, range) | 84 (51–107) | 84 (39–115) | 0.5047 |
| AFP (ng/mL) (median, range) | 11 (2–1509) | 10 (2–175) | 0.5692 |
| Creatinine (mg/dL) (median, range) | 0.74 (0.40–0.95) | 0.74 (0.50–1.03) | 0.7315 |
4 Discussion
We clearly demonstrated that the risk of HCC occurrence persists for a long time after NUC treatment initiation in the present study. Additionally, we revealed that older age, the presence of cirrhosis, and a high γ-GTP level are risk factors for subsequent HCC occurrence 5 years after the start of NUC treatment, whereas the presence of cirrhosis and diabetes at baseline are risk factors for subsequent incident HCC 10 years after the start of NUC treatment. The mean observation period after the initiation of NUC treatment in the present study exceeded 12 years, making this study valuable.
Our study revealed that in noncirrhotic patients, the risk persists at a comparable level even more than 10 years after starting NUC treatment. The factors related to HBV-induced incident HCC include the accumulation of genetic abnormalities due to chronic inflammation and regeneration, genome instability due to the integration of the HBV genome into the host genome [14, 15], DNA methylation [16], and direct carcinogenic effects of the HBx protein [17] or HBV preS/S proteins [18]. NUC treatment suppresses chronic hepatitis and inhibits incident HCC by suppressing serum HBV DNA but does not eliminate HBV genome integration, DNA methylation, the HBx protein, or the HBV preS/S proteins. The persistence of the risk of incident HCC more than 15 years after the initiation of NUC treatment in noncirrhotic patients may reflect that the risk of incident HCC due to HBV genome integration, DNA methylation, the HBx protein, and the HBV preS/S proteins persists over time. In cirrhotic patients, the cumulative HCC incidence after 5 and 10 years of NUC treatment was lower than that immediately after NUC treatment initiation. The observation of a suppressive effect on incident HCC after long-term NUC administration in cirrhotic patients suggests that chronic inflammation plays a significant role in the development of incident HCC in this population. However, the data in Table 5 suggest that patients who developed HCC within 5 years were more likely to have advanced cirrhosis than patients who developed HCC more than 5 years after treatment initiation. The reduction in HCC incidence after long-term NUC treatment in cirrhotic patients may simply be due to a decline in the number of high-risk patients over time.
Previous studies reported that advanced-stage liver fibrosis at the start of NUC treatment is an important predictor of incident HCC despite antiviral therapy [19-21]. Additionally, it has been reported that an elevated FIB-4 index, which estimates fibrosis, after starting NUC treatment is a risk factor for the development of incident HCC during NUC therapy [22, 23]. Although previous research has showed that NUC treatment can improve fibrosis, even in patients with cirrhosis [24, 25], our study revealed that individuals with cirrhosis at the initiation of NUC therapy remain at a significantly greater risk of developing HCC than those without cirrhosis, even more than 10 years after starting NUC treatment. From the perspective of suppressing incident HCC, initiating NUC treatment in HBV patients before they progress to cirrhosis may be critical.
Huang CF et al. reported that a γ-GTP level > 25 U/L at 6 months after NUC treatment initiation is a risk factor associated with the development of HCC in the following 6 months [26]. Sometani E et al. reported that a high γ-GTP level was a risk factor for HCC in CHB patients treated with NUCs for more than 1 year who had suppressed HBV DNA titers [27]. γ-GTP is a well-known biological marker of excessive ethanol consumption and is associated with metabolic dysfunction-associated steatotic liver disease (MASLD) [28]. In patients with chronic hepatitis C without alcohol consumption or diabetes mellitus, γ-GTP at the time of sustained virological response (SVR) has been reported to be correlated with growth differentiation factor 15 (GDF15), a marker of mitochondrial dysfunction. Additionally, single nucleotide polymorphisms (SNPs) in ZNF827 and GDF15 have been associated with high GGT levels [29]. In the present study, GDF15 and SNPs were not examined, which is a limitation of the present study. Patients with higher alcohol intake had higher γ-GTP levels than did those with lower alcohol intake, and patients with diabetes mellitus had higher γ-GTP levels than did those without diabetes mellitus. The finding that a high γ-GTP level is a risk factor for HCC suggests that the presence of comorbid alcohol-associated liver disease (ALD) or MASLD is a risk factor for HCC.
Diabetes was identified as contributing to the development of HCC more than 10 years after NUC treatment initiation. Because the comorbidity rate of diabetes is high in MASLD patients [30], the coexistence of MASLD may have contributed to the development of HCC more than 10 years after NUC treatment initiation. It has been reported that in CHB patients with diabetes, the hyperglycemic burden is associated with the development of HCC, regardless of the use of anti-HBV therapy or baseline HBV DNA levels [31]. Furthermore, sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce the risk of incident HCC in CHB patients with diabetes [32]. Future studies are needed to determine whether glycemic control reduces the risk of incident HCC in patients with diabetes receiving NUC treatment.
Interestingly, in the present study, the HBs antigen, HBe antigen, hepatitis B virus core-related antigen (HBcrAg), and HBV DNA were not risk factors for incident HCC under NUC treatment initiation. Recently, new HBV markers, including HBV RNA [33], iTACT-HBcrAg [12], and the hepatitis B surface antigen glycan isomer (HBsAgGi)/HBs antigen ratio [34], have been reported to be valuable predictors of HCC incidence during NUC therapy. These novel HBV markers were not examined in the present study. In the future, the predictive ability of these novel HBV markers for incident HCC during NUC treatment is expected to be examined in cohorts with longer observation periods, such as in the present study.
In conclusion, the HCC incidence rate persists for at least 15 years after NUC treatment initiation. Cirrhosis at the start of NUC therapy was strongly associated with a higher risk of HCC, even after 10 years. However, in noncirrhotic patients, the risk remains at a similar level even after a decade of NUC treatment.
Acknowledgments
This study was funded by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP24fk0310512 and JP25fk0310534.
Conflicts of Interest
Hayato Hikita and Tomohide Tatsumi are Editorial Board members of Hepatology Research and coauthors of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication. Tetsuo Takehara is a former member of the Hepatology Research Editorial Board and a coauthor of this article. Tetsuo Takehara received research grants from Gilead Sciences Inc., GSK plc, and Bristol Myers Squibb. Tetsuo Takehara and Hayato Hikita received lecture fees from Gilead Sciences.