Time trend of outcomes according to systemic therapy for patients with unresectable hepatocellular carcinoma: A single-institution study
Abstract
Background
We have been able to use molecular targeted agents for unresectable hepatocellular carcinoma since 2009, and immune checkpoint inhibitors have been approved in recent years. We assessed the efficacy of systemic therapy in Hiroshima University Hospital by each era.
Methods
A total of 357 patients who were treated with sorafenib, lenvatinib, atezolizumab plus bevacizumab combination therapy, or durvalumab plus tremeliumab combination therapy as first-line systemic therapy in our hospital from November 2009 to December 2023 were enrolled in this retrospective cohort study. We divided the years from 2009 to 2023 into the following three periods: cohort I, 2009–2016, the single-molecular targeted agent era; cohort II, 2017–2020, the multi-molecular targeted agent era; and cohort III, 2020–2023, the immuno-oncology era.
Results
The median survival time was 9.5 months in cohort I, 15.8 months in cohort II, and 20.2 months in cohort III. The median survival time in cohort III was significantly (p < 0.01) longer than in the other cohorts. The overall response rate by mRECIST was 4.1% in cohort I, 28.7% in cohort II, and 47.2% in cohort III. The disease control rate was 41.6% in cohort I, 61.2% in cohort II, and 73.6% in cohort III. Both overall response rate and disease control rate significantly increased by era.
Conclusions
We consider that advancements in systemic therapy, along with changes in treatment strategies, such as sequential therapy after progression, contribute to the prolonged prognosis across different eras.
Graphical Abstract
Abbreviations
-
- AFP
-
- α-fetoprotein
-
- BCLC
-
- Barcelona Clinic Liver Cancer
-
- EHM
-
- extrahepatic metastasis
-
- HCC
-
- hepatocellular carcinoma
-
- MTA
-
- molecular targeted agent
-
- MVI
-
- macroscopic vascular invasion
-
- OS
-
- overall survival
-
- TNM
-
- tumor node metastasis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related mortality worldwide.1 HCC commonly occurs in patients with chronic hepatitis or liver cirrhosis due to viral infection, alcohol use, or metabolic dysfunction-associated steatohepatits.2
Previously, the only treatment for patients with unresectable HCC was intrahepatic locoregional therapy with fluorouracil-based continuous infusion therapy through a venous port system3-6 or with cisplatin arterial infusion through a catheter,7, 8 but patients with metastatic HCC could not be treated. In the SHARP study9 and an Asia–Pacific study,10 sorafenib (SOR) was shown to be efficacious and well-tolerated in patients with advanced HCC. After 2008, the treatment strategy for HCC changed due to the availability of sorafenib, which enabled the treatment of patients with metastatic HCC. In 2017, lenvatinib (LEN) demonstrated a treatment effect on overall survival (OS) by statistical confirmation of non-inferiority when compared with SOR in the REFLECT study.11 Next, the combination of atezolizumab, a programmed cell death ligand 1 inhibitor, with bevacizumab showed longer OS and progression-free survival than SOR in the IMbrave 150 trial.12 Recently, combination therapy with durvalumab, a programmed cell death ligand 1 inhibitor, and tremelimumab, a cytotoxic T-lymphocyte-associated antigen 4 inhibitor, was approved for patients with unresectable HCC based on the results of the HIMALAYA study,13 and systemic treatment for unresectable HCC has changed to immune therapy. In a previous report,14 the prognosis of hepatitis B virus- and hepatitis C virus-related HCC patients by era group was compared. Following this study, we assessed the outcome of systemic therapy in Hiroshima University Hospital (Hiroshima, Japan) in each era to devise a new treatment strategy in the era of immunotherapy for HCC.
METHODS
Patients
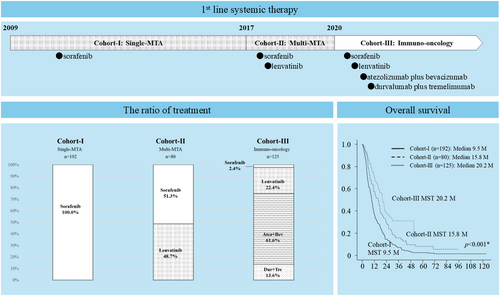
A total of 397 patients with unresectable HCC who were treated with SOR, lenvatinib (LEN), atezolizumab plus bevacizumab combination therapy (Atez + Bev), or durvalumab plus tremeliumab combination therapy (Dur + Tre) as first-line systemic therapy in our hospital from November 2009 to October 2023 were enrolled in the present retrospective cohort study. Their functional hepatic reserve score was Child–Pugh 5–7, and Eastern Cooperative Oncology Group performance status was 0 or 1. Unresectable HCC was defined as a liver cancer not eligible for liver resection given the disease extension, patients who were not suitable for resection because of the location of tumor, or who rejected surgery. We divided the years from 2009 to 2023 into the following three periods: cohort I, 2009–2016, the single-molecular targeted agent (MTA) era; cohort II, 2017–2020, the multi-MTA era; and cohort III, 2020–2023, the immuno-oncology era (Figure 1).
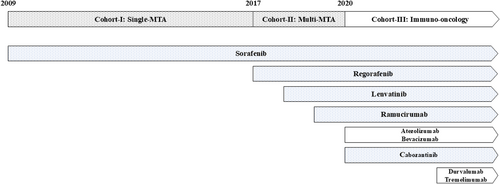
The history of systemic therapy for unresectable hepatocellular carcinoma divided into three eras. MTA, molecular targeted agent.
The present study was carried out in accordance with the ethical principles of the Declaration of Helsinki, and was approved by the Institutional Review Board of Hiroshima University. Written informed consent was obtained from each patient after a detailed explanation of the study procedures. Patients continued therapy until death or until they met one of the following criteria: (i) adverse events that required termination of treatment; (ii) deterioration of Eastern Cooperative Oncology Group performance status; (iii) worsening liver function; or (iv) withdrawal of consent.
Treatment regimens
Sorafenib
Patients received 400 mg SOR (Bayer AG) twice daily. Treatment interruptions and dose reductions were permitted for adverse drug reactions.
Lenvatinib
Patients received 8 mg/12 mg Len (Eisai) once daily based on bodyweight.
Atezolizumab plus bevacizumab combination therapy
The patients were treated with combination therapy with 1200 mg of atezolizumab (Chugai Pharmaceutical) plus 15 mg/kg bevacizumab (Chugai Pharmaceutical).
Durvalumab plus tremelimumab combination therapy
The patients were treated following the STRIDE regimen, comprising a single priming dose of tremelimumab (AstraZeneca) 300 mg and durvalumab (AstraZeneca plc) 1500 mg followed by durvalumab every 4 weeks.
Radiation therapy for macroscopic vascular invasion
After 2004, patients in our hospital with severe macroscopic vascular invasion (MVI) were treated with radiation therapy for MVI (39 Gy/13-Fr) in combination with hepatic arterial infusion chemotherapy before MTA therapy. In our hospital, the patients who had Vp 3, 4, or Vv 3 tumor thrombosis were principally administered radiation therapy + hepatic arterial infusion chemotherapy.
Statistical analysis
Univariate analysis was performed with the log-rank test using Fisher's exact test or the Kruskal–Wallis test. Variables were identified as significant if they had p-values <0.05 by univariate analysis. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), a graphical user interface for R (The R Foundation for Statistical Computing). More precisely, it is a modified version of R Commander designed to add statistical functions frequently used in biostatistics.
RESULTS
Patient background characteristics
The background characteristics of the 397 patients are summarized in Table 1.
| Variable | Cohort I | Cohort II | Cohort III | p-value |
|---|---|---|---|---|
| n = 192 | n = 80 | n = 125 | ||
| Regimen, SOR/LEN/Atez + Bev/Dur + Tre (n) | 192/0/0/0 | 41/39/0/0 | 3/28/77/17 | <0.001a |
| Age, range (years)b | 68 (20–86) | 72 (40–88) | 74 (37–92) | <0.001c |
| Sex, M/F (n) | 163/29 | 63/17 | 97/28 | 0.211a |
| Etiology, HBV/HCV/NBNC/B + C (n) | 62/78/49/3 | 14/29/36/1 | 15/43/67/0 | <0.001a |
| Serum albumin (range), g/dLb | 3.8 (2.2–4.9) | 3.6 (2.5–4.8) | 3.7 (2.4–4.9) | 0.007c |
| Serum total bilirubin, range (mg/dL) | 0.8 (0.3–2.7) | 0.8 (0.4–1.9) | 0.8 (0.3–1.9) | 0.841c |
| Prothrombin activity, range (%)b | 84 (18–114) | 87 (50–117) | 88 (23–129) | 0.003c |
| Ascites (none/mild/moderate) | 191/1/0 | 78/2/0 | 112/12/1 | 0.001a |
| Encephalopathy (none/mild/moderate) | 192/0/0 | 80/0/0 | 125/0/0 | NAa |
| Modified ALBI grade, 1/2a/2b (n) | 72/53/64/3 | 20/23/35/2 | 35/45/43/2 | 0.266a |
| Child–Pugh score, 5/6/7 (n) | 120/53/19 | 41/33/6 | 67/37/21 | 0.044a |
| No. intrahepatic tumors, ≤3/≥4 (n) | 85/107 | 28/52 | 67/58 | <0.001a |
| Macroscopic vascular invasion, absent/present (n) | 121/71 | 64/16 | 82/43 | 0.022a |
| Extrahepatic metastasis, absent/present (n) | 42/150 | 39/41 | 71/54 | <0.001a |
| TNM stage (III/IVa/IVb) | 35/38/119 | 29/20/31 | 56/33/36 | <0.001a |
| BCLC stage (B/C) | 29/163 | 27/53 | 46/79 | <0.001a |
| Tumor size relative to the liver, <50%/≥50% (n) | 150/42 | 71/9 | 113/12 | 0.006a |
| Serum α-fetoprotein, range (ng/mL)b | 238.5 (0.5–2 650 000) | 75.6 (0.5–1 647 200) | 42.9 (1.2–689 460) | 0.001c |
| Serum des-γ-carboxy prothrombin, range (mAU/mL)b | 1294.5 (0.5–4 376 200) | 1385.5 (13.0–1 083 990) | 637.0 (12.0–530 130) | 0.529c |
- Abbreviations: ALBI, albumin-bilirubin; Atez + Bev, atezolizumab plus bevacizumab combination therapy; BCLC, Barcelona Clinic Liver Cancer; F, female; HBV, hepatitis B virus; HCV, hepatitis C virus; LEN, lenvatinib; M, male; NBNC, non-B-non-C; SOR, sorafenib; TNM, tumor nodule metastasis.
- a Fisher's exact test.
- b Median.
- c Kruskal–Wallis test.
Cohort I: The single-MTA era
The median age of the 192 patients analyzed in this era was 68 years (range 20–86 years), and 163 patients (84.9%) were men. All patients were treated with SOR. Out of the 192 patients, 71 (37.0%) had MVI, and 150 (78.1%) had extrahepatic metastasis (EHM).
Cohort II: The multi-MTA era
The median age of the 80 patients analyzed in this era was 72 years (range 40–88 years) years, and 63 patients (78.8%) were men. Approximately half (39; 48.8%) of the patients were treated with LEN, and 41 (51.3%) patients were treated with SOR. Out of the 80 patients, 16 (20.0%) had MVI, and 41 (51.3%) had EHM.
Cohort III: The immuno-oncology era
The median age of the 125 patients analyzed in this era was 74 years (range 37–93 years), and 97 patients (77.6%) were men. Three (2.4%) patients were treated with SOR, 28 patients with LEN (22.4%), 77 with Atez + Bev (61.6%), and 17 with Dur + Tre (13.6%). Out of the 125 patients, 43 (34.4%) had MVI and 54 (43.2%) had EHM.
Differences in patient backgrounds in each cohort
The median age of the patients was 68, 72, and 74 years in cohort I, II, and III, respectively. Patient age was significantly (p < 0.001) higher in cohort III. The ratios of patients with non-B-non-C (NBNC) hepatitis were 25.5%, 45.0%, and 53.6% in cohort I, II, and III, respectively. The ratio of NBNC-HCC increased. The ratios of the patients with Child–Pugh score 7 hepatic reserve were 9.9%, 7.5%, and 16.8% in cohort I, II, and III respectively. Hepatic functional reserve was significantly (p = 0.044) worse in cohort III. Clinical stage and tumor markers were higher in the earlier eras (Table 2).
| Cohort I | Cohort II | Cohort III | p-valuea | |
|---|---|---|---|---|
| mRECIST | ||||
| CR | 1 (0.5%) | 5 (6.2%) | 6 (4.8%) | |
| PR | 7 (3.6%) | 18 (22.5%) | 53 (42.4%) | |
| SD | 72 (37.5%) | 26 (32.5%) | 33 (26.4%) | |
| PD | 94 (49.0%) | 26 (32.5%) | 28 (22.4%) | |
| NE | 18 (9.4%) | 5 (6.2%) | 5 (4.0%) | |
| ORR | 8 (4.1%) | 23 (28.7%) | 59 (47.2%) | <0.001 |
| DCR | 80 (41.6%) | 49 (61.2%) | 92 (73.6%) | <0.001 |
| RECIST | ||||
| CR | 1 (0.5%) | 1 (1.2%) | 0 | |
| PR | 5 (2.6%) | 13 (16.2%) | 43 (34.4%) | |
| SD | 74 (38.5%) | 33 (41.2%) | 50 (40.0%) | |
| PD | 94 (49.0%) | 27 (33.8%) | 30 (24.0%) | |
| NE | 18 (9.4%) | 6 (7.5%) | 2 (1.6%) | |
| ORR | 6 (3.1%) | 14 (17.4%) | 43 (34.4%) | <0.001 |
| DCR | 80 (41.6%) | 47 (58.6%) | 93 (74.4%) | <0.001 |
- Abbreviations: CR, complete response; DCR, disease control rate; mRECIST, Modified Response Evaluation Criteria in Solid Tumors; NE, not evaluated; ORR, over all response rate; PD, progression disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
- a Fisher's exact test.
Overall survival
The median survival time (MST) was 9.5 months in cohort I, 15.8 months in cohort II, and 20.2 months in cohort III. The MST in cohort III was significantly (p < 0.001) longer than in the other eras (Figure 2a). In patients with Child–Pugh score 7 liver function, the MST was 6.3 months in cohort I, 6.9 months in cohort II, and 7.1 months in cohort III, and there were no significant differences among eras (Figure 2b). In the Barcelona Clinic Liver Cancer (BCLC) stage B group, the MST was 9.6 months in cohort I, 17.9 months in cohort II, and 20.1 months in cohort III, which was significantly longer than in earlier eras (p < 0.001; Figure 2c). In the BCLC stage C group, the MST was 9.5 months in cohort I, 12.8 months in cohort II, and 21.2 months in cohort III, with MST again significantly longer in cohort III (p < 0.001). In the MVI present group, the MST was 8.3 months in cohort I, 11.1 months in cohort II, and 23.6 months in cohort III. The MST in cohort III was significantly (p < 0.001) longer than the other cohorts (Figure 2e).
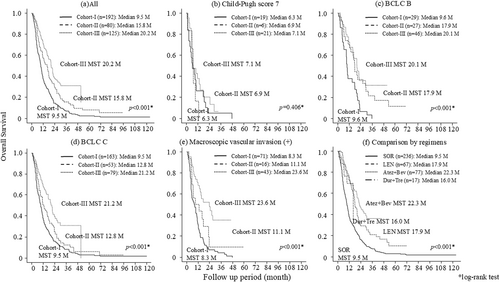
The overall survival of the patients by cohort; (a) all patients, (b) among ChildPugh 7 patients, (c) among BCLC B patients, (d) among BCLC C patients, (e) among patients with macroscopic vascular invasion. (f) The over all survival comparison by regimens. Atez + Bev, atezolizumab plus bevacizumab combination therapy; BCLC C, Barcelona Clinic Liver Cancer stage C; Dur + Tre, durvalumab plus tremeliumab combination therapy; LEN, lenvatinib; M, months; MST, median survival time; SOR, sorafenib.
Figure 2f shows comparison by regimens, the MST of SOR, LEN, Atez + Bev, and Dur + Tre were 9.5, 17.9, 22.3, and 16.0 months, respectively. There were significant differences between SOR and the other regimens; however, there were no significant differences among LEN, Atez + Bev, and Dur + Tre.
Best response
The overall response rate (ORR) by mRECIST was 4.1% in cohort I, 28.7% in cohort II, and 47.2% in cohort III. The disease control rate was 41.6% in Cohort-I, 61.2% in cohort II, and 73.6% in cohort III. The ORR by RECIST was 3.1%, 17.4%, and 34.4% in cohort I, cohort II, and cohort III, respectively, whereas disease control rate by RECIST was 41.6%, 58.6%, and 74.4% in cohort I, II, and III, respectively. Both the ORR and disease control rate became higher by era.
Treatment duration
The median treatment durations were 5.1 months for cohort I, 4.5 months for cohort II, and 6.2 months for cohort III.
Sequential therapy
The rate of sequential transition in discontinued cases was three out of 189 cases (1.5%) for cohort I, 45 out of 80 cases (56.3%) for cohort II, and 59 out of 118 cases (50.0%) for cohort III.
In cohort I, all three cases followed a sequential regimen from SOR to regorafenib.
In cohort II, among patients who received SOR as their first-line treatment, 16 (35.6%) were subsequently treated with regorafenib, and three (15.6%) with LEN as second-line therapies. For those initially treated with lenvatinib, the number of patients who received SOR, Atez + Bev, ramucirumab, and regorafenib as second-line options were 16 (35.6%), eight (15.6%), two (4.4%), and one (2.2%), respectively.
In cohort III, patients who started with LEN received second-line treatments with Atez + Bev in 10 cases (16.9%), Dur + Tre in two cases (3.4%), cabozantinib in two cases (3.4%), and both SOR and ramucirumab in one case each (1.7%). For patients initially treated with Atez + Bev, second-line regimens included LEN in 27 cases (45.8%), Dur + Tre in seven cases (11.9%), and ramucirumab in one case (1.7%).
DISCUSSION
In the present study, we analyzed the history of systemic therapy in our institutions. We divided this 15-year span into three eras, as follows: cohort I, the era of single-MTA; cohort II, the era of multi-MTA; and cohort III, the era of immno-oncology.
There were many differences in the backgrounds of the patients in each era. The age of the patients increased in each era. A previous report determined that there were no significant differences in the incidence of severe adverse effects and therapeutic effects between elderly and non-elderly patients treated with SOR or LEN.15 Therefore, we should decide patient eligibility based on hepatic functional reserve and general condition instead of age.
The ratio of patients with HCC derived from NBNC hepatitis increased between cohort I and cohort III. In Japan, the percentage of patients with HCV-induced HCC was 70% in the 1990s, but the incidence has decreased over time.16 Nagaoki et al. reported that the ratio of NBNC-HCC increased from 26.5% in 2009 to 46.3% in 201817 because of precautions against hepatitis C virus and hepatitis B virus infection, and the evolution of antiviral drugs.
The ratio of patients with BCLC B stage HCC treated with systemic therapy increased. The patients with BCLC B stage HCC were eligible for transarterial chemoembolization, but it is known that repeated transarterial chemoembolization leads to deterioration of hepatic functional reserve.18 It has been reported that initiation of systemic therapy leads to a favorable prognosis before deterioration of functional reserve induced by repeated transarterial chemoembolization.18
For patients with Child–Pugh B liver function, MST of patients with Child–Pugh 7 in cohort III in the present study was 7.1 months, which was a longer prognosis than the previous eras, although the difference was not significant among eras. Our previous study reported that the patients treated with Atez + Bev had significantly better progression-free survival than patients treated with LEN and SOR among patients with Child–Pugh B liver function.19 However, in the Child–Pugh B group, the effect of systemic therapy remains unclear, more studies are needed.
In cohort II, MST was longer than in cohort I, with an MST of 9.5 months in cohort I and 15.8 months in cohort II. We conclude from this that LEN and sequential therapy improved the prognosis. In cohort III, MST was 20.2 months, which suggests that immune checkpoint inhibitors improved the prognosis further. The ORR was 4.1% in era I, similar to the SHARP study9 and the Asia–Pacific study,10 suggesting that SOR might improve MST, but not lead to tumor shrinkage. After the era of cohort II, patients treated with LEN, sequential therapy, and immune checkpoint inhibitors experienced tumor shrinkage at a higher rate; the ORR was 28.7% in cohort II and 47.2% in cohort III. In real-world practice, several reports said that sequential therapy and immune checkpoint inhibitors improved the prognosis of HCC patients.20-25
The prognosis for advanced cases of HCC with vascular invasion, which is considered a poor prognostic factor, has been improving. In addition, there is no significant difference in prognosis when comparing LEN, Atez + Bev and Dur + Tre. In the present study, the rate of sequential transition in discontinuation cases were higher in cohorts II and III than cohort I. The reason for improvement in prognosis is not only improvement in regimens or admission of systemic drug therapy at earlier stages, but also the change in treatment strategy, switching to the next therapy even after disease progression, so-called sequential therapy.
The present study had some limitations in that this research was retrospective and performed only in our institution.
We assessed the efficacy of treatment for patients with unresectable HCC in our hospital. The prognosis was improved as the eras progressed because of changes in treatment strategies, although there were many differences in the backgrounds of the patients, and the drugs for systemic therapy improved.
ACKNOWLEDGMENTS
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
Tomokazu Kawaoka received honoraria (lecture fees) from Chugai Pharmaceutical and AstraZeneca. The other authors declare no Conflict of Interests for this article.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: This study was conducted in accordance with the ethics principles of the Declaration of Helsinki, and was approved by the Institutional Review Board of Hiroshima University (IRB number 695).
Informed consent: Written informed consent was obtained from each patient after a detailed explanation of the study procedure.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.