Efficacy and safety of apararenone (MT-3995) in patients with nonalcoholic steatohepatitis: A randomized controlled study
Abstract
Aim
To evaluate the efficacy, safety, and tolerability of apararenone 10 mg/day in patients with nonalcoholic steatohepatitis (NASH).
Methods
In this multicenter, randomized, double-blind, placebo-controlled phase II study, patients received apararenone 10 mg or placebo once daily for 72 weeks. The primary efficacy end-point was percent change in serum alanine aminotransferase (ALT) from baseline to 24 weeks after randomization. Secondary efficacy end-points included changes in liver fibrosis markers. Adverse drug reactions (ADRs) and serum potassium levels were evaluated.
Results
Forty-eight patients were randomly assigned to treatment (placebo, 23; apararenone, 25). The percent change in ALT at 24 weeks was −3.0% and −13.7% with placebo and apararenone, respectively (p = 0.308). The apararenone group showed greater reductions from baseline in fibrosis markers (type IV collagen 7S and procollagen-3 N-terminal peptide) and noninvasive tests of fibrosis (enhanced liver fibrosis score and Fibrosis-4 index) at all time points versus placebo. The percentage of patients with improvement of 1 point or more in fibrosis stage/without nonalcoholic fatty liver disease activity score worsening was 41.7% with apararenone and 26.1% with placebo (p = 0.203). Adverse drug reactions were reported in three (13.0%) and three (12.5%) patients in the placebo and apararenone groups, respectively. Serum potassium levels increased in the apararenone group during the study and decreased to near baseline after the end of treatment.
Conclusions
In patients with NASH, apararenone 10 mg/day for 72 weeks was effective in decreasing ALT levels, improved multiple potential fibrosis markers, and was safe and well tolerated. Pathological findings showed anti-inflammatory and antifibrotic effects of apararenone.
Abbreviations
-
- ADR
-
- adverse drug reaction
-
- AE
-
- adverse event
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- eGFR
-
- estimated glomerular filtration rate
-
- ELF
-
- enhanced liver fibrosis
-
- FAS
-
- full analysis set
-
- FIB-4
-
- fibrosis-4
-
- GGT
-
- gamma-glutamyl transpeptidase
-
- HCC
-
- hepatocellular carcinoma
-
- LS
-
- least-squares
-
- MMRM
-
- mixed model repeated measures
-
- MR
-
- mineralocorticoid receptor
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NAS
-
- NAFLD activity score
-
- NASH
-
- nonalcoholic steatohepatitis
-
- P3NP
-
- procollagen-3 N-terminal peptide
-
- PNPLA3
-
- phospholipase domain-containing protein 3
-
- REML
-
- restricted maximum likelihood method
-
- SD
-
- standard deviation
-
- SNP
-
- single nucleotide polymorphism
-
- TE
-
- transient elastography
INTRODUCTION
Nonalcoholic fatty liver disease encompasses a clinical and histological spectrum of diseases that ranges from nonalcoholic fatty liver, which rarely progresses to cirrhosis and HCC, and NASH, which could progress to cirrhosis or HCC.1 In Japan, the estimated prevalence of patients with NAFLD is 29.7% of the population and the prevalence of patients with NASH is estimated to be 1.9%–2.7%.2
There are no specific symptoms or physical signs in patients with NASH, although subjective symptoms, such as fatigue and insomnia, have been reported and could reduce the quality of life. Liver biopsy is required for a definitive diagnosis of NASH.1, 3, 4
The histological features of NASH are steatosis, inflammation, and hepatocellular ballooning with or without fibrosis. A 5–10-year follow-up study reported that 5%–20% of patients with NASH progressed to cirrhosis, and the rate of liver carcinogenesis from NASH cirrhosis was 11.3% at 5 years.5 In patients with NASH, known risk factors for the progression of fibrosis include diabetes,6 insulin resistance,7 and obesity.8
Multiple drugs have shown efficacy in preclinical NASH models. Some of these drugs are currently being studied in clinical trials,2, 9 but no drug has yet been approved. Mineralocorticoid receptor antagonists have shown anti-inflammatory and antifibrotic effects in NASH animal models.10, 11
Apararenone (MT-3995) is a nonsteroidal compound with highly selective MR antagonistic activity.12-14 Apararenone has been evaluated in several clinical studies in healthy adults; its safety and pharmacokinetic profiles have been confirmed,12-14 and it was well tolerated in healthy subjects and in patients with diabetic nephropathy.14
In an animal model of metabolic syndrome (SHR/NDmicro-cp), repeated administrations with oral apararenone at a dose range of 1–10 mg/kg was reported to show anti-inflammatory and antifibrotic effects with cardiovascular and renal protection. Similarly, repeated administrations with oral apararenone at doses of 1–10 mg/kg inhibited the development of hepatic fibrosis in a NASH model of rats (Mitsubishi Tanabe Pharma Corp., 2014 and 2015).15 In addition, repeated administrations with oral apararenone at a dose of 30 mg/kg improved inflammatory markers in trans-fat diet-loaded mice (Mitsubishi Tanabe Pharma Corp., 2016).16 These animal studies suggest that apararenone could have the potential to improve hepatic fibrosis in patients with NASH.
The present study was designed to evaluate the efficacy, safety, and tolerability of apararenone at 10 mg/day in Japanese patients with NASH.
METHODS
Study design and treatment
This multicenter, randomized, double-blind, placebo-controlled phase II study was undertaken at 20 centers in Japan from September 2016 to April 2019. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and in compliance with Japanese legal and regulatory requirements, and was approved by the institutional review boards of each participating center.
The study consisted of a preobservation period (10 weeks), a treatment period (72 weeks, double-blind period), and a follow-up period (8 weeks) (Figure S1). Patients were treated with apararenone 10 mg or placebo once daily for 72 weeks. As this was the first clinical study of apararenone in patients with NASH, the highest dose of apararenone used in a previous study of patients with diabetic nephropathy (10 mg/day)14 was selected to confirm the effect of apararenone in patients with NASH.
Patients were randomly assigned to either the apararenone 10 mg group or placebo group using a permuted block method. The investigational products were indistinguishable in appearance. Patients were stratified according to serum ALT levels at 2 weeks before randomization (ALT level 60–90 or ≥91 U/L) and Kleiner fibrosis stage17 at 7 weeks before randomization (stage 2 or stage 3).
Patients
The key inclusion criteria were Japanese patients aged 20–75 years who were histologically diagnosed with NASH (per the criteria of the NASH Clinical Research Network17). Patients who had both NAS of 4 or higher and Kleiner fibrosis stage 2 or 3, as confirmed by central pathology review of liver biopsy specimens obtained at 7 weeks before randomization, were included. Patients undergoing diet or exercise therapy at commencement of the preobservation period and who complied with the restrictions for the concomitant use of therapy were included. Diet or exercise therapies that were ongoing at the start of the run-in period were required to remain unchanged, wherever possible, until the end of the treatment period. Patients who had ALT 60–300 U/L at both 10 and 2 weeks before randomization, and who were undergoing diet or exercise therapy at commencement of the preobservation period, were also included. The key exclusion criteria were BMI 40 kg/m2 or more, and patients whose daily dose of insulin preparation exceeded 60 units. A complete list of inclusion and exclusion criteria is included in the Document S1.
Efficacy
The primary efficacy end-point was the percent change in ALT from baseline to 24 weeks after randomization. The secondary efficacy end-points were: (i) percent change and/or absolute change from baseline in ALT, AST, FIB-4 index, and type IV collagen 7S at each assessment time point (including at 24 weeks after randomization) and up to 72 weeks; (ii) change from baseline in ELF score and P3NP up to 72 weeks; (iii) pathological findings at 72 weeks after randomization (including the percentages of the following: patients with improvement in each item of the NAS, patients in whom hepatocellular ballooning disappeared with an inflammatory cell infiltration score of 0–1 point, patients with improvement in Matteoni’s classification to type 1 or type 2, patients without worsening of Kleiner fibrosis stage, patients with improvement of 1 point or more in Kleiner fibrosis stage [post hoc analysis], patients with improvement of 1 point or more in Kleiner fibrosis stage without worsening of NAS [post hoc analysis], patients with improvement of 2 points or more in NAS and without worsening of Kleiner fibrosis stage); (iv) change in other biomarkers (v) change in body weight, and BMI from baseline to 72 weeks, change from baseline in liver stiffness at each time point, as measured by TE.
The AST and ALT levels were used as markers of cell damage that is suggested to be related to inflammation,18 and type IV collagen 7S,19, 20 FIB-4 index,21 P3NP,22 and ELF score23, 24 were used to assess improvement in liver fibrosis. The results of pathological findings at 72 weeks were also used as markers of inflammation and fibrosis. Liver biopsies were evaluated centrally by an independent committee comprising two pathologists.
Pharmacogenomics testing
It has been reported that SNPs of the PNPLA3 gene correlate with the onset and development of NASH.25, 26 We undertook SNP genotyping to explore the relationship between the PNPLA3 SNP, rs738409, and response to apararenone. Blood samples were collected 2 weeks after randomization. Genotyping was undertaken by LSI Medience Corporation.
Safety and tolerability
Adverse events, ADRs, serum potassium levels, and eGFR were evaluated for safety. Adverse events were tabulated using the Medical Dictionary for Regulatory Activities (version 19.1) Preferred Term and System Organ Class.
Statistical methods
The target sample size was 40 patients (20 per group). Efficacy was assessed in the FAS, which included all randomly assigned patients except for patients without a diagnosis of NASH, not receiving investigational product, or without efficacy data available. Safety was assessed in the safety analysis set, which included all randomly assigned patients except for those not receiving apararenone or without safety data available.
Descriptive statistics were used for baseline demographic and clinical characteristics. Categorical variables were described using n (%), and continuous variables using mean (SD) and median (range). Descriptive statistics and the percent change in ALT at 24 weeks were presented by treatment group. The log-transformed ALT was analyzed for percent change using the REML (MMRM). The LS mean for each group, the between-group difference in the LS mean values, and the 95% CI and p-values are shown at 24 weeks after randomization (primary end-point) and at each time point (secondary end-points). The percent change in AST, ALT, and FIB-4 index at each time point was analyzed in the same manner as the primary endpoint. Descriptive statistics by time point and by treatment group were calculated for AST, ALT, and type IV collagen 7S. Mean changes in AST, ALT, and type IV collagen 7S were analyzed using the REML (MMRM) method. The ELF score, P3NP, ferritin, hyaluronic acid, tissue inhibitor of metalloproteinase-1, and FM-fibro index at each time point were analyzed in the same manner as above. Descriptive statistics by time point and by treatment group were calculated for other efficacy end-points. For pathological findings, 72 weeks after randomization, n (%), 95% CIs, and the odds ratio (using logistic regression analysis) for the apararenone group versus placebo group were calculated.
It has been reported that various factors affect the progression and suppression of natural course in NASH.18, 24, 27, 28 After unblinding of the data, post hoc univariate and multivariate analyses were undertaken to investigate the presence of factors that affect the improvement of fibrosis when using the FIB-4 index as a potential marker of liver fibrosis. For multivariate analysis, factors were extracted using the forward and backward methods. Details of the statistical analyses are described in Document S1.
All tests were two-sided, with a significance level of 5%, and CIs were two-sided with a confidence factor of 95%. SAS for Windows (version 9.4; SAS Institute) was used for the statistical analysis.
RESULTS
Patients
The disposition of patients is shown in Figure S2. Informed consent was obtained from 152 patients; 145 began the preobservation period, and 97 discontinued during preobservation. Forty-eight patients (placebo, n = 23; apararenone, n = 25) were randomly assigned to treatment (Table S1). Five patients discontinued after randomization (placebo, n = 2; apararenone, n = 3); one patient in the apararenone group discontinued before starting the treatment period, and all four remaining patients discontinued due to AEs. Overall, 47 patients (placebo, n = 23; apararenone, n = 24) received treatment, of whom 43 (91.5%; placebo, n = 21; apararenone, n = 22) completed the treatment period.
Patient demographic and clinical characteristics are shown in Table 1, and were generally well-balanced between treatment groups. The respective percentages of patients in the placebo and apararenone groups with type 2 diabetes (34.8% and 50.0%), dyslipidemia (56.5% and 62.5%), or hypertension (52.2% and 66.7%) was consistent with NASH epidemiology.1 The ratio of patients with Kleiner fibrosis stage 2 : stage 3 was 6:4. The percentage of patients with the PNPLA3 GG genotype was 39.1% in the placebo group and 58.3% in the apararenone group.
| Placebo | Apararenone | Total | |
|---|---|---|---|
| n = 23 | n = 24 | n = 47 | |
| Sex (male) | 13 (56.5) | 12 (50.0) | 25 (53.2) |
| Age, years | 53 (31–75) | 52 (40–67) | 53 (31–75) |
| ≥50 | 13 (56.5) | 14 (58.3) | 27 (57.4) |
| Weight, kg | 76.7 (51.8–100.5) | 81.1 (45.6–108.3) | 78.2 (45.6–108.3) |
| BMI, kg/m2 | 29.3 (21.8–36.3) | 30.2 (21.7–39.8) | 29.7 (21.7–39.8) |
| Complications | |||
| Type 2 diabetes | 8 (34.8) | 12 (50.0) | 20 (42.6) |
| Dyslipidemia | 13 (56.5) | 15 (62.5) | 28 (59.6) |
| Hypertension | 12 (52.2) | 16 (66.7) | 28 (59.6) |
| ALT, U/L | 90 (64–168) | 103 (49–174) | 93 (49–174) |
| AST, U/L | 73 (41–124) | 74 (28–123) | 73 (28–124) |
| AST/ALT ratio | 0.7 (0.6–1.1) | 0.7 (0.6–1.0) | 0.7 (0.6–1.1) |
| GGT, U/L | 105 (32–373) | 84 (33–423) | 92 (32–423) |
| Platelets, 104/μl | 19.7 (11.9–29.9) | 17.8 (10.2–29.1) | 19.5 (10.2–29.9) |
| HbA1c, % | 6.3 (5.0–8.2) | 6.0 (5.2–8.2) | 6.1 (5.0–8.2) |
| Fasting blood glucose, mg/dl | 114 (95–250) | 117 (81–213) | 114 (81–250) |
| Fasting insulin, µU/ml | 22.70 (8.24–83.15) | 20.27 (4.52–104.80) | 20.59 (4.52–104.80) |
| HOMA-IR, µU⋅mg/ml⋅dl/405, blood glucose ≤140 | 5.1 (2.1–13.2) | 5.4 (0.9–19.2) | 5.2 (0.9–19.2) |
| (n = 19) | (n = 18) | (n = 37) | |
| Total cholesterol, mg/dl | 196 (142–319) | 192 (144–304) | 196 (142–319) |
| Triglycerides, mg/dl | 168 (59–733) | 123 (67–725) | 149 (59–733) |
| HDL cholesterol, mg/dl | 43 (26–72) | 48 (28–74) | 47 (26–74) |
| LDL cholesterol, mg/dl | 112 (84–231) | 120 (80–217) | 117 (80–231) |
| eGFR, ml/min/1.73 m2 | 83 (50–120) | 84 (66–131) | 84 (50–131) |
| Serum potassium, mmol/L | 4.1 (3.5–4.3) | 4.1 (3.5–4.4) | 4.1 (3.5–4.4) |
| Kleiner fibrosis stage 2 | 14 (60.9) | 15 (62.5) | 29 (61.7) |
| Kleiner fibrosis stage 3 | 9 (39.1) | 9 (37.5) | 18 (38.3) |
| NAS 4 | 2 (8.7) | 2 (8.3) | 4 (8.5) |
| NAS 5 | 5 (21.7) | 4 (16.7) | 9 (19.1) |
| NAS 6 | 9 (39.1) | 11 (45.8) | 20 (42.6) |
| NAS 7 | 4 (17.4) | 5 (20.8) | 9 (19.1) |
| NAS 8 | 3 (13.0) | 2 (8.3) | 5 (10.6) |
| TE (FibroScan), kPa | 8.7 (4.1–21.3) | 11.3 (6.5–22.3) | 10.8 (4.1–22.3) |
| n = 15 | n = 15 | n = 30 | |
| PNPLA3 genotype | |||
| GG | 9 (39.1) | 14 (58.3) | 23 (48.9) |
| CG | 10 (43.5) | 7 (29.2) | 17 (36.2) |
| CC | 4 (17.4) | 3 (12.5) | 7 (14.9) |
| Type IV collagen 7S, ng/ml | 5.8 (4.4–7.8) | 5.9 (3.0–10.2) | 5.8 (3.0–10.2) |
| FIB-4 index | 1.8 (0.9–6.3) | 2.0 (0.8–4.3) | 2.0 (0.8–6.3) |
| IL-6, pg/ml | 2.570 (0.699–247.000) | 1.735 (0.748–7.470) | 2.130 (0.699–247.000) |
| Ferritin, ng/ml | 302.0 (10.3–1210.0) | 316.5 (32.5–2230.0) | 302.0 (10.3–2230.0) |
| TNF-α, pg/ml | 3.38 (1.84–12.80) | 2.77 (1.26–4.59) | 3.18 (1.26–12.80) |
| Hyaluronic acid, ng/ml | 83.9 (21.6–493.0) | 100.9 (13.8–876.0) | 91.1 (13.8–876.0) |
| P3NP, ng/ml | 15.50 (9.61–35.70) | 14.90 (8.26–23.20) | 15.20 (8.26–35.70) |
| TIMP-1, ng/ml | 298 (194–382) | 255 (184–329) | 264 (184–382) |
| M2BPGi | 0.98 (0.46–3.86) | 0.97 (0.46–3.45) | 0.97 (0.46–3.86) |
| ELF score | 10.1 (8.9–12.3) | 10.4 (8.4–12.6) | 10.3 (8.4–12.6) |
| FM-fibro index (HA, COL4-7S) | 0.58 (0.41–0.89) | 0.62 (0.27–0.91) | 0.60 (0.27–0.91) |
- Note: Data are presented as n (%) or median (range).
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; COL4-7S, type IV collagen 7S; eGFR, estimated glomerular filtration rate; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4; GGT, γ-glutamyl transpeptidase; HA, hyaluronic acid; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model of assessment for insulin resistance; IL-6, interleukin-6; LDL, low-density lipoprotein; M2BPGi, Mac-2 binding protein glycan isomer; NAS, nonalcoholic fatty liver disease activity score; P3NP, procollagen-3 N-terminal peptide; PNPLA3, patatin-like phospholipase domain-containing protein 3; TE, transient elastography; TIMP-1, tissue inhibitor of metalloproteinase-1; TNF-α, tumor necrosis factor-α.
Efficacy
Primary end-point
The percent change in ALT from baseline to 24 weeks was −3.0% in the placebo group and −13.7% in the apararenone group. The treatment ratio for this change (ratio of ALT level in the apararenone group at 24 weeks to a baseline value of 1 [0.863] / ratio of ALT level in the placebo group at 24 weeks to a baseline value of 1 [0.970] [95% CI]) was 0.890 (0.708–1.118), indicating a −11.0% (p = 0.308) decrease in ALT from baseline in the apararenone group compared with the placebo group (relative percent change) at 24 weeks.
Secondary end-points
The percent change in ALT from baseline to each assessment time point up to 72 weeks is shown in Figure 1a. The relative percent change in ALT from baseline to 48 and 72 weeks was −15.9% and −23.6% in the placebo group, and −26.4% and −33.2% in the apararenone group. Although the differences between the groups were not statistically significant, reductions in ALT in the apararenone group were greater than those in the placebo group at all time points except for 4 weeks after randomization onwards, and ALT consistently decreased over time throughout the evaluation from 8 weeks after randomization onwards. The LS mean change in ALT from baseline (95% CI) to 24 weeks was −0.6 (−17.2–15.9) U/L in the placebo group and −12.1 (−28.1–4.0) U/L in the apararenone group (between-group difference [95% CI], −11.4 [−34.5–11.6]; p = 0.322).
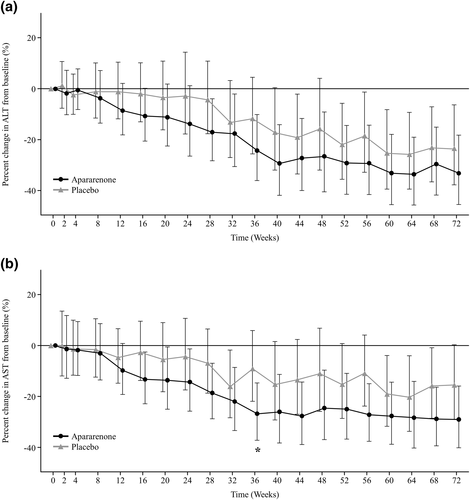
Percent change in (a) alanine aminotransferase (ALT) and (b) aspartate aminotransferase (AST) from baseline to each assessment time point up to week 72 of treatment with apararenone or placebo in patients with nonalcoholic steatohepatitis. Data are shown as geometric least-squares mean (95% confidence interval) (mixed-effect model repeated measure). *p < 0.05
The percent change in AST from baseline to each assessment time point up to 72 weeks is shown in Figure 1b. The percent change in AST from baseline to 24, 48, and 72 weeks was −4.4%, −11.0%, and −15.5% in the placebo group and −14.4%, −24.5%, and −29.0% in the apararenone group, respectively. Reductions in AST in the apararenone group were greater than those in the placebo group at all time points, and AST consistently decreased over time throughout the evaluation. The LS mean change in AST from baseline (95% CI) to 24 weeks was −2.3 (−13.9–9.2) U/L in the placebo group and −10.2 (−21.4–1.0) U/L in the apararenone group (between-group difference [95% CI], −7.8 [−23.9–8.2]; p = 0.329).
The percent change in FIB-4 index from baseline to 24 weeks was 4.8% in the placebo group and −8.5% in the apararenone group (between-group ratio, −12.8%; p = 0.103). The LS mean change in ELF score from baseline (95% CI) to 72 weeks was −0.06 (−0.35 to 0.23) in the placebo group and −0.51 (−0.80 to −0.23) in the apararenone group (between-group difference [95% CI], −0.45 [−0.85–−0.05]; p = 0.027). The percent change in FIB-4 index and the LS mean change in ELF score from baseline was greater in the apararenone group versus the placebo group at all time points (Figure 2a,b).
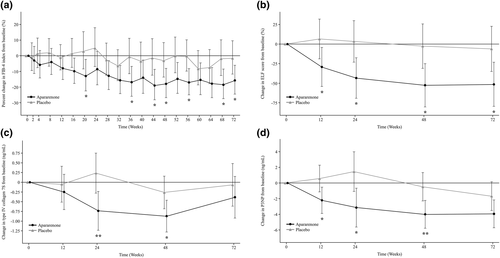
Percent change in (a) fibrosis-4 (FIB-4) index and change in (b) enhanced liver fibrosis (ELF) score, (c) type IV collagen 7S, and (d) procollagen-3 N-terminal peptide (P3NP) from baseline to week 72 of treatment with apararenone or placebo in patients with nonalcoholic steatohepatitis. Data are shown as geometric least-squares mean (95% confidence interval) (mixed-effect model repeated measure). *p < 0.05, **p < 0.01
The change in type IV collagen 7S (95% CI) from baseline to 24 weeks was 0.24 (−0.28 to 0.75) ng/ml in the placebo group and −0.74 (−1.23 to −0.24) ng/ml in the apararenone group (Figure 2c); the between-group difference was −0.97 (−1.68 to −0.26) ng/ml (p = 0.008). The change from baseline in type IV collagen 7S in the apararenone group decreased over time up to 48 weeks. At all times points, the reduction in type IV collagen 7S from baseline was greater in the apararenone group versus the placebo group.
The LS mean change in P3NP from baseline (95% CI) to 72 weeks was −1.664 (−3.489 to 0.161) ng/ml in the placebo group and −3.930 (−5.714 to −2.146) ng/ml in the apararenone group (between-group difference [95% CI], −2.266 [−4.795 to 0.263] ng/ml; p = 0.078). The change in P3NP from baseline to each assessment time point was greater in the apararenone group compared with the placebo group up to 72 weeks (Figure 2d).
Histological findings
The changes in liver histological features after 72 weeks of treatment are shown in Table 2. Regarding each item of NAS, the percentage of patients whose score improved by 1 point or more at 72 weeks for lobular inflammation and hepatocellular ballooning was higher in the apararenone group than in the placebo group. The percentage of patients with improvement of 1 point or more in Kleiner fibrosis stage and without worsening of NAS was greater in the apararenone group versus the placebo group. The percentage of patients with improvement of 2 points or more in NAS and without worsening of Kleiner fibrosis stage was slightly higher in the placebo group versus the apararenone group (Table 2 and Figure 3). The results of histological findings did not yield any statistically significant differences between the groups, which is likely due to the small sample size of this exploratory study.
| Placebo (n = 23) | Apararenone (n = 24) | |||
|---|---|---|---|---|
| n (%) | Odds ratio (95% CI) | p-value | ||
| Patients with improvement in NAS by ≥2 points without worsening of Kleiner fibrosis stage | 6 (26.1) | 5 (20.8) | 0.789 (0.199–3.129) | 0.736 |
| ≥1 point improvement in steatosis | 7 (30.4) | 5 (20.8) | 0.704 (0.176–2.809) | 0.619 |
| ≥1 point improvement in lobular inflammation | 9 (39.1) | 14 (58.3) | 2.188 (0.662–7.228) | 0.199 |
| ≥1 point improvement in hepatocellular ballooning | 10 (43.5) | 13 (54.2) | 1.448 (0.447–4.685) | 0.537 |
| Percentage of patients in whom hepatocellular ballooning disappeared with an inflammatory cell infiltration score of 0–1 point | 1 (4.3) | 3 (12.5) | 2.577 (0.232–28.619) | 0.441 |
| Percentage of patients with an improvement in Matteoni’s classification to type 1 or type 2 | 0 (0.0) | 0 (0.0) | – | – |
| Without worsening of Kleiner fibrosis stage | 17 (73.9) | 18 (75.0) | 1.099 (0.279–4.333) | 0.893 |
| ≥1 point improvement in Kleiner fibrosis stage | 7 (30.4) | 11 (45.8) | 1.930 (0.570–6.542) | 0.291 |
| ≥1 point improvement in Kleiner fibrosis stage and without worsening of NAS | 6 (26.1) | 10 (41.7) | 2.293 (0.640–8.221) | 0.203 |
- Note: Data are presented as n (%) unless otherwise stated.
- Abbreviations: CI, confidence interval; NAS, nonalcoholic fatty liver disease activity score.
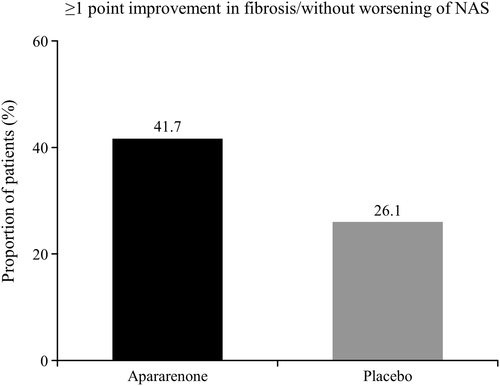
Percentage of patients with nonalcoholic steatohepatitis with improvement of 1 point or more in fibrosis by Kleiner fibrosis stage and without worsening of nonalcoholic fatty liver disease activity score (NAS) following treatment with apararenone or placebo
Other efficacy end-points
The results of changes in other biomarkers from baseline to 72 weeks are shown in Table 3. The change in liver stiffness, as measured by TE, tended to be lower in the apararenone group versus the placebo group at all time points except 24 weeks after randomization. In the apararenone group, the change in liver stiffness decreased over time. At all time points, the apararenone group experienced greater reductions in body weight and BMI changes from baseline compared with the placebo group.
| LS mean (95% CI) | |||
|---|---|---|---|
| MMRM | Placebo (n = 23) | Apararenone (n = 24) | Between-group LS mean difference (95% CI) |
| Ferritin, ng/ml | −143.44 (−283.34 to −3.55) | −165.58 (−302.08 to −29.09) | −22.14 (−217.15 to 172.87) |
| Hyaluronic acid, ng/ml | 15.67 (−11.65 to 42.99) | −32.02 (−58.74 to −5.31) | −47.69 (−85.54 to −9.85) |
| TIMP-1, ng/ml | −20.8 (−41.4 to −0.1) | −25.8 (−45.5 to −6.0) | −5.0 (−34.0 to 24.1) |
| FM-fibro index (HA, COL4-7S) | 0.012 (−0.041 to 0.065) | −0.060 (−0.112 to −0.008) | −0.072 (−0.145 to 0.002) |
| Mean (95% CI) | |||
|---|---|---|---|
| Descriptive statistics | Placebo (n = 23) | Apararenone (n = 24) | Between-group mean difference (95% CI) |
| IL-6, pg/ml | |||
| Mean (SD) | 14.719 (68.619) | 0.095 (1.690) | −14.625 (−44.162 to 14.913) |
| Median (min, max) | −0.180 (−6.750 to 314.000) | 0.046 (−5.330 to 3.840) | |
| M2BPGi | |||
| Mean (SD) | 0.06 (0.47) | 0.07 (0.37) | 0.006 (−0.254 to 0.266) |
| Median (min, max) | 0.06 (−0.70 to 1.21) | 0.05 (−0.5 to 0.85) | |
| TE (FibroScan), kPa | |||
| Mean (SD) | 0.5 (3.5) | −1.4 (4.0) | - |
| Median (min, max) | 1.2 (−6.0 to 6.3) | −0.7 (−10.9 to 5.2) | |
| HbA1c, % | |||
| Mean (SD) | −0.06 (0.44) | 0.16 (0.55) | 0.22 (−0.09 to 0.53) |
| Median (min, max) | 0.00 (−1.1 to 0.7) | 0.15 (−1.5 to 1.6) | |
| Fasting blood glucose, mg/dl | |||
| Mean (SD) | −3.4 (24.2) | −5.0 (30.1) | −1.6 (−18.5 to 15.3) |
| Median (min, max) | −2.0 (−87.0 to 28.0) | 1.5 (−99.0 to 38.0) | |
| Fasting insulin, µU/ml | |||
| Mean (SD) | 1.866 (11.583) | −5.674 (22.791) | −7.540 (−19.051 to 3.970) |
| Median (min, max) | −0.715 (−19.300 to 34.770) | 3.350 (−92.730 to 9.510) | |
| HOMA-IRa, µU⋅mg/ml⋅dl/405, blood glucose ≤140 mg/dl | |||
| Mean (SD) | 0.10 (4.15) | −2.86 (11.66) | −2.97 (−8.41 to 2.47) |
| Median (min, max) | −0.10 (−6.40 to 9.90) | 1.05 (−51.70 to 3.90) | |
| Total cholesterol, mg/dl | |||
| Mean (SD) | −20.3 (27.7) | 6.6 (28.0) | 26.9 (9.8 to 44.1) |
| Median (min, max) | −14.0 (−110.0 to 12.0) | 1.5 (−51.0 to 71.0) | |
| Triglycerides, mg/dl | |||
| Mean (SD) | −30.3 (138.6) | 16.8 (221.7) | 47.2 (−67.4 to 161.7) |
| Median (min, max) | −12.0 (−488.0 to 325.0) | 6.0 (−498.0 to 869.0) | |
| HDL cholesterol, mg/dl | |||
| Mean (SD) | −0.1 (8.1) | 2.9 (9.4) | 3.0 (−2.4 to 8.4) |
| Median (min, max) | −2.0 (−14.0 to 15.0) | 2.0 (−12.0 to 32.0) | |
| LDL cholesterol, mg/dl | |||
| Mean (SD) | −10.4 (31.5) | 7.5 (24.5) | 18.0 (0.6 to 35.3) |
| Median (min, max) | −9.0 (−99.0 to 50.0) | 8.0 (−47.0 to 57.0) | |
| Platelets, 104/μl | |||
| Mean (SD) | −0.02 (2.86) | 1.30 (1.94) | - |
| Median (min, max) | 0.10 (−4.60 to 6.40) | 1.70 (−3.60 to 4.30) | |
| Weight, kg | |||
| Mean (SD) | −0.78 (2.55) | −2.21 (3.10) | −1.43 (−3.18 to 0.32) |
| Median (min, max) | −1.30 (−5.50 to 3.70) | −0.90 (−9.60 to 1.20) | |
- Abbreviations: CI, confidence interval; COL4-7S, type IV collagen 7S; HA, hyaluronic acid; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model of assessment for insulin resistance; IL-6, interleukin-6; LDL, low-density lipoprotein; LS, least-squares; max, maximum; min, minimum; M2BPGi, Mac-2 binding protein glycan isomer; MMRM, mixed model repeated measures; TE, transient elastography; TIMP-1, tissue inhibitor of metalloproteinase-1.
- a Placebo, n = 19; apararenone, n = 18.
Pharmacogenomics testing
Consent for genetic testing was obtained from all 47 patients included in the FAS. There were 9, 10, and 4 patients in the placebo group, and 14, 7, and 3 patients in the apararenone group with GG, CG, and CC genotypes, respectively. The change in ALT and AST from baseline to 24 weeks was lower in patients with a CG genotype in the apararenone group versus patients with a CC or GG genotype in either group. In the apararenone group, the change from baseline in type IV collagen 7S was lower in patients with a CG or CC genotype versus patients with a GG genotype. Patients with a CG genotype in the apararenone group had a smaller change from baseline in the FIB-4 index at each assessment time point versus patients with a CC or GG genotype in either group. Similar pharmacogenomic results to those of the FIB-4 index were shown for hyaluronic acid, P3NP, and ELF scores.
Exploratory analysis of factors affecting improvement of fibrosis (post hoc analyses)
Factors influencing the improvement of fibrosis were analyzed using the FIB-4 index. Univariate analysis showed that multiple factors significantly influenced the FIB-4 index (p < 0.05) (Table S2). With a multivariate analysis using the forward and backward methods, it was shown that total bilirubin, GGT, ELF score, high-density lipoprotein cholesterol, soluble vascular cell adhesion molecule-1, and baseline Kleiner fibrosis staging significantly influenced improvement in the FIB-4 index (Tables 4 and S3).
| Factor | F-value | p-value |
|---|---|---|
| Total bilirubin, mg/dl | 20.83 | <0.0001 |
| GGT, U/L | 17.57 | <0.0001 |
| ELF score | 16.72 | <0.0001 |
| HDL cholesterol, mg/dl | 5.52 | 0.0207 |
| sVCAM-1, ng/ml | 5.44 | 0.0215 |
| Baseline Kleiner fibrosis staging | 4.46 | 0.0408 |
| FIB-4 index baseline value | 3.16 | 0.0829 |
| Apararenone | 2.40 | 0.1276 |
| Analysis visit | 1.21 | 0.3163 |
| Apararenone analysis visit | 0.39 | 0.7614 |
- Abbreviations: ELF, enhanced liver fibrosis; GGT, γ-glutamyl transpeptidase; HDL, high-density lipoprotein; sVCAM-1, soluble vascular cell adhesion molecule-1.
Safety and tolerability
The number of patients with AEs was 19 (82.6%) in the placebo group and 23 (95.8%) in the apararenone group (Table 5). No deaths were reported in this study. The rate of serious AEs was lower in the apararenone group than in the placebo group. The incidence of AEs leading to study drug discontinuation was similar between the two groups.
| Placebo (n = 23) | Apararenone (n = 24) | |
|---|---|---|
| TEAEs | 19 (82.6) | 23 (95.8) |
| Death | 0 (0.0) | 0 (0.0) |
| Serious TEAEs | 4 (17.4) | 2 (8.3) |
| Significant TEAEs | 0 (0.0) | 0 (0.0) |
| TEAEs with drug discontinuation | 2 (8.7) | 2 (8.3) |
| TEAEs resolved with treatment | 19 (82.6) | 22 (91.7) |
| TEAEs (reasonable possibility) | 3 (13.0) | 3 (12.5) |
| Death (reasonable possibility) | 0 (0.0) | 0 (0.0) |
| Serious TEAEs (reasonable possibility) | 0 (0.0) | 0 (0.0) |
| Significant TEAEs (reasonable possibility) | 0 (0.0) | 0 (0.0) |
| TEAEs with drug discontinuation (reasonable possibility) | 0 (0.0) | 1 (4.2) |
| TEAEs resolved with treatment (reasonable possibility) | 2 (8.7) | 2 (8.3) |
| TEAEs occurring in ≥2 patients in any group | ||
| Infections and infestations | 14 (60.9) | 17 (70.8) |
| Nasopharyngitis | 7 (30.4) | 12 (50.0) |
| Influenza | 4 (17.4) | 2 (8.3) |
| Tonsillitis | 1 (4.3) | 2 (8.3) |
| Gastroenteritis | 2 (8.7) | 1 (4.2) |
| Bronchitis | 2 (8.7) | 0 (0.0) |
| Helicobacter gastritis | 2 (8.7) | 0 (0.0) |
| Metabolism and nutrition disorders | 5 (21.7) | 5 (20.8) |
| Type 2 diabetes mellitus | 3 (13.0) | 4 (16.7) |
| Nervous system disorders | 5 (21.7) | 4 (16.7) |
| Headache | 2 (8.7) | 2 (8.3) |
| Respiratory, thoracic, and mediastinal disorders | 3 (13.0) | 5 (20.8) |
| Cough | 0 (0.0) | 2 (8.3) |
| Gastrointestinal disorders | 7 (30.4) | 12 (50.0) |
| Diarrhea | 3 (13.0) | 3 (12.5) |
| Abdominal discomfort | 0 (0.0) | 2 (8.3) |
| Nausea | 0 (0.0) | 2 (8.3) |
| Gastroesophageal reflux disease | 3 (13.0) | 1 (4.2) |
| Hepatobiliary disorders | 0 (0.0) | 2 (8.3) |
| Gallbladder polyp | 0 (0.0) | 2 (8.3) |
| Musculoskeletal and connective tissue disorders | 2 (8.7) | 4 (16.7) |
| Back pain | 1 (4.3) | 3 (12.5) |
| General disorders and administration site conditions | 1 (4.3) | 4 (16.7) |
| Puncture site pain | 0 (0.0) | 3 (12.5) |
| Injury, poisoning, and procedural complications | 5 (21.7) | 3 (12.5) |
| Wound | 2 (8.7) | 0 (0.0) |
- Note: Data are presented as n (%). Adverse events were coded using Medical Dictionary for Regulatory Activities version 19.1.
The most common AEs (those occurring in two or more patients in any group, preferred term) observed in the present study were nasopharyngitis, influenza, tonsillitis, gastroenteritis, bronchitis, Helicobacter gastritis, type 2 diabetes, headache, cough, diarrhea, abdominal discomfort, nausea, gastroesophageal reflux disease, gallbladder polyp, back pain, puncture site pain, and wound. Of these, nasopharyngitis, tonsillitis, type 2 diabetes, cough, abdominal discomfort, nausea, gallbladder polyp, back pain, and puncture site pain occurred at a higher rate (by at least one patient) in the apararenone group compared with the placebo group. The number of patients with severe AEs was one (4.3%) in the placebo group and zero in the apararenone group.
Adverse drug reactions were reported in three (13.0%) patients in the placebo group and three (12.5%) patients in the apararenone group. All events judged to be ADRs were mild in severity.
Time course changes in serum potassium levels and changes in serum potassium levels from baseline to 72 weeks are shown in Figure S3. The mean (SD) change in serum potassium from baseline to 72 weeks and that from baseline to the end of the 8-week follow-up period was 0.00 (0.25) and 0.04 (0.22) mmol/L, respectively, in the placebo group and 0.09 (0.29) and −0.01 (0.23) mmol/L, respectively, in the apararenone group.
Serum potassium increased in the apararenone group until week 8 and then remained stable until week 72, and levels remained in the range of 0.2 to 0.3 mmol/L. After the end of the study treatment, the values in the apararenone group decreased to near baseline values. During the treatment period, three patients had a serum potassium level between 5.0 and 5.5 mmol/L at some time points. No patient had a serum potassium level greater than 5.5 mmol/L.
DISCUSSION
In patients histologically diagnosed with NASH in this study, there were no significant differences between placebo and apararenone groups in the baseline characteristics. Treatment with apararenone 10 mg/day for 72 weeks was associated with reductions in ALT levels and fibrosis improvement. Pathological findings also suggested that apararenone has an anti-inflammatory and an antifibrotic effect.
Fibrosis is an important prognostic factor in NASH, and fibrosis improvement has been considered to be the critical finding in clinical trials.27, 29-32 In this study, the proportions of patients with an improvement of 1 point or more in Kleiner fibrosis stage, and an improvement of 1 point or more in Kleiner fibrosis stage without worsening of NAS were higher in the apararenone group versus the placebo group. In addition, patients in the apararenone group had greater reductions from baseline in potential fibrosis markers, including type IV collagen 7S, hyaluronic acid, P3NP, ELF score, FIB-4 index, and FM-fibro index at all time points, compared with patients in the placebo group. Given that multiple potential markers of fibrosis were significantly reduced in the apararenone group, all showing a tendency to improve, these findings suggest that apararenone could have an antifibrotic effect on the liver. Apararenone has also been reported to have renal-protective effects in patients with diabetic nephropathy14; therefore, it could show a potent antifibrotic effect in both the liver and the kidney. Further studies are needed to confirm this in the future.
It has been reported that a weight loss of 5% or more correlates with an improvement in histological findings in patients with NAFLD/NASH.33, 34 It is unclear from the results of this study whether treatment with apararenone directly causes a reduction in body weight.
It has previously been reported that there are several PNPLA3 SNPs that are significantly associated with the onset and progression of NASH.35 In this study, we investigated the association between the SNP rs738409 in the PNPLA3 gene and its effect on apararenone response. The PNPLA3 genotype GG has been reported to be more progressive than the CG genotype in terms of fibrosis.25, 26 In the present study, there were nine patients in the placebo group and 14 patients in the apararenone group with the GG genotype. The change from baseline in various biomarkers such as ALT, AST, and type IV collagen 7S, tended to be lower in patients with the CG or GG genotypes than in patients with the CC genotype.
Various factors have been reported to influence the progression and suppression of natural course in NASH.18, 24, 27, 28 For example, fibrosis improvement has previously been associated with weight loss.33, 34 In the present study, post hoc univariate analyses were undertaken after unblinding to identify factors that contributed to an improvement in fibrosis using the FIB-4 index as a fibrosis indicator. Lifestyle-related factors (including body weight) and factors related to the pathophysiology of NAFLD and NASH were found to significantly influence the FIB-4 index. In a subsequent multivariate analysis, body weight was no longer found to be a significant factor and, instead, total bilirubin, GGT, and ELF score were shown to be factors that contributed significantly to an improvement in the FIB-4 index (p < 0.0001). These factors have previously been reported to be related to the pathophysiology of NAFLD and NASH24, 28, 36, 37; GGT is known to be an oxidative stress marker, and the inhibition of oxidative stress is also known to contribute to the improvement of fibrosis.36, 38, 39 However, further investigation is necessary to confirm the findings from our post hoc analyses.
In this study, although serum potassium levels increased during the first 8 weeks of treatment with apararenone, the levels subsequently stabilized, and tended to decrease to the baseline level after treatment. It is therefore possible to control serum potassium levels by regular monitoring. No safety concerns were raised regarding changes in eGFR throughout the study period.
The present study has some limitations. This was an exploratory trial, and the sample size was small, and included only Japanese patients. However, the study also has several strengths. Notably, this is the first double-blind controlled clinical trial to evaluate the efficacy of an MR antagonist in patients with NASH. Only patients with a definitive diagnosis of NASH and Kleiner fibrosis stage 2 and 3 based on liver biopsy results were included. Although many clinical trials primarily evaluate the efficacy of investigational drugs for NASH based on liver imaging tests, in the present study, in addition to pathological findings, several biomarkers including many potential fibrosis markers (such as ELF score) were measured over a relatively long period (72 weeks). Finally, SNP genotyping of the PNPLA3 gene was carried out.
In conclusion, the present study indicated that apararenone 10 mg/day for 72 weeks was effective in decreasing ALT levels in patients with NASH and improving multiple potential fibrosis markers. The pathological findings also showed the anti-inflammatory and antifibrotic effects of apararenone, which are important prognostic factors in these patients. Although serum potassium levels increased with treatment, these did not continuously increase over time and levels could be maintained within acceptable limits by regular monitoring. No other major safety concerns were raised. Thus, apararenone 10 mg/day for 72 weeks was safe and well tolerated in this study.
ACKNOWLEDGMENTS
This study was funded by Mitsubishi Tanabe Pharma Corporation. The authors thank all investigators involved in this study. The list of principal investigators is provided in Document S1. We also thank Michelle Belanger, MD, of Edanz Pharma, for providing medical writing support, which was funded by Mitsubishi Tanabe Pharma Corporation.
CONFLICT OF INTEREST
T. Okanoue received remuneration from Mitsubishi Tanabe Pharma Corporation during the conduct of the study. M. Sakamoto received research funding from Mitsubishi Tanabe Pharma Corporation during the conduct of the study, and has received research funding from Eisai Co., Ltd., Olympus Corporation, FUJI FILM Corporation, and CYTLIMIC Inc. outside the submitted work. K. Harada received remuneration from Mitsubishi Tanabe Pharma Corporation during the conduct of the study, and has received remuneration from Gilead Sciences Inc., Otsuka Pharmaceutical, and Taisho Toyama Co., Ltd outside the submitted work. M. Inagaki, N. Totsuka, and G. Hashimoto are employees of Mitsubishi Tanabe Pharma Corporation. H. Kumada received remuneration from Mitsubishi Tanabe Pharma Corporation during the conduct of the study, and has received remuneration from AbbVie Inc, Eisai Co., Ltd., Gilead Sciences K.K., Sumitomo Dainippon Pharma Co., Ltd., and MSD K.K. outside the submitted work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.




