Assessment of clinical and magnetic resonance imaging features of de novo hypervascular hepatocellular carcinoma using gadoxetic acid-enhanced magnetic resonance imaging
Abstract
Aim
To clarify the clinical and magnetic resonance imaging (MRI) features of de novo hypervascular hepatocellular carcinoma (HCC) using serial gadoxetic acid-enhanced MRI.
Methods
The institutional review board approved this retrospective study. After review of 1007 MRI examinations in 240 patients with chronic liver disease, 17 newly developed hypervascular HCCs in 16 patients detected by follow-up from initial MRI examination without hepatocellular nodules were evaluated. The clinical and MRI findings such as previous treatment history for HCC, period to hypervascular HCC onset, presence or absence of hypovascular hypointense nodules on hepatobiliary phase before hypervascularization, and intralesional fat component were recorded or evaluated. Statistical evaluations included Fisher's exact test, χ2-test, and Mann–Whitney U-test.
Results
In 17 HCCs, 12 (71%) were de novo hypervascular HCC without showing hypovascular hypointense nodule on hepatobiliary phase before hypervascularization (de novo group) and 5 (29%) were hypervascularized HCC developed during multistep hepatocarcinogenesis (multistep group). The incidence of previous treatment history for HCC in the de novo group (91%) was significantly higher than that in the multistep group (20%) (P = 0.013). The duration to hypervascular HCC onset from initial examination was shorter in the de novo group (mean, 291 days) than in the multistep group (mean, 509 days) (P = 0.035). The incidence of fat-containing lesion in the de novo group (0%) was lower than that in the multistep group (40%) (P = 0.074).
Conclusion
De novo hypervascular HCC is characterized by rapid growth, patients with previous treatment history for HCC, and lack of intralesional fat, compared to hypervascular HCC with multistep progression.
Introduction
In hepatocellular nodules seen in patients with chronic liver disease (CLD), two types of hepatocarcinogenesis are now considered. One is de novo hepatocarcinogenesis and the other is multistep progression from dysplastic nodule (DN) to early hepatocellular carcinoma (HCC), and finally to classical HCC.1-3 Therefore, it is extremely important to understand the features of clinical and imaging findings of these HCCs for treatment strategies. Prompt treatment intervention using interventional and surgical resection for hypervascular HCC will be required for de novo HCC, as it occurs as a hypervascular tumor. In contrast, hypovascular early-stage multistep hepatocellular nodules such as DN and early HCC will be followed up until the development of hypervascularization.
Gadoxetic acid with hepatobiliary uptake have been shown to improve the detection of HCC relative to other imaging methods, including dynamic computed tomography (CT) and dynamic MRI using extracellular contrast agents.4-7 In gadoxetic acid-enhanced MRI, lesions with reduced or absent hepatocyte function appear hypointense on the hepatobiliary phase (HP), which is generally acquired within 20 min of contrast media administration. This new application for contrast-enhanced MRI can result in the improvement of detection of early-stage HCC. In fact, several clinical studies have reported that gadoxetic acid-enhanced MRI can distinguish hepatocellular nodules in the early stage of multistep hepatocarcinogenesis as hypovascular hypointense nodules on HP, so-called “high-risk nodules” (probably DNs or early HCC).8-22 Furthermore, recent observation studies have reported that the high-risk nodules show hypervascularization at follow-up and the risks of hypervascularization include initial tumor size, intratumoral fat, increase in size during follow-up, and high signal intensity on T2-weighted imaging.10-12, 15, 18, 20-22 Conversely, we have also noticed that some hypervascular HCCs without such high-risk nodules developed suddenly in cirrhotic liver, corresponding to de novo hepatocarcinogenesis. To our knowledge, however, no clinical studies focusing on de novo HCC using gadoxetic acid-enhanced MRI have been reported. Therefore, the aim of this study was to clarify the clinical and MRI features of de novo HCC (equivalent to hypervascular HCC without high-risk nodules) using serial gadoxetic acid-enhanced MRI in patients with CLD.
Methods
Subjects
The institutional review board approved this retrospective study, and the need for informed consent from patients was waived.
Between January 2008 and January 2014, 957 consecutive patients underwent 1877 gadoxetic acid-enhanced MRI examinations for suspected liver diseases in our institution. Inclusion criteria for this study were: (i) ≥20 years old; (ii) CLD; and (iii) patients who had undergone MR examination at least twice. Eight hundred and seventy examinations were excluded based on the following criteria: patients <20 years (17 examinations), single examination (629 examinations), and patients without CLD (224 examinations). A total of 1007 examinations in 240 patients (156 men, 84 women) with a mean age of 68 years (range, 35–88 years) were thus included in this study. There were no patients with poor MR images that could influence diagnostic quality. All patients had CLD and had been referred to MRI for detection and characterization of hepatocellular nodule related to CLD.
Magnetic resonance imaging protocol
All MRI scanning was carried out using one of three MR scanners including two 1.5-T systems (Signa Excite high speed; GE, Milwaukee, WI, USA; or EXCELART Vantage Powered by Atlas; Toshiba Medical Systems, Tochigi, Japan) with a 4-channel (GE) or 16-channel (Toshiba) phased array coil as the receiver coil and a 3-T system (Vantage Titan 3 T; Toshiba Medical Systems) with a 16-channel (Toshiba) phased array coil as the receiver coil.
Imaging was carried out in the axial plane under fasting conditions in all subjects. In our institution, the protocols for liver MRI consisted of in- and opposed-phase T1-weighted imaging, breath-hold fast spin-echo T2-weighted imaging with fat suppression, single-shot fast spin-echo heavily T2-weighted imaging with fat suppression, diffusion-weighted imaging (b values of 0 and 800 s/mm2), and dynamic contrast-enhanced (DCE)-MRI.
Dynamic contrast-enhanced MRI was carried out using a 3-D T1-weighted gradient-echo sequence with fat-suppression technique (liver acquisition with volume acceleration, GE or Quick 3D, Toshiba). Data acquisition for DCE-MRI began simultaneously with initiation of i.v. injection of gadoxetic acid (Primovist; Bayer Schering Pharma, Berlin, Germany) at a dose of 0.1 mL/kg (0.025 mmol/kg body weight) through a power injector as a rapid bolus at the rate of 1 or 3 mL/s, followed by a 30-mL saline flush at the rate of 1 or 3 mL/s. For multiphase DCE images, precontrast phase, arterial phase (fixed scan timing [25 s] or modified scan timing using a fluoroscopic triggering [Fluoro Trigger, GE Healthcare or Visual Prep, Toshiba] or an automated bolus detection algorithm [SmartPrep, GE Healthcare]), portal phase (70 s), late phase (3 min), and HP (20 min). Imaging parameters for DCE-MRI sequences were: repetition time, 3.3–5.5 ms; echo time, 1.3–2.2 ms; flip angle, 12° or 15°; bandwidth, 62.5–78.08 kHz; parallel imaging factor, 1.5–2; field of view, 35 × 35 cm; slice thickness, 2.2–3.0 mm; matrix, 288–320 × 160–256; and acquisition time, 19–22 s.
Image analysis
Magnetic resonance images were evaluated using a PACS viewer (Rapidey Core; Toshiba Medical Systems, Tokyo Japan) by two radiologists with 20 years and 8 years of experience in abdominal MRI in consensus. In MRI analysis, images from precontrast-, arterial-, portal venous-, late-phases, and HP were reviewed in all subjects.
Reviewers were aware that patients with CLD had been referred for MRI on suspicion of hepatocellular nodule but were blinded to all other clinical information, MRI findings from clinical interpretation reports, and the final diagnosis in order to avoid bias.
First, in each patient, serial MR examinations were reviewed in chronological order from initial to most current MR examination until the development of hypervascular HCC. Patients who had hepatocellular nodule (hypervascular HCC or high-risk nodule) at the initial MR examination were excluded because this study tried to investigate “newly” developed hypervascular HCCs. Hypervascular HCC was defined as round or oval-shaped nodules of 5 mm or more that showed hypervascularity at the arterial phase, hypointensity at the portal phase or late phase, and hypointensity or heterogeneous isointensity or hyperintensity at the HP, regardless of findings of other MR images including T1, T2, and diffusion-weighted MR sequences.21 High flow hepatic hemangiomas, which show DCE-MRI findings similar to hypervascular HCC, were carefully excluded by reviewing T2- and heavily T2-weighted MR images as well as abdominal ultrasonography (US).23 Second, patients with developed hypervascular HCC during serial MR examinations were evaluated whether the stage of high risk nodule was present before hypervascularization. High-risk nodules were defined as round or oval-shaped hepatocellular nodules of 5 mm or more that showed hypovascularity at the arterial phase and hypointensity at the HP, regardless of findings of other MR images including T1, T2, and diffusion-weighted MR sequences.21 Hepatic cysts with hypovascular and hypointensity at the hepatobiliary phase were carefully excluded by reviewing T2- and heavily T2-weighted MR images, as well as abdominal US, DCE-CT, or DCE-MR images. We classified hypervascular HCC with the stage of high-risk nodule before hypervascularization as multistep hepatocarcinogenesis (multistep group), and classified hypervascular HCC without the stage of high-risk nodule before hypervascularization as de novo hepatocarcinogenesis (de novo group). In patients with a treatment history for pre-existing hypervascular HCC, de novo hypervascular HCC was defined as a lesion (with enough distance from the site after the treatment) which developed six months or more after treatment by reviewing from initial MR examination without hepatocellular nodule in whole liver parenchyma after the treatment, to distinguish it from intrahepatic metastases. Third, the patients with examination intervals of 6 months or more among serial MR examinations in each patient were excluded. Finally, to detected hypervascular HCCs in the selected study subjects, the tumor size, presence or absence of intralesional fat component on in- and opposed-phase T1-weighted imaging, qualitative signal intensity of lesion relative to the surrounding liver parenchyma in diffusion-weighted imaging and T2-weighted imaging using a 5-point grading scale (0, hypointense; 1, slightly hypointense; 2, isointense; 3, slightly hyperintense; and 4, hyperintense), and quantitative signal intensity ratio (SIR) of lesion in HP (signal intensity [SI] of lesion / SI of surrounding liver parenchyma near the lesion) were also analyzed. The SI was measured using a region of interest (ROI). The same two radiologists set all ROIs by consensus. Each ROI was a circle or oval. The size of each ROI was as large as possible. When the ROI of the liver parenchyma was set, great care was taken to exclude the large vessels to reduce any errors in SI measurements from macroscopic flow.
Clinical data collection
A study coordinator reviewed the medical record and MRI report for clinical data collection in patients targeted for analysis. As clinical and MRI data, the age, sex, cause of CLD (type C viral hepatitis, type B viral hepatitis or others), degree of liver damage (chronic hepatitis or liver cirrhosis), previous treatment history for HCC (interventional or surgical), and duration of hypervascular HCC onset from initial MRI examination were obtained from the medical record and MRI report.
Statistical analyses
Statistical analyses were carried out using spss for Windows version 19.0 software (SPSS, Chicago, IL, USA). All tests were two-sided and values of P < 0.05 were considered statistically significant unless otherwise specified. The clinical data and MRI features were compared between the de novo group and multistep group using Fisher's exact test, χ2-test, and Mann–Whitney U-test. Sex, tumor size, period to hypervascular HCC from initial MRI examination onset, SI of tumor in T2-weighted imaging, SI of tumor in diffusion-weighted imaging, and SIR of tumor in HP were compared by using a Mann–Whitney U-test. The sex, cause of chronic liver disease, degree of liver damage, frequency of previous treatment history for HCC, and frequency of intralesional fat were compared by using Fisher's exact test or χ2-test.
Results
AFTER SERIAL REVIEW from initial MRI examination without hepatocellular nodule using 1007 MRI examinations in 240 patients with CLD, 17 hypervascular HCCs in 16 patients (101 examinations) were newly developed. Only 2 lesions of these hypervascular HCCs were diagnosed pathologically after surgical resection. The remaining 224 patients (906 examinations) were excluded based on the following reasons: 109 patients (413 examinations) without the presence of hypervascular HCC, 107 patients (450 examinations) who had hepatocellular nodule at all MR examinations, and 8 patients (43 examinations) with the presence of hypervascular HCC who had examination intervals of 6 months or more among serial MR examinations. Of these 17 hypervascular HCCs, 12 HCCs (71%) in 11 patients were in the de novo group without the stage of high-risk nodule before the development of hypervascular HCC (Fig. 1). Five HCCs (29%) in 5 patients were in the multistep group with the stage of high-risk nodule before the development of hypervascular HCC (Fig. 2).
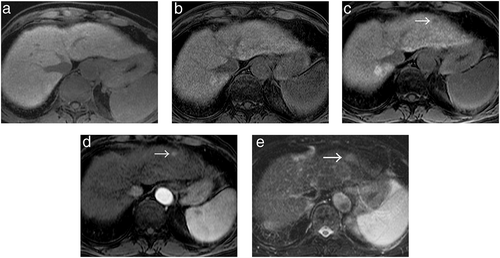
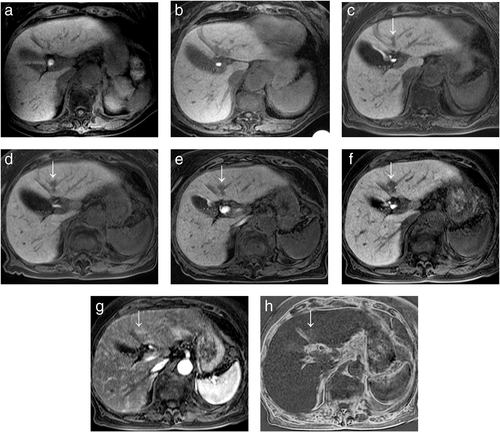
Clinical features of de novo hypervascular HCC
The results of a comparison of clinical findings between the de novo and multistep groups are summarized in Table 1.
| Data | De novo group (n = 11) | Multistep group (n = 5) | P-value |
|---|---|---|---|
| Tumor number† | 12 (71) | 5 (29) | |
| Age, years‡ | 71.2 ± 7.93 | 70.9 ± 12.5 | 0.609 |
| Sex† | 0.036 | ||
| Male | 9 (81.8) | 1 (20) | |
| Female | 2 (18.2) | 4 (80) | |
| Cause of chronic liver disease (C / B / others)† | 0.800 | ||
| HCV | 5 (45.5) | 3 (60) | |
| HBV | 2 (18.2) | 1 (20) | |
| Others | 4 (36.3) (cryptogenic hepatitis in 4 patients) | 1 (20) (autoimmune hepatitis in one patient) | |
| Liver damage† | 0.093 | ||
| Chronic hepatitis | 6 (54.5) | 0 | |
| Liver cirrhosis
(Child–Pugh class A in all 10 patients) |
5 (45.5) | 5 (100) | |
| Treatment history for HCC, interventional or surgical† | 0.013 | ||
| Presence | 10 (90.9) | 1 (20) | |
| Absence | 1 (9.1) | 4 (80) | |
| Tumor size of hypervascular HCC, mm‡ | 11.6 ± 3.06 | 11.0 ± 1.93 | 0.916 |
| Duration of hypervascular HCC onset from initial MRI examination, days‡ | 291.3 ± 298.6 | 509.2 ± 255.7 | 0.035 |
- Data are in 12 de novo hypervascular HCC without showing hypovascular hypointense nodule on hepatobiliary phase (HP) before hypervascularization (de novo group) and 5 multistep hypervascular HCC showing hypovascular hypointense nodule on HP before hypervascularization (multistep group).
- † Numbers in parentheses are percentages.
- ‡ Data are mean ± standard deviation.
- HBV, hepatitis B virus; HCV, hepatitis C virus.
The frequency of male patients in the de novo group was significantly higher than in the multistep group (P = 0.036). The patient's age, cause of CLD, degree of liver damage, and tumor size of hypervascular HCC between both groups had no statistical significant difference (P = 0.093 to 0.916). In comparisons between both groups, the frequency of treatment history for HCC was significantly higher in the de novo group (90.9% [10/11 patients]) than in the multistep group (20% [1/5 patients]) (P = 0.013). In 10 patients with treatment history for HCC in the de novo group, the median duration between most recent treatment and onset of hypervascular HCC was 463 days (range, 180–923 days). In addition, 80% (8/10 lesions) of hypervascular HCC with treatment history in the de novo group developed from a segment that was different from the treated lesion. Previous treatment for HCC included surgical resection in 6 patients, transarterial chemoembolization in 1 patient, radiofrequency ablation in 2 patients, and radiofrequency ablation + transarterial chemoembolization in 1 patient in the de novo group; 1 patient with treatment history in the multistep group was treated with surgical resection.
In addition, the duration of hypervascular HCC onset from initial MRI examination in the de novo group (median, 181 days; range, 83–1125 days) was significantly shorter than that in the multistep group (median, 454 days; range, 253–879 days) (P = 0.035).
MRI features of hypervascular HCC
The results of a comparison of MRI findings between both groups are summarized in Table 2.
| Data | De novo group (n = 11) | Multistep group (n = 5) | P-value |
|---|---|---|---|
| Tumor number† | 12 (71) | 5 (29) | |
| Fat-containing lesion† | 074 | ||
| Presence | 0 (0) | 2 (40) | |
| Absence | 12 (100) | 3 (60) | |
| Tumor SI of T2WI‡ | 2.83 ± 0.72 | 2.60 ± 0.55 | 558 |
| Tumor SI of DWI‡ | 2.91 ± 0.83 | 3.00 ± 0.71 | 808 |
| Tumor SIR of HP‡ | 0.64 ± 0.20 | 0.52 ± 0.05 | 246 |
- Data are in 12 de novo hypervascular HCC without showing hypovascular hypointense nodule on hepatobiliary phase (HP) before hypervascularization (de novo group) and 5 multistep hypervascular HCC showing hypovascular hypointense nodule on HP before hypervascularization (multistep group).
- † Numbers in parentheses are percentages.
- ‡ Data are mean ± standard deviation.
- DWI, diffusion-weighted imaging; HP, hepatobiliary phase; SI, signal intensity; SIR, signal intensity ratio; T2WI, T2-weighted imaging.
The incidence of fat-containing lesion of hypervascular HCC in the de novo group (0% [0/12 lesions]) was lower than in the multistep group (40% [2/5 lesions]), with marginally significant difference (P = 0.074) (Fig. 2). There were no significant differences in the tumor SI of T2-weighted imaging, tumor SI of diffusion-weighted imaging, or tumor SIR of HP between both groups (P = 0.246 to 0.808).
Discussion
IN THE PRESENT study, de novo hypervascular HCC showed four different characteristic clinical and MRI imaging findings as compared to multistep hypervascular HCC. We showed for the first time that 71% (12/17 lesions) of hypervascular HCCs that newly developed during follow-up survey from initial MRI examination, without the presence of hepatocellular nodules in the whole liver, was de novo hypervascular HCC without the stage of high-risk nodule. Therefore, hepatologists need to recognize the high incidence of de novo hepatocarcinogenesis, as compared with multistep hepatocarcinogenesis, for the detection of hepatocellular nodules using gadoxetic acid-enhanced MRI in patients with chronic hepatitis. In addition, for the detection of de novo hypervascular HCCs of such small size (mean, 11.6 mm), CT or MRI examinations using optimal dynamic-contrast enhanced protocol including arterial dominant, portal venous, and late (or equilibrium) phases with excellent tumor detectability is indispensable.24, 25
Second, 90.9% (10/11 patients) of patients with de novo hypervascular HCC had previous treatment history for HCC, such as interventional and surgical resection, and the frequency was significantly higher than patients with multistep hypervascular HCC (20%, 1/5 patients). This fact might suggest patients with previous treatment history for HCC already have the predisposition, such as genetic factors, that liver parenchyma of background is easy to cause de novo carcinogenesis.26, 27 Therefore, strict follow-up study using gadoxetic acid-enhanced MRI, dynamic contrast-enhanced CT, and contrast-enhanced US for the onset of newly developed HCC may be required even in patients with complete disappearance of hepatocellular nodules in whole liver after treatment for HCC.
Third, the duration to hypervascular HCC onset from initial MRI examination in the de novo group (median, 181 days; mean, 291.3 days; range, 83–1125 days) was significantly shorter than that of the multistep group (median, 454 days; mean, 509.2 days; range, 253–879 days). The onset of HCC in the short period in the de novo group may be explained by sudden hepatocarcinogenesis without early-stage hepatocellular nodule, such as high-risk nodule including DN and early HCC.1-3 In addition, the initial follow-up examination for the detection of hypervascular HCC after treatment in patients with treatment history for HCC should be carried out within 3 months after the treatment, as the shortest period to hypervascular HCC onset in de novo group was 83 days. Furthermore, if hypervascular HCC is not detected in the initial examination after treatment, we recommend subsequent examinations every 6 months. However, these recommendations should also consider the clinical information of patients without the onset of new hypervascular HCC by follow-up survey after treatment for HCC.
In patients with treatment history of hypervascular HCC, most newly developed hypervascular HCC appeared in a short period after treatment in the de novo group. Therefore, we cannot exclude the possibility that these lesions were intrahepatic metastases. However, in the present study, such de novo hypervascular HCCs newly developed in the liver in patients with lesions that had completely disappeared after treatment, and were far from the treated site. Furthermore, the median duration between onset of hypervascular HCC and most recent treatment was 463 days.
In the discrimination between de novo tumor and intrahepatic spread in HCC, Finkelstein et al. reported that, as the underlying CLD is thought to give rise to HCC tumor formation, it is hardly surprising that bilobar tumors could arise independently of one another and do not always represent metastatic spread.27
Finally, the presence of fat within hypervascular HCCs in the de novo group was 0% (0/12 lesions) and, although there was a marginally significant difference, the frequency was substantially lower than in the multistep group (40%, 2/5 patients). Several previous studies have reported that fatty change within the hepatocellular nodule is one of the characteristic histopathological findings of early HCC.28-31 Furthermore, recent clinical studies that have evaluated the outcome of high-risk nodules on gadoxetic acid-enhanced MRI have suggested that the presence of intratumoral fat, indicating high risk for development of hypervascularization, is the critical change suggesting malignant transformation.10, 18, 21 These facts suggested that hypervascular HCCs in the de novo group were advanced HCCs from the onset that did not have the stage of high-risk nodule nor early HCC with intratumoral fat.
There were several potential limitations of our study. First, because our study was carried out in a retrospective manner, there may have been selection bias. However, the research design would demonstrate limitlessly prospective implications. Second, the number of patients was relatively small. Accordingly, further large-scale prospective clinical studies in patients with CLD should be undertaken to confirm the clinical and imaging characteristics of de novo hypervascular HCC obtained with the present study. Third, we used three different MRI scanners of 1.5-T or 3-T systems for assessment of hepatocellular nodules. Although this difference may influence the enhancement effects of hepatic lesions and the signal intensity of nodules on HP, the injection protocol of contrast material and imaging parameters was based on the latest recommended method in each era for the detection of hepatocellular nodule using gadoxetic acid-enhanced MRI in either MR scanners. However, in particular, the infusion rate should be fixed at 1 or 2 mL/s for optimal arterial phase acquisition in further studies.25 Fourth, the interval between follow-up examinations varied. However, all intervals in our study were less than 6 months, and the interval would be proper in both the daily clinical use of gadoxetic acid-enhanced MRI for the detection of hepatocellular nodules in patients with CLD and the observation of hypervascular HCC with or without the onset of high risk nodule from initial examination as mentioned above.19 Fifth, as the interval between follow-up examinations was 6 months, we cannot exclude the possibility of the existence of hypervascular HCC that develops in multistep hepatocarcinogenesis within 6 months.32 Finally, pathological diagnosis was not obtained in all lesions except two. However, we believe this widely accepted imaging-based diagnosis was sufficient for the diagnosis of hypervascular HCC. In addition, high-risk nodules are often detected only by HP of gadoxetic acid-enhanced MRI, and generally, pathological evaluation by means of image-guided needle biopsy, such as US or surgery, would be difficult for these nodules.
In conclusion, de novo hypervascular HCC is characterized by rapid growth, patients with previous treatment history for HCC, and lack of intralesional fat, compared to hypervascular HCC with multistep progression. These clinical and MRI features of de novo HCC may play an important role for the early detection and proper treatment intervention for hypervascular HCC, particularly in patients with previous treatment history for HCC.




