Flagellar Assembly Factor FliW2 De-Represses Helicobacter pylori FlaA-Mediated Motility by Allosteric Obstruction of Global Regulator CsrA
Funding: This study was supported by the Ministry of Science and Technology, as well as the National Science and Technology Council in Taiwan (MOST 108-2320-B-010-002, 108-2811-B-010-536, 109-2320-B-010-040, 109-2811-B-010-535, and 110-2811-B-A49A-027; NSTC 111-2811-B-468-001, NSTC 112-2811-B-468-001, and NSTC 113-2811-B-468-002).
ABSTRACT
Background
Helicobacter pylori colonizes the human stomach as a dominant member of the gastric microbiota and constitutively expresses flagellar motility for survival. Carbon storage regulator A (CsrA) is a posttranscriptional global regulator and a critical determinant of H. pylori's motility and pathogenicity. The regulation of H. pylori CsrA is still uncertain although in other species CsrA is reported to be antagonized by small RNAs and proteins. In this study, we attempted to unveil how CsrA is regulated and hypothesized that H. pylori CsrA activity is antagonized by a flagellar assembly factor, FliW2, via protein allosteric obstruction.
Materials and Methods
Multiple sequence comparisons indicated that, along its length and in contrast to fliW1, the fliW2 of H. pylori J99 is conserved. We then generated an isogenic ΔfliW2 strain whose function was characterized using phenotypic and biochemical approaches. We also applied a machine learning approach (AlphaFold2) to predict FliW2-CsrA binding domains and investigated the FliW2-CsrA interaction using pull-down assays and in vivo bacterial two-hybrid systems.
Results
We observed the reduced expression of major flagellin FlaA and impaired flagellar filaments that attenuated the motility of the ΔfliW2 strain. Furthermore, a direct interaction between FliW2 and CsrA was demonstrated, and a novel region of the C-terminal extension of CsrA was suggested to be crucial for CsrA interacting with FliW2. Based on our AlphaFold2 prediction, this C-terminal region of FliW2-CsrA interaction does not overlap with CsrA's N-terminal RNA binding domain, implying that FliW2 allosterically antagonizes CsrA activity and restricts CsrA's binding to flaA mRNAs.
Conclusions
Our data points to novel regulatory roles that the H. pylori flagellar assembly factor FliW2 has in obstructing CsrA activity, and thus FliW2 may indirectly antagonize CsrA's regulation of flaA mRNA processing and translation. Our findings reveal a new regulatory mechanism of flagellar motility in H. pylori.
Abbreviations
-
- Amp
-
- ampicillin
-
- BACTH system
-
- Bacterial Adenylate Cyclase-based Two-Hybrid system
-
- cDNA
-
- complementary deoxyribonucleic acid
-
- Cm
-
- chloramphenicol
-
- CsrA
-
- carbon storage regulator
-
- FliW2
-
- flagellar assembly factor FliW2
-
- IPTG
-
- isopropyl-β-D-thiogalactopyranoside
-
- Km
-
- kanamycin
-
- LB
-
- Lurie-Bertani medium
-
- LC-MS/MS
-
- liquid chromatography with tandem mass spectrometry
-
- ODU
-
- optical density unit
-
- PBS
-
- phosphate-buffered saline
-
- qPCR
-
- quantitative polymerase chain reaction
-
- SEM
-
- Scanning electron microscope
-
- WT
-
- wild-type
1 Introduction
Gram-negative microaerophilic Helicobacter pylori of Epsilonproteobacteria colonizes the human stomach as a dominant member of the gastric microbiota and is a causative agent of gastric infections that may lead to the development of gastroduodenal diseases and gastric cancer [1-3]. Unlike Gammaproteobacteria, which are mostly found in intestinal niches, H. pylori's gastric milieu constitutes a uniquely hostile ecological niche. Because of the restriction of small absorptive nutrients and the acidity of the stomach [4], H. pylori's environment may favor the evolution of multilayered hierarchical regulatory networks [5]. H. pylori constitutively expresses flagellar biosynthesis, chemotaxis, and motility as essential housekeeping functions in vivo [6]. Due to the lack of the master regulator FlhDC in the flagellar regulatory network of Epsilonproteobacteria, the biogeneses of flagella in H. pylori and Campylobacter jejuni are fundamentally different from those of Escherichia coli and Salmonella (Gammaproteobacteria) [7]. Flagella-mediated motility is one of the crucial virulence determinants and it is tightly regulated. The regulation of the flagellar motility in Epsilonproteobacteria includes three transcriptional sigma factors (RpoD/σ80, RpoN/σ54, and FliA/σ28), regulators (CsrA, FlgR/S, FlhA/F, FlgM, HrcA, and HspR), and small RNAs (CncR1 and FlmE/R) during the early (class I), middle (class II), and late (class III) stages of flagellar biosynthesis, respectively [8-15]. Of these, the global posttranscriptional regulator CsrA (carbon storage regulator A) has been widely studied for its modulation of carbon metabolism, stress responses, virulence, biofilm formation, flagellar biosynthesis, and motility [13, 14, 16-18].
CsrA regulates the expression of downstream genes via mechanisms that operate posttranscriptionally and translationally. When a CsrA dimer binds to NGGA motifs on the leader sequence of a transcript, it can stabilize target mRNAs, promote Rho-dependent transcriptional termination, change RNA secondary structure to repress or activate translation, or occupy the Shine-Dalgarno (SD) sequence to occlude ribosome binding [19]. In E. coli, CsrA activates the expression of several motility genes, including the flhDC operon that encodes the transcription factor FlhDC for flagellar biosynthesis and chemotaxis [20, 21]. However, studies of CsrA regulation in Bacillus and Campylobacter show that genes for flagellar filament formation and motility are repressed by CsrA [14, 22]. The regulation of CsrA antagonists in the different species also reveals interesting patterns. In E. coli and Gammaproteobacteria, the non-coding small RNAs, CsrB and CsrC, form a secondary structure known as a “hedgehog ball”, where many NGGA sequences are presented as CsrA binding sites [23-27]. The CsrB binds to E. coli CsrA thus inhibiting the CsrA activity and attenuating the CsrA modulation of gene expression through a competitive antagonism. In addition to small RNA regulation, antagonizing CsrA activity through protein interaction is reported in the cases of enteropathogenic E. coli (EPEC), Bacillus and Campylobacter. The chaperone CesT required for EPEC to colonize in host intestinal epithelial cells reduces CsrA activity by binding to the RNA binding domain of E. coli CsrA as a competitive antagonist [28]. Unlike the mechanism of CesT-CsrA binding, a flagellar assembly factor FliW found in Bacillus subtilis and C. jejuni employs a three-node negative-feedback regulation between FliW, CsrA, and the major flagellin FlaA homolog of flagellar biosynthesis [14, 29-31]. In B. subtilis, FliW proteins spatially bind to the different residues of CsrA required for RNA binding, thus inhibiting CsrA activity through an allosteric noncompetitive mechanism. This FliW-CsrA dimeric interaction strictly controls the intracellular concentration of flagellar filament protein Hag (FlaA homolog) for the homeostasis of flagellar biosynthesis and the maintenance of intracellular architecture [29, 31]. A similar but more complicated FlaA-FliW-CsrA negative-feedback regulatory circuit has been reported in C. jejuni. C. jejuni CsrA primarily binds flagellar mRNAs and/or their 5′ un-translational regions (UTRs) that repress their RNA processing and translation [14]. C. jejuni CsrA binds to the abundant flaA mRNA and its UTR that translationally represses flaA mRNA. In turn, the CsrA activity is modulated by the flaA titration. The concentration of the flaA mRNA accordingly controls the expression of other flagellar genes through CsrA-mediated posttranscriptional regulation [14]. More interestingly, C. jejuni CsrA activity is also antagonized by FliW proteins, which bind the N-terminal subdomain of FlaA flagellins for optimizing the homeostasis of flagellins [14, 30].
In H. pylori, CsrA is shown to maintain full motility and facilitate adaptation to environmental stresses by mediating its effect at the posttranscriptional level [16, 18]. The CsrA regulons include oxidant-induced transcriptional response and heat shock response (ahpC, NapA, GroESL, and HspR), virulence, and acid adaptation (napA, cagA, vacA, fur, and urease operon) [14]. Loss of csrA also impairs flagellar motility. In the non-motile N6 ∆csrA and 26695 ∆csrA mutant strains, the processing and translation of the elevated flaA and flaB mRNAs may be influenced by CsrA, in contrast to the reduced flaA and flaB mRNAs and decreased FlaA/FlaB of the non-motile J99 ∆csrA mutant strain [16, 18]. Although CsrA is a critical determinant of gene regulation and flagellar biosynthesis, the regulation of CsrA is uncertain. In this study, we attempted to understand whether H. pylori CsrA activity is regulated by protein antagonism and how this mechanism affects H. pylori motility. We employed a comparative genomic analysis to identify two fliW homologs (jhp1081-encoded FliW1 and jhp1291-encoded FliW2) from the H. pylori J99 genome. We characterized the isogenic ΔfliW2 mutant strain by phenotypic and biochemical analyses, and determined the FliW2-CsrA interaction using a machine learning approach and an in vivo bacterial two-hybrid system. Our findings revealed the crucial roles of FliW2 in flagellar motility and in de-repression of H. pylori motility by allosteric obstruction of posttranscriptional regulator CsrA.
2 Materials and Methods
2.1 Bacterial Strains and Inoculation
H. pylori strain J99 was incubated at 37°C under microaerobic conditions (5% O2, 10% CO2, 85% N2). For agar plate culturing, strains were grown on Brucella (BD Biosciences, USA) agar plates supplemented with 10% horse serum (Gibco BRL, Life Technologies, USA) for 32 to 36 h. For liquid culturing, H. pylori was inoculated in Brucella broth containing 10% horse serum with a starting optical density at 600 nm of 0.2 optical density units (ODU) using a shaker at a speed of 150 rpm. H. pylori cells were collected when bacteria reached mid-log phase (1.5 ODU) or early stationary phase (4.0 ODU) [32]. When needed, 10 μg/mL chloramphenicol (Cm) or 10 μg/mL kanamycin (Km) was added to Brucella medium during inoculation. For E. coli inoculation, E. coli was grown on Luria-Bertani (LB) (BD Biosciences) agar plates or in broth media at 37°C. If required, 100 μg/mL ampicillin (Amp), Cm (25 μg/mL), or Km (25 μg/mL) were supplemented in LB media. The bacterial strains and plasmids used in this study are described in Table S1.
2.2 Construction of In-Frame Deletion ΔfliW2 Mutant of H. pylori
An in-frame deletion approach was employed to construct a ΔfliW2 knockout mutant strain of H. pylori J99 (genome accession number: NC_000921.1). We used the genomic DNA (gDNA) of J99 wild-type (WT) strain as a template, along with primers jhp1291(fliW2)-PCR-1-XbaI and jhp1291(fliW2)-PCR-2-BamHI (Table S2) to amplify a ~ 1.5 kb fragment containing fliW2 and its upstream and downstream flanking sequences. This amplicon was then ligated to XbaI/BamHI-cleaved pGEMTeasy to generate pGEMTeasy-fliW2 (Table S1). Next, we applied inverse PCR approach to generate an in-frame deletion of fliW2 with primers jhp1291(fliW2)-mut-3 and jhp1291(fliW2)-mut*-4, followed by ligation with HincII-cleaved Cm resistance cassette. The resultant pGEMTeasy::fliW2::Cm (pKO-fliW2) was delivered to H. pylori J99 WT strain using natural transformation. After homologous recombination occurred, the Cm-resistant transformants were inoculated for gDNA isolation to validate the deletion of fliW2 gene. The mutation of the ΔfliW2 #5 strain was confirmed by Southern blotting and Sanger sequencing analyses. In addition, we performed cDNA-qPCR analysis to rule out polar effect in this ΔfliW2 mutant strain (Figure S1).
2.3 RNA Isolation, cDNA Conversion, and cDNA-Quantitative PCR Analysis
Bacterial pellets were collected at 1.5 ODU by centrifugation and preserved in RNALater solution (Thermo Fisher Scientific, USA). Total RNAs were extracted using GENEzol TriRNA Bacteria Kit (Geneaid, Taiwan) according to the manufacturer's recommendations. The RNA concentration and its quality were determined using a Nanodrop spectrophotometer (NanoDrop ND-1000, USA) and agarose gel electrophoresis. Subsequently, a total of 0.5 μg RNA was converted to cDNAs using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan) according to the manufacturer's protocols. Standard PCR was performed to ensure that there was no residual gDNA. For cDNA-qPCR analysis, cDNA samples were diluted ten-fold using an elution buffer (50 mM Tris–HCl, pH 8.0). The qPCR reaction was prepared containing diluted cDNA, 2× Fast SYBR Green Master mix (Thermo Fisher Scientific) and qPCR primers (Table S2), followed by execution in a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). The amplification cycling was set as follows: 95°C for 20 s, followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. Data was collected during the extension step. The melting curve was included for qPCR primer quality control. Relative quantification of gene expression was determined and analyzed using the ABI software and 2−ΔΔCt method [33], compared to gyrA Ct value as endogenous gene control.
2.4 Motility Assay Using Soft Agar Plates
A tip-full of H. pylori cells were inoculated by being vertically touched to the surface of soft agar (0.3%) plates (pH 7 and 6; Brucella agar/10% horse serum). After a seven-day incubation, we measured the diameter of the migrated bacteria. The motility of each strain was calculated as mean ± SD from at least three independent experiments. The ΔflaA mutant strain serves as a non-motile control.
2.5 Flagellar Filament Examination Using Scanning Electron Microscopy
The grown H. pylori J99 WT, ΔflaA, and ΔfliW2 strains were diluted in Brucella broth containing 2% horse serum to have an initial OD600 of 0.2. Next, we transferred 1.5 mL of diluted H. pylori cells to 24-well plates, which contained lysine-coated glass coverslips. After 30 to 60 h of inoculation under microaerophilic conditions at 37°C, the supernatants were discarded, and the cells were fixed with 2.5% glutaraldehyde in PBS buffer at 4°C for overnight. Fixed cells were washed twice in PBS buffer, and dehydrated by adding 1 mL of ethanol in water in increasing concentration of 70%, 85%, 95%, and 100% (v/v) at room temperature for 10 min. An additional two dehydrations were conducted using 100% ethanol for 15 min for each time, followed by a critical point drying at the Electron Microscopy Facility (National Yang Ming Chiao Tung University). The coverslips containing the adherent cells were immediately coated with Au/Pd on a sputter coater. The examination was performed using a field emission scanning electron microscope (Jeol JSM-7600F, Japan) at an acceleration voltage of 5 kV. The micrographs were taken from at least two different fields from two independent experiments.
2.6 Generation of Mouse Polyclonal Anti-FlaA and Anti-FliW2 Sera
We over-expressed and purified recombinant FlaA and FliW2 proteins from E. coli BL21 harboring pET29b-FlaA and pET22b-FliW2 (Table S1), respectively, according to the recommendations from the manufacturer (Sigma-Aldrich, USA). One hundred micrograms of purified recombinant FlaA and FliW2 proteins were mixed with complete Freund's adjuvant (1:1, v/v), and subcutaneously injected into female C57BL/6 mice (six to eight-week-old). Mice were boosted three times biweekly for 6 weeks. In the seventh week, the sera were collected from the immunized mice and stored at −20°C.
2.7 SDS-PAGE and Western Blotting Analysis
Total proteins of collected H. pylori cells were extracted and subjected to 10% SDS-PAGE. In brief, after transferring proteins to PVDF membranes and blocking in 5% skimmed milk, we probed the membranes in TBS buffer containing 0.1% Tween 20 at 4°C overnight with diluted (1:5000) mouse anti-FlaA, anti-FliW2 polyclonal antibodies (in-house), or mouse anti-GroEL (Hsp60) monoclonal antibody (Product number H3524, Sigma-Aldrich, USA). GroEL served as an internal control.
2.8 In Silico Predictions of FliW2, CsrA, and flaA RNA Secondary Structure
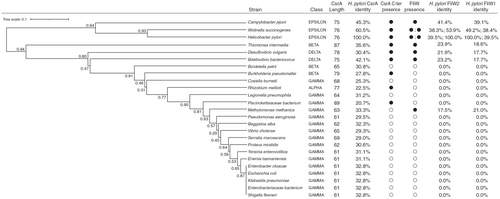
We used H. pylori J99 CsrA as a query to construct the phylogenetic tree analysis (Figure 1, left panel). The CsrA homologs (Table S3) from a range of representative taxa spanning proteobacteria were aligned using (standalone) MAFFT v7.526, the L-INS-i method, and default settings [34]. With the alignment as input, a distance matrix was generated using the WAG amino acid model implemented in phangorn v2.12.1 in R v4.4.1 [35]. A tree topology was generated via UPGMA (unweighted pair group method with an arithmetic mean) and also implemented in phangorn. Bootstrap analysis was carried out and the results were plotted using phangorn's plotBS() function. Protein homolog comparison (Figure 1, right panel) was performed using the H. pylori J99 CsrA (UniProt ID: Q9ZJH4), FliW1 (UniProt ID: Q9ZK60), and FliW2 (UniProt ID: Q9ZJL5) as queries for protein identity analysis. The alignments were executed using Clustal O (version 1.2.4) on the UniProt website with default settings. The UniProt accession numbers for the CsrA homologs and FliW homologs are listed in Tables S3 and S4, respectively.
To compare the structure and conservation of H. pylori J99 FliW1 and FliW2 (Figure S2), we used MMseqs2 (version 71dd32ec43e3ac4dabf111bbc4b124f1c66a85f1) for a single-against-many search for homologous sequences using FliW1 (UniProt ID: Q9ZK60) and FliW2 (UniProt ID: Q9ZJL5) as query sequences. This strategy was implemented in ColabFold v1.5.5 [36], a structural prediction tool containing AlphaFold2 and hosted as a Jupyter Notebook (https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2.ipynb). ColabFold uses three databases: UniRef30 (a clustered version of UniRef100), PDB70 and a bespoke environmental dataset hosted by ColabFold (https://colabfold.mmseqs.com) in the homology search. The procedure employed limits the number of sequences in each multiple sequence alignment (MSA), reducing redundancy therein, while collecting sequences within (and retaining similar sequences between) buckets with different degrees of similarity to the query sequence, that is, it samples diverse sequences inferred by the query while reducing the overall alignment size. ColabFold was run with default parameters via the online notebook. For the MSA these parameters were “msa_mode = mmseq2_uniref_env and pair_mode = unpaired_paired.”
Protein sequence alignment of the H. pylori J99 CsrA (UniProt ID: Q9ZJH4), FliW1 (UniProt ID: Q9ZK60), and FliW2 (UniProt ID: Q9ZJL5) homologs were carried out using CLC Genomic Workbench (version 24.0.1). The 3D structure of FliW2-CsrA interacting regions was predicted using ColabFold based on DeepMind AlphaFold2 [36] and reconstructed using PyMoL. The RNA secondary structure of the 5'UTR of flaA mRNA (50 bases) was executed in the RNA Folding Form V2.3 at the UNAFold Web Server (http://www.unafold.org/) with a default setting.
2.9 In Vitro Pull-Down Assays
2.9.1 H. pylori Experiment
To demonstrate that FliW2 interacts with CsrA in H. pylori, we first over-expressed recombinant C-terminal histidine-tagged CsrA protein (CsrAHis) from pET22b-CsrA (Table S1) in E. coli BL21(DE3) host cells. After sonication, we applied the E. coli lysate containing His-tagged CsrA proteins and protease inhibitors to the nickel-charged (Ni2+) magnetic beads in Lysis Equilibration (LE) Buffer (50 mM phosphate buffer, 300 mM NaCl, 0.05% Tween 20, and 1.5 mg/mL Lysozyme; pH 8.0) at 4°C for overnight. This generated CsrAHis-bound Ni2+-magnetic beads (CsrA-beads), which were washed twice in Wash Buffer (50 mM phosphate buffer, 300 mM NaCl, 10 mM imidazole; pH 8.0) and ready for protein–protein interaction. To prepare H. pylori lysates, 10 mL of freshly grown H. pylori cells were collected when they reached early stationary phase (OD600 = 4–6). The H. pylori cells were washed and resuspended in ice-cold LE Buffer containing lysozyme, DNase I, RNase A, and protease inhibitors, followed by sonication. After centrifugation at 2000 × g for 15 min at 4°C to remove cell debris, the supernatants of H. pylori lysates were saved and ready for protein pulldown assay. We then pooled the CsrAHis-bound beads with the lysates of H. pylori WT or ΔfliW2 as prey proteins and mixed using an inverter at 4°C for 2 h. After washing, we added 100 μL Elution Buffer (50 mM phosphate buffer, 300 mM NaCl, 500 mM imidazole; pH 8.0) to elute CsrAHis and its interacting proteins. The eluents were examined using Western blotting. FlaA and FliW2 were probed using mouse anti-FlaA and anti-FliW2 polyclonal antibodies, respectively. The negative control group was the H. pylori ΔfliW2 lysate pooled with CsrAHis.
2.9.2 E. coli Experiment
We over-expressed CsrAHis (pET22b-CsrA) and tag-free FliW2 (pET22b-FliW2-noHis) (Table S1) in E. coli BL21 (DE3) cells, respectively, by induction using isopropyl-β-D-thiogalactopyranoside (IPTG) at 0.4–0.6 ODU. The E. coli cells were incubated at 25°C for 4 h, harvested by centrifugation at 9020 g for 20 min, and resuspended in Binding Buffer (20 mM HEPES, 500 mM NaCl, 10% glycerol, 1 mM β-mercaptoethanol, pH 7.5). We pooled 3 L of E. coli (pET22b-FliW2-noHis) and 1 L of E. coli (pET22b-CsrA) cells, and underwent bacterial disruption by sonication. The resulting supernatant was loaded onto a nickel-NTA affinity column (Ni Sepharos 6 Fast Flow, GE Healthcare) that was previously equilibrated in Binding Buffer. The column was sequentially washed in Buffer A (20 mM HEPES, 300 mM NaCl, 1 mM β-mercaptoethanol, pH 7.5) containing 25 mM imidazole. Proteins were eluted in Buffer A supplemented with 300 mM imidazole. The eluted proteins were examined by SDS-PAGE, followed by Coomassie brilliant blue staining and analysis.
2.10 Bacterial Two-Hybrid Analysis
We chose an in vivo approach, the Bacterial Adenylate Cyclase-based Two-Hybrid (BACTH) system (Euromedex, France), to examine the interaction between CsrA and FliW2. In brief, pKT25 (N-terminal T25 segment fusion) serves as a vector for constructing a bait protein, while pUT18 (C-terminal T18 segment fusion) vector is used for a target protein. The fliW2 gene and csrA gene carrying various deletions were individually PCR-amplified, BamHI/Acc65I-digested, and cloned into the BamHI/Acc65I-cleaved pKT25 and pUT18 vectors (Table S1). After Sanger sequencing validation on the constructed clones, pKT25- and pUT18-derivative clones were co-transformed into an E. coli reporter strain DHM1. The transformants were grown in LB broth with ampicillin, kanamycin, and IPTG for 16 h, then the cells were lysed and extracted for β-galactosidase activity measurement. An increase of β-galactosidase activity in Miller units is an indication of positive protein–protein interactions. E. coli DHM1 cells harboring vectors pKT25 and pUT18 served as a negative control, whereas the cells harboring pKT25-FeoB and pUT18-FeoC were a positive control [37]. We also included background signal controls to distinguish false-positive protein interactions.
2.11 Ethics Statement
Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of National Cheng Kung University, Taiwan (approval number: 109127). Animal well-being, sedation, and analgesia were monitored and administered as indicated to minimize stress and pain associated with any veterinary procedures.
2.12 Statistical Analysis
Statistical analysis was assessed using an unpaired t test with Welch's correction, one-way or two-way analysis of variance (ANOVA) (GraphPad Prism 8). Statistical significance was represented as *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001 unless indicated.
3 Results
3.1 Evolutionary Characteristics of the CsrA and FliW2 Homologs in H. pylori and Proteobacteria
We identified two fliW homologs (jhp1081-encoded FliW1 and jhp1291-encoded FliW2) from the H. pylori J99 genome using the BLAST analysis. Subsequently, we constructed multiple sequence alignments (MSAs) using MMseqs2 for comparison against diverse sequences (Figure S2). Our result showed a more consistent sequence coverage distribution across the length of FliW2 than across the length of FliW1, particularly among the bulk of the sequences that have intermediate similarities to the query. It appears that the N-terminal region of FliW1 is less well-represented in MSAs than that of FliW2. This analysis implied that the H. pylori FliW2 is more likely to be conserved than the FliW1. Our proteome analysis of the H. pylori J99 wild-type strain also showed that FliW2 production was ten-fold more than that of FliW1 in neutral or acidic media (Table S5). Further phylogenetic tree analysis was carried out to investigate whether H. pylori CsrA has an association with the FliW2 protein over macroevolutionary time scales. We identified the presence of csrA and fliW genes and extracted their protein sequences from the genomes of the representative members of Proteobacteria. Interestingly, H. pylori's CsrA sequence is approximately 15 residues longer at the carboxyl terminus than that of Gammaproteobacteria (Figure 1, right panel). Based on evidence presented elsewhere in this paper, H. pylori CsrA likely interacts with FliW2. As well, we found that most Gammaproteobacteria do not contain FliW proteins (Figure 1, right panel). Therefore, the genomic characteristics of these CsrA and FliW2 show that H. pylori differs from other Proteobacteria (Figure 1, left panel). We also predicted the RNA secondary structure of the 5′ un-translational region (UTR) of the major flagellin flaA transcript, revealing two hairpin structures containing the CsrA binding motif NGGA (Figure S3). This finding was in agreement with the hypothesis that CsrA dimers bind to the flaA mRNAs in C. jejuni [14]. We therefore hypothesized that FliW2 modulates CsrA by allosteric obstruction of target transcripts (i.e., the major flagellin flaA), thus de-repressing the motility of H. pylori.
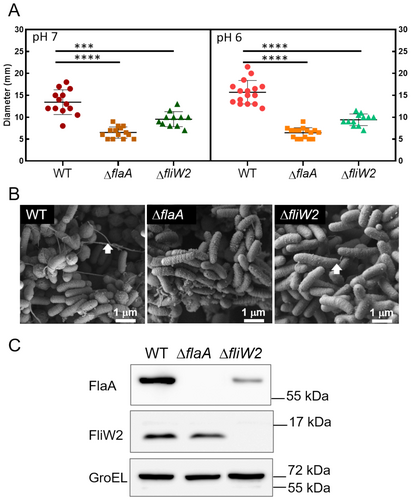
3.2 Disruption of fliW2 Impairs the Flagellar Motility of H. pylori
To discover the function of FliW2, we constructed an in-frame deletion mutant of fliW2 using a gene replacement approach. The resultant ∆fliW2 mutant was validated using Sanger sequencing and cDNA-qPCR analysis, confirming that the ∆fliW2 mutant had no polar effects (Figure S1). We examined the motility of the ∆fliW2 mutant in the soft agar plates. Our results showed that, unlike the WT cells, the ∆fliW2 mutant cells lost motility in both the neutral and acidic media (pH 7 and 6), similar to the non-motile ∆flaA cells (Figure 2A). We also studied the flagellar structure of the ∆fliW2 mutant cells using scanning electron microscopy. The ∆fliW2 mutant cells were primarily aflagellate or had only short-protruding flagella, differing from the long tangled flagellar filaments observed in the WT cells (Figure 2B). To further investigate the non-motile ∆fliW2 mutant cells, we analyzed the expression of the major flagellin FlaA by Western blotting. The FlaA expression was substantially reduced in the ∆fliW2 mutant cells (Figure 2C), indicating that FliW2 modulates FlaA expression through direct or indirect regulations. In order to test our hypothesis, we investigated whether or not FliW2 interacts with CsrA.
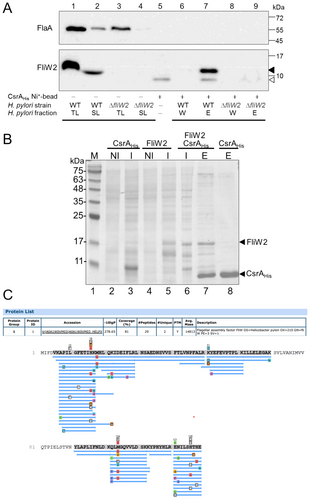
3.3 Interaction Between FliW2 and CsrA Proteins
To demonstrate protein interactions between FliW2 and CsrA, we carried out pull-down assays. We generated nickel ion magnetic beads bound with recombinant histidine-tagged CsrA protein (CsrAHis). By applying the total lysate of the H. pylori WT strain to the beads, it allowed us to detect the proteins that interact with CsrAHis under native conditions. After washing, the FliW2 signal was revealed in the eluted fraction by Western blotting (Figure 3A, Lane 7). To determine whether or not FliW2 binds to CsrA directly, we overexpressed the recombinant CsrAHis (pET22b-CsrA) (Figure 3B, Lane 3) and the recombinant tag-free FliW2 (pET22b-FliW2-noHis) (Figure 3B, Lane 5), respectively, in E. coli BL21 (DE3) cells. Then we pooled the E. coli lysate containing FliW2 proteins with the other E. coli lysate, which contained CsrAHis proteins. As we hypothesized, the tag-free FliW2 was co-eluted with the CsrAHis through nickel column separation, showing that the FliW2 directly interacted with the CsrA (Figure 3B, Lane 7). The overexpressed protein band (~ 15 kDa) was then excised, purified, and analyzed by liquid chromatography with tandem mass spectrometry. The matched peptide fragments of H. pylori FliW2 were identified (Figure 3C). The combined results of these investigations demonstrate that H. pylori FliW2 interacts directly with CsrA.
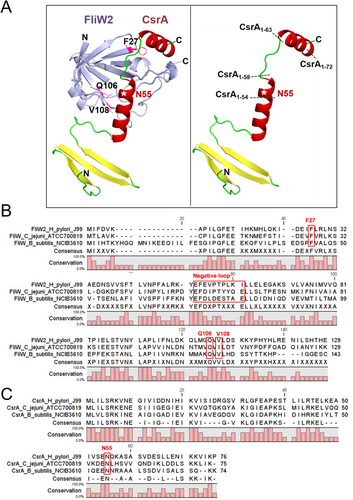
3.4 AlphaFold2 Prediction of FliW2-CsrA Interacting Regions
To identify the FliW2-CsrA interacting regions, we took advantage of the deep learning algorithm AlphaFold2 (AF2) to predict the pattern of protein interactions. As shown in Figure 4A (Left panel), the β-barrel region of FliW2 formed a cleft that is predicted to interact with CsrA. The structure of H. pylori CsrA contains the N-terminal β-strands region and a loop–helix–loop–helix region, where we predicted that the C-terminal extension helix–loop–helix (residues 55–76) would interact with FliW2 (Figure 4A, right panel). The findings of earlier loss-of-function studies of the FliW and CsrA in B. subtilis and C. jejuni [38, 39] allowed us to postulate the crucial residues of FliW2 (F27, Q106, and V108) and CsrA's core proximal cluster (N55) for protein binding (Figure 4B,C). Subsequently, we designed successive truncations of CsrA to determine the region that is essential for the CsrA-FliW2 binding based on our predictions. The deletion of the C-terminal extended helix–loop–helix structure of CsrA occurs in CsrA1-72 (4-residue deletion), CsrA1-63 (13-residue deletion), CsrA1-58 (18-residue deletion), and CsrA1-54 (22-residue deletion) (Figure 4A, right panel).
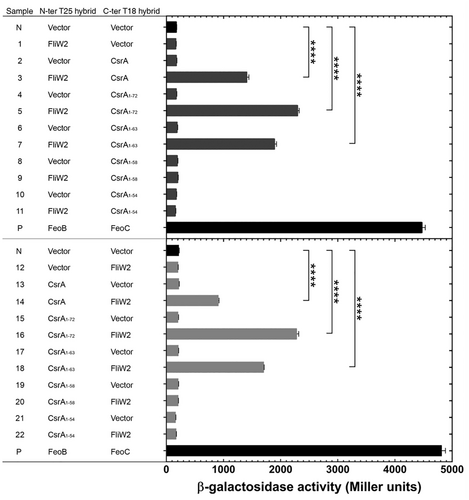
3.5 Determination of the Critical Regions of CsrA for FliW2 Interaction In Vivo
Following our AF2 prediction, we performed in vivo bacterial two-hybrid analysis to determine FliW2-CsrA interactions and the regions required for the FliW2-CsrA binding. We chose the Bacterial Adenylate Cyclase-based Two-Hybrid (BACTH) system to generate T25-fused bait proteins using pKT25 (N-terminal T25 fusion) and T18-fused target proteins using pUT18 (C-terminal T18 fusion). In our first set of experiments, we detected the activity of β-galactosidase by analyzing T25-fused FliW2 and T18-fused CsrA, and T18-fused CsrA truncating proteins (Figure 5, upper panel). The increase in β-galactosidase activity observed in samples 3, 5, and 7 showed that FliW2 was bound to full length CsrA, CsrA1-72, and CsrA1-63. With the elimination of the C-terminus end 18 and 22 residues of CsrA, FliW2 could not bind to CsrA1-58 or CsrA1-54 and the β-galactosidase activity was not significantly elevated compared to negative controls (Samples 9 and 11). To verify whether the C-terminal extension of CsrA for FliW2 binding was not an artificial defect, we created T25-fused CsrA proteins (full-length or with truncations) and T18-fused FliW2 in our second set of experiments (Figure 5, lower panel). Consistently, the binding between CsrA and FliW2 was absent when the C-terminal 18 and 22 residues of CsrA were deleted (Samples 20 and 22). Taken together, these results showed that the C-terminal region of CsrA is crucial for interaction with FliW2.
4 Discussion
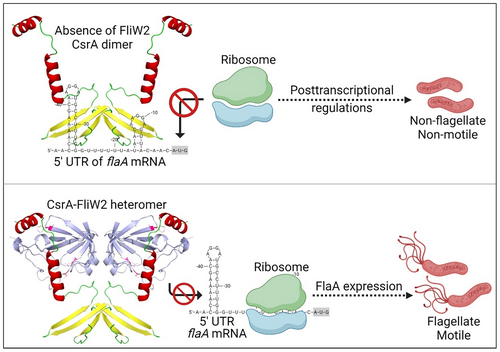
In this study we have characterized the H. pylori flagellar assembly factor FliW2 that interacts directly with the posttranscriptional global regulator CsrA. Hence, FliW2 may affect CsrA's repression of the major flagellin FlaA expression and flagellar biosynthesis (Figure 6). Based on our in silico analyses, we postulated that the H. pylori FliW2 protein modulates CsrA functionality based on (i) CsrA binding motifs predicted at the 5'-UTR of the flaA mRNA; (ii) the region of CsrA for binding to FliW homologs; and (iii) the identification of fliW homologs, fliW1 and fliW2, in the genome of H. pylori. The phenotypic analyses of the isogenic ∆fliW2 mutant cells clearly demonstrated that the loss of FliW2 diminishes H. pylori's flagellar formation and motility, as well as the production of FlaA. Further prediction of the CsrA-FliW2 interactive domains by the AlphaFold2 algorithm revealed the crucial binding regions that were then examined using in vitro pull-down assays and in vivo bacterial two-hybrid system analysis. Our data points to novel regulatory roles that the H. pylori flagellar assembly factor FliW2 has in obstructing CsrA activity and in antagonizing CsrA's regulation of flaA mRNA processing and translation. This may have implications for the control of flagellar motility in H. pylori.
The biogenesis of flagella in H. pylori and C. jejuni is tightly regulated by regulators and small RNAs, and significantly different from that in the Gammaproteobacteria E. coli due to the lack of the master regulator FlhDC. Interestingly, Konig et al. recently discovered novel posttranscriptional non-coding small RNAs, the RpoN-dependent CJnc230 and the FliA-dependent CJnc170, that fine tune the hierarchical regulation of flagellar biogenesis in C. jejuni [8]. In contrast to this finding, small RNA-mediated posttranscriptional regulation in stress response and virulence control has been reported in H. pylori [11, 40-42]. The cag-PAI encoded small RNA CncR1 down-regulates a flagellar checkpoint factor fliK and promotes the expression of H. pylori adhesins [11]. As well, the small RNA RepG (regulator of polymeric G-repeats) found in H. pylori not only controls lipopolysaccharide phase variation but also the variable G-repeat in the mRNA leader of a chemotaxis receptor gene tlpB [40]. However, there has been no small RNA study reported in relation to the modulation of CsrA in H. pylori or C. jejuni. Even though we could not rule out the possibility of small RNAs regulating CsrA, our study evidently supports the possibility of an alternative mechanism in which FliW2 indirectly modulates flagellar genes posttranscriptionally via protein allosteric antagonism on riboregulator CsrA.
We also give an explanation for the biological meaning of FliW1 and FliW2 in the H. pylori of Epsilonproteobacteria. Since the genome of H. pylori is merely 1.6 M base-pairs in length and the co-occurrence of fliW paralog is widespread (Figure 1), it seems unusual that it possesses two fliW homologous genes in H. pylori. We postulated that H. pylori requires FliW1 and FliW2 to antagonize CsrA repression of flagellar biogenesis for H. pylori's survival in the human gastric niche under heterogeneous conditions. Previous studies have reported that flagellar and chemotactic motility remarkably increases the survival of H. pylori in the extremely acidic environment of the stomach by allowing the bacteria to rapidly swim and colonize the mucin layer of the gastric epithelium [6, 12]. Therefore, despite the known regulatory cascade of flagellar biosynthesis in H. pylori, CsrA aids in governing the expression and assembly of flagellar filaments [18, 43]. This implies the importance of H. pylori utilizing FliW1 and FliW2 to antagonize CsrA repression in different ways. The supporting data includes our genomic analyses (Figure 1 and Figure S2) showing that the fliW2 gene is likely more conserved than the fliW1 gene, and our proteome analysis of H. pylori J99 indicates that the expression of FliW2 is approximately ten-fold higher than FliW1 (Table S5). This could explain our observation on the short flagellar filament and trace amount of FlaA production in the ∆fliW2 mutant (Figure 2B,C), possibly due to the positive FliW1-CsrA interaction in the bacterial two-hybrid assay (data not shown). Nonetheless, Niehus et al. propose a transcriptional regulation model in the flagellar system in several H. pylori strains. In their model, the fliW1 (HP1154) gene belongs to the middle stage (class 2) flagellar genes whose expression is modulated by the alternative sigma factor RpoN54 and FlgR regulator. On the other hand, the expression of the fliW2 (HP1377) gene is presumably under the control of the housekeeping RpoD [7]. It seems possible that H. pylori FliW1 and FliW2 modulate CsrA at the different stages of flagellar biosynthesis, though there has been no evidence demonstrating that fliW1 and fliW2 are differentially regulated. This mechanism is not yet understood and remains to be explained. Additionally, investigating the ∆fliW1 knockout and/or the ∆fliW1∆fliW2 double knockout strains of H. pylori would significantly improve our understanding of whether fliW1 also plays a role in the antagonism of CsrA and in the FlaA regulation. More interestingly, the key residues of H. pylori FliW1-FliW2-CsrA interaction are worthy of further investigation to distinguish the physiological roles in the FliW1-CsrA and the FliW2-CsrA. Discovering how the FliW1-FliW2-CsrA interaction alters the modulation of flaA mRNA would require delicate investigations such as protein–protein and protein-RNA crystal structures to find the key conformations and residues of the FliW1-FliW2-CsrA-flaA mRNA complex. Collectively, our findings support the hypothesis that FliW2 is the dominant antagonist of CsrA and de-represses H. pylori's motility.
The truncations on the C-terminal extension of H. pylori CsrA demonstrate the importance of its interaction with the FliW2 protein. Our in vivo bacterial hybrid-system showed the residues 59–63 of CsrA (Figure 5), which was predicted to be a loop structure (Figure 4A), might have an uncovered significant role in binding to FliW2. Nonetheless, the truncated CsrA proteins may be unstable as they have not been tested. Another interesting finding is that the residues constituting the negative loop of H. pylori FliW2 obtains only two negatively charged residues (E53 and E55) (Figure 4B), compared to that of B. subtilis FliW (E71, D73, D75, E76, and E80) [38]. This suggests that the negative loop of the H. pylori FliW2 might not cause a strong electrostatic repulsion with the SD sequence like the B. subtilis FliW does to hinder RNA interaction. Hence, the H. pylori flagellar biosynthesis system would serve as an interesting model to study the co-evolution between the loss of CsrA's C-terminal extension in association with FliW1, FliW2, and small RNAs (CsrB/D) in proteobacteria. The outcome would help us to understand why in Gammaproteobacteria the CsrA proteins without the C-terminal extension are modulated by small RNAs but not FliW antagonism.
In conclusion, we propose a model of the allosteric obstruction of FliW2 to CsrA that de-represses the motility of H. pylori (Figure 6). In the absence of FliW2, CsrA proteins form homodimers binding to the overlapping CsrA motif-ribosomal binding sites of a target transcript. This may affect the translation of the target transcripts and/or their mRNA processing, thus influencing the expression of FlaA and flagellar formation. As a consequence, CsrA modulates the motility of H. pylori through a negative regulatory mechanism at the posttranscriptional level. However, when FliW2 is present, it interacts with CsrA to form heterodimers. The conformation of the FliW2-CsrA heterodimers prevents them from binding to the target transcripts. This allows ribosomes to bind and start the translation of the target transcripts, attenuating the negative regulation of CsrA. Taken together, FliW2 de-represses the flagellar motility of H. pylori by the allosteric obstruction of CsrA.
Author Contributions
M.S.-W.S. conceptualized research; M.S.-W.S., B.D., F.Y.K., W.-J.T., Y.-L.C., J.-W.C., S.W., and P.-J.T. performed research; M.S.-W.S. and B.D. wrote and edited the manuscript. J.-J.W. supervised the project, edited the manuscript, and obtained research funding. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank Drs. Ryan Holroyd and Shiau-Ting Hu for their helpful comments and editing on this manuscript. We are grateful for the technical support from Professor Tsuey-Ching Yang Lab and Yui-Ying Yu at the Electron Microscopy Facility in the National Yang Ming Chiao Tung University, and for the graphic design from Shu-Chen Su. This study was supported by the Ministry of Science and Technology, as well as the National Science and Technology Council in Taiwan (MOST 108-2320-B-010-002, 108-2811-B-010-536, 109-2320-B-010-040, 109-2811-B-010-535, and 110-2811-B-A49A-027; NSTC 111-2811-B-468-001, NSTC 112-2811-B-468-001, and NSTC 113-2811-B-468-002). Figures were created with BioRender.com.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The dataset supporting the conclusions of this article is included in the article.




