Helicobacter macacae MazF interplays with Escherichia coli homologs and enhances antibiotic tolerance
Xi Zeng and Limiao Hu contributed equally to this work.
Abstract
Background
Toxin-antitoxin systems are highly variable, even among strains of the same bacterial species. The MazEF toxin-antitoxin system is found in many bacteria and plays important roles in various biological processes such as antibiotic tolerance and phage defense. However, no interplay of MazEF systems between different species was reported.
Materials and Methods
MazEF toxin-antitoxin system of Helicobacter macacae was examined in three Escherichia coli strains with and without endogenous MazEF knockout. In vivo toxicity, antibiotic tolerance, and live/dead staining followed by flowcytometry analysis were performed to evaluate the functionality and interplay of the toxin-antitoxin system between the two species.
Results
Controlled ectopic expression of MazF of H. macacae (MazFhm) in E. coli did not affect its growth. However, in endogenous MazEF knockout E. coli strains, MazFhm expression caused a sharp growth arrest. The toxicity of MazFhm could be neutralized by both the antitoxin of MazE homolog of H.macacae and the antitoxin of MazE of E. coli, indicating interplay of MazEF toxin-antitoxin systems between the two species. Induced expression of MazFhm enhanced tolerance to a lethal dose of levofloxacin, suggesting enhanced persister formation, which was further confirmed by live/dead cell staining.
Conclusions
The MazEF toxin-antitoxin system of H. macace enhances persister formation and thus antibiotic tolerance in E. coli. Our findings reveal an interplay between the MazEF systems of H. macacae and E. coli, emphasizing the need to consider this interaction while evaluating the toxicity and functionality of MazF homologs from different species in future studies.
1 INTRODUCTION
Toxin–antitoxin systems were initially discovered in plasmids that maintain their stability by selectively killing daughter cells lacking the plasmid.1 Typically, the unstable antitoxin forms a complex with the stable toxin to neutralize its toxicity. However, in a daughter cell that did not inherit the plasmid containing the toxin–antitoxin system, no continuous antitoxin is produced, leading to the death of the cell by the toxin. Toxin–antitoxin systems have also been found in chromosomes, which are believed to have been acquired via horizontal gene transfer.2 The function of them is controversial and enigmatic, which arranged from genome stability, nutritional stress, biofilm and persister formation in antibiotic tolerance, oxidative stress to phage defense.3-5 Based on the properties and action mode of antitoxins, the toxin–antitoxin system could be divided into eight groups.2 Among them, Type II is the most common and most studied, with both toxins and their cognate antitoxins being proteins.
MazEF is a Type II toxin–antitoxin system in Escherichia coli that has been extensively studied. Its toxin, MazF, is an endonuclease that specifically recognizes the ACA trinucleotide and induces global RNA cleavage.6, 7 The antitoxin MazE could bind to MazF in a heterohexamer form of MazF2-MazE2-MazF2 and neutralize the enzymatic activity of MazF.8 There are also DNA binding spaces formed by the two MazEs, which control the transcription of the MazEF operon. Induction of MazF expression results in a bacteriostatic state rather than programmed cell death, which can be reversed by reintroducing the cognate antitoxin MazE, provided that toxin expression is not prolonged.7, 9 A moderate direct interaction between MazEF and rifampicin has also been reported, and the dissociation constant is approximately in the range of dozens of micromoles, which may vary depending on the dye employed in the fluorescence binding assay.10 MazF-mediated bacteriostatic status or persister formation is important for bacterial response to environmental stress.11
MazEF systems are present in many bacteria species besides E. coli. While most MazF homologs recognize a unique RNA sequence and exert endonuclease activity, the exact recognized sequence, target RNA types, and exertion mechanisms are diverse. The Mycobacterium tuberculosis genome contains nine MazF homologs (MazF-mts). Expression of MazF-mt3, MazF-mt6, and MazF-mt9 could induce bacteriostasis. These MazF-mts of M. tuberculosis synergistically respond to environmental changes such as oxidative stress, nutrient depletion, and antibiotic resistance.12 In addition to their mRNA endonuclease activity, MazF-mt3 cleaves 16S and 23S rRNA; MazF-mt6 specifically recognize UU^CCU and cleaves the 23S rRNA helix 70 in the ribosomal A site (^ indicates cleavage site), thus inhibiting translation13, 14; MazF-mt9 recognize tRNALys and cleaves at UUU of the anticodon loop, regardless the sequences outside of the loop.15, 16 In Staphylococcus aureus, MazF recognizes and cleaves mRNA at UACAU, causing a global change in the transcriptome, translatome, and proteome, resulting in bacterial dormancy and antibiotic resistance.17-19 In Nitrosomonas europaea, MazF specifically recognizes and cleaves at UGG trinucleotide and specifically, and two transcripts: hydroxylamine dehydrogenase (hao) and a large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase (rbcL), are among its targets.20 MazF of Methanohalobium evestigatum specifically cleaves mRNA at CUGGU/UUGGU.21 Although these MazF homologs share similar overall tetra-structure, they recognize different sequences and target different RNAs, and often they are toxic to E. coli. No interplay between them and MazF from E. coli has been reported.20-22
Here we reported a MazF homolog from Helicobacter macacae (MazFhm), a bacterium colonizing rhesus monkey and causes digestive symptoms. H. macacae infection of rhesus was used as a model for human H. pylori infection,23 and a systemic search of MazF homolog in the latter species resulted no subject.24, 25 MazFhm is only toxic to E. coli in endogenous MazF knockout strains, and its induced expression results in bacteriostasis and increased resistance to levofloxacin. To the best of our knowledge, this is the first report of MazEF system in Helicobacter genus and the interplay of MazEF homologs from different species.
2 MATERIALS AND METHODS
2.1 Plasmids
The MazEF sequences of H. macacae MIT 99-5501 strain26 was retrieved from Genbank (acc. AZJI00000000.1) and chemically synthesized (Qinlan Biotech). Polymerase chain reaction (PCR) was performed to amplify MazF from the synthesized DNA, then cloned to KpnI and HindIII double digested pBAD33, with a ligation-independent method (Vazyme), designated as pBAD33-MazFhm. MazEhm was also amplified from the synthesized constructed and cloned to BamHI and HindIII double digested pQE80L, designated as pQE80L-MazEhm. MazF and MazE from E. coli was similarity amplified with genomic DNA from E. coli as templates and cloned to pBAD33 to pQE80L, designated as pBAD33-MazFec and pQE80L-MazEec, respectively. The primers used in the PCR amplification are listed in Table S1.
2.2 Knockout strains
The CRISPR/Cas9 knockout protocol followed the method described in Ref [27]. Briefly, primers targeting E. coli MazF (MazFec) were designed and used to amplify pTargetF, which was then transformed into competent cells and screened for positive colonies, designated as pTargetF-MazF. To obtain the homologous donor template, two homology arms flanking the MazEF locus were amplified from E. coli genomic DNA via PCR (primers listed in Table S1). Subsequently, an assembly PCR was conducted using the forward primer of the left arm and the reverse primer of the right arm as a primer pair, and the mixture of PCR products of the two homology arms was utilized as a template. The PCR product was purified with agarose gel electrophoresis, resulting the homologous donor template. The temperature-sensitive vector pCas, which stably expresses Cas9 and contains an inducible guide RNA targeting the replication origin of pTargetF, was transformed into MG1655 or BW25113 (kindly provided by the National Institute of Genetics, Japan28), or DH5α and maintained at 30°C with 50 μg/mL kanamycin. A positive colony, which was confirmed with PCR using primers targeting the coding region of Cas9, was selected and made into electroporation competent cells. Then, 100 ng of pTargetF-MazF together with 1 μg of the homologous donor template were co-electroporated into the competent cells, which subsequently spread to plates containing 50 μg/mL kanamycin and 50 μg/mL spectinomycin. Positive knockout colonies were verified by PCR (primers listed in Table S1) and Sanger sequencing. The pTargetF-MazF vectors were erased by the addition of isopropyl 1-thio-B-d-galactopyranoside (IPTG) to induce guide RNA expression, which is encoded in pCas and targets the pTargetF-MazF replication origin. The culture system was then brought to 37°C to further erase pCas. The knockout strains were confirmed by high-throughput sequencing of genomic DNA. The primer sequences are listed in Table S1.
2.3 In vivo toxicity assay of MazF
The pBAD33-MazFhm plasmid was introduced into WT or knockout strains (MG1655, BW25113, DH5α, and their MazEF knockout strains MG1655ΔMazEF, BWΔMazEF, DHΔMazEF, respectively) which were then cultivated at 37°C overnight on Luria Broth (LB) agar plates containing 30 μg/mL chloramphenicol to screen out positive colonies. Individual colonies were picked out, verified for positive transformation, cultured overnight. The colonies were diluted 1:100 and then cultured at 37°C in a shaking incubator. Optical density at 600 nm (OD600) was monitored using a spectrophotometer. Arabinose was added at a final concentration of 0.2% when OD600 reached 0.4. Then OD600 was measured at indicated time points. For antitoxin rescue experiments, pQE80L-MazEhm or pQE80-MazEec was co-introduced to knockout strains. At OD600 0.4, arabinose and IPTG were added at a final concentration of 0.2% and 0.2 mM, respectively. OD600 was measured at indicated time points. Simultaneously, at each time point (5, 10, 30, 60 min post-induction) the samples were 10-fold serially diluted using LB medium without antibiotics and 3 μL aliquots were spot inoculated in LB agar plates without antibiotics and incubated at 37°C overnight.
2.4 Antibiotic tolerance assay
Overnight cultures of knockout strains transformed with pBAD33-MazFhm were diluted 1:100 in 50 mL of LB medium containing 30 μg/mL chloramphenicol and incubated at 37°C with shaking until OD600 reached 0.4. Arabinose was then added to a final concentration of 0.2%. After 5 min, an aliquot of the cultures was collected and washed twice before being serially diluted and inoculated onto LB agar plates containing 0.2% glucose to serve as the initial colony formation unit (cfu). The same strain without arabinose induction was used as a control. At the same time, the bacteria were exposed to 10 times the minimum inhibitory concentration (MIC) of levofloxacin (0.32 μg/mL) for 4 h, washed twice, serially diluted, and plated onto agar plates containing 0.2% glucose. The survival colonies were then calculated. The percentage of survival was obtained by dividing the number of colonies after levofloxacin exposure by the initial cfu.
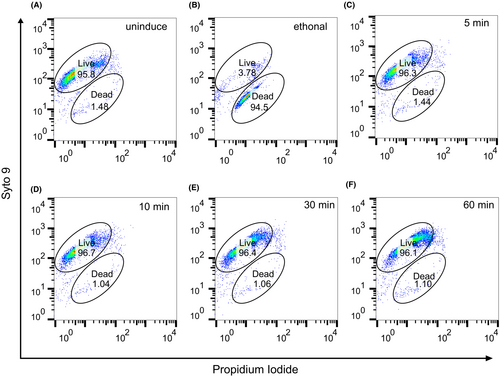
2.5 Live/Dead staining and flow cytometry analysis to determine the percentage of live and dead bacterial cells
Cell preparation was performed as described above for MazFhm toxicity assays. At 5, 10, 30, and 60 min post-induction, the cultures were centrifuged, and the pellet was washed three times with phosphate-buffered saline (PBS). The cells were then standardized to McFarland 0.5 (1.5 × 108 cfu/mL). Staining was performed according to the manual of the Bacterial Viability and Counting Kit (Thermo; #L34856). Briefly, the cells were diluted 1:100 into solutions containing propidium iodide and Syto 9. Dead control cells were prepared by treating cells with 75% ethanol. Four single staining samples were used for setting up flow cytometry (BD FACS Arial II).
3 RESULTS AND DISCUSSION
3.1 The MazEF locus in H. macacae
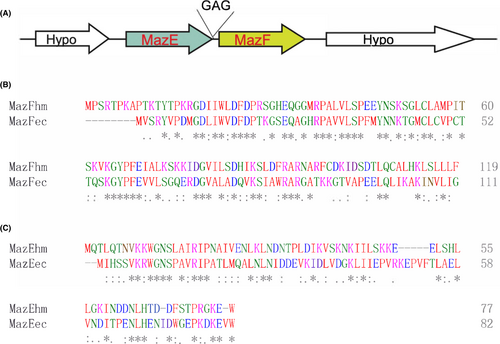
MazEhm is located upstream of MazFhm, there are three nucleotides between the stop codon of MazEhm and the start code of MazFhm (Figure 1A). This is consistent with classical type II toxin-antitoxin settings in the operon.29 The surrounding two genes are both hypothetical. The MazFhm and MazEhm contain 119 and 77 amino acid residues respectively, which is of similar size with other MazEF homologs.22 The isoelectric point of MazEhm and MazFhm are 9.06 and 9.38, respectively, both higher than pH 7.0, which is different from classical MazEF systems, whose toxin MazF is usually acidic, and the cognate antitoxin are basic at neutral pH.21, 22 MazFhm shared 42.5% amino acid identity with MazFec, and MazEhm shared 30.6% amino acid identity with MazEec, which is comparable to similarities of MazF homologs from other species with MazFec.21, 22, 30
3.2 MazFhm ectopic expression is only toxic in MazEFec knockout cells
MazFhm was cloned into an arabinose-controlled expression vector pBAD33 and introduced into E. coli strain MG1655. Arabinose was added to induce MazFhm expression, to our surprise, no growth arrest was observed as determined by measuring the OD value at a wavelength of 600 nm (Figure 2A). Similar growth curves were obtained when MazFhm was expressed in E. coli strains BW25113 and BL21 (Figure S1A,B). We presumed that the low-level expression of MazFhm controlled under araBAD promoter of pBAD33 vector was neutralized by endogenous MazEec of E. coli. To verify this, we introduced MazFhm to a high expression vector pET28a, whose expression is under control of T7 promoter and Lac operon, which generates far more transcripts than pBAD33 vector system.31 As expected, MazFhm expression in the pET28a system successfully inhibited growth of E. coli strain BL21 (Figure S2). To further confirm that endogenous MazEec neutralized the toxicity of MazFhm, we utilized the CRISPR/Cas9 system to construct MazEF locus knockout strains of E. coli. As schematic in Figure 2B, a homologous donor template containing upstream and downstream sequences of MazEF locus was co-transfected with a CRISPR vector targeting the locus in MG1655. PCR amplification using primers spanning the region confirmed the deletion of MazEF gene locus, with a 940 bp fragment amplified from the genomic DNA of the knockout strain, and a 1500 bp fragment from the wild type MG1655 strain (Figure 2C). Additionally, high throughput sequencing of the knockout strain's genomic DNA confirmed the deletion of the MazEF gene locus, with no reads aligned to the locus (Figure 2D), indicating successful deletion without transfer to other genomic regions. We designated the resulting strain as MG1655ΔMazEF.
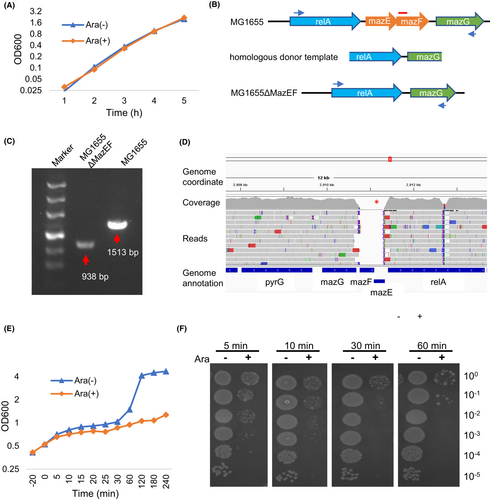
pBAD33-MazFhm was introduced into MG1655ΔMazEF, and the expression of MazFhm was induced by arabinose addition, results showed that ectopic expression of MazFhm caused growth arrest of MG1655ΔMazEF (Figure 2E). To investigate the time course of MazFhm toxicity, bacteria were spread to agar plates (without arabinose), and the colonies growth out were observed. The results showed that even a 5-min induction of MazFhm expression greatly inhibited the growth of MG1655ΔMazEF (Figure 2F). Similar results were obtained in other E. coli strains with endogenous MazEF knockout, including BW25113 and DH5α (BWΔMazEF and DHΔMazEF, respectively, Figure S3).
3.3 MazEhm is an antitoxin of MazFhm and could be neutralized by MazEec
We introduced pBAD33-MazFhm and pQE80L-MazEhm into MG1655ΔMazEF by co-transformation. As previously observed, the induced expression of MazFhm by addition of arabinose inhibited bacterial growth. However, when IPTG was also added to induce MazEhm expression, the growth inhibition caused by MazFhm was reversed (Figure 3A), indicating that MazFhm-MazEhm formed a toxin-antitoxin pair. This neutralizing effect of MazEhm on MazFhm was also observed in BWΔMazEF and DHΔMazEF (Figure S4). To further examine the growth effects, the bacteria was also spread into agar plates to observe the colonies. Consistent with the growth curve, the number of colonies in agar plates with MazFhm induction was significantly decreased, while the number of colonies with MazFhm-MazEhm co-induction was similar to that of the non-induced cells (Figure 3B).
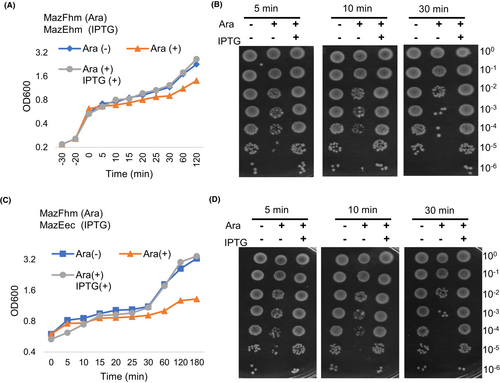
The above results indicated that MazFhm is a toxin that may be counteracted by the endogenous MazEec of E. coli. To confirm whether MazEec can neutralize MazFhm in vivo, we co-expressed MazFhm and MazEec in MG1655ΔMazEF and induced their expression with arabinose and IPTG, respectively. The growth curve measured by OD600 (Figure 3C) and colony growth on agar plates (Figure 3D) showed that MazEec perfectly reversed the toxicity of MazFhm, indicating that MazEec can indeed neutralize MazFhm. Similar neutralization experiments were also performed in BWΔMazEF and DHΔMazEF, both further verified that MazEec could neutralize the toxicity of MazFhm (Figure S5). MazF homologs from various species have been evaluated for toxicity in E. coli, but the interplay between these homologs and MazF of E. coli has often been overlooked. In many studies, MazF homologs were cloned to araBAD promoter-controlled expression system (for example pBAD vector system) to evaluate the toxicity, and no interference of endogenous E. coli MazEF was reported.20-22, 32, 33 The MazF homolog of Legionella pneumophila and Agrobacterium tumefaciens cloned to pBAD24 showed significant growth arrest of E. coli strain BW25113.22, 32 For generation of large amount of proteins, in some studies, MazF homologs were cloned into high expression vectors such as pColdTF, which contains a large chaperone tag to help solubility. The tag may also hinder the toxic activity of MazF.15, 21 Still some cloned MazF homologs into vectors such as pET vector systems whose protein expression is controlled by phage T7 promoter, which may unintentionally ignored the interaction of different MazEF systems.20 Our results showed MazFhm showed no growth effect to E. coli while cloned to pBAD33, while growth effect was observed if MazFhm was expressed under control of T7 promoter. The knockout of endogenous MazEF system recovered the toxicity of pBAD33-MazFhm, which could also be neutralized by MazF of E. coli. These results verified the interplays of MazF homolog from H. macace and E. coli, which should be taken into account when evaluating the toxicity of MazF homologs from different species.
3.4 MazFhm expression enhanced resistance to levofloxacin
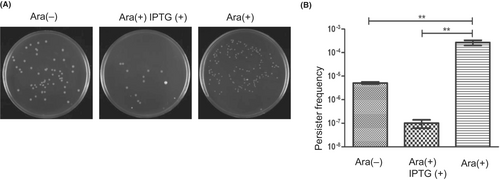
One of the functions of the toxin-antitoxin system is to respond to stress, such as antibiotics, with the toxin facilitating the formation of persister bacteria that can tolerate antibiotics.34 We investigated the possible effect of MazFhm expression on antibiotic tolerance. MG1655ΔMazEF was co-transformed with pBAD33-MazFhm and pQE80-MazEhm, and MazFhm expression was induced for 5 min or co-induced with MazEhm for 5 min. A lethal dose of antibiotics (10 times the MICs) was added, and the bacteria were cultured for an additional 4 h before rinsing with PBS twice. The washed bacteria were then spread onto agar plates, and the colonies were counted. MazFhm expression showed more than 10-fold increase in tolerance to levofloxacin (Figure 4A,B). Interestingly, co-expression of MazFhm and MazEhm significantly reduced the tolerance to levofloxacin (Figure 4A,B), which was probably due to the overproduce of antitoxin MazEhm. Similar experiments were performed with tetracycline and clarithromycin, but no visible enhanced tolerance was observed upon MazFhm expression (data not shown).
3.5 MazFhm expression caused cell growth arrest but not death
It has been suggested that the expression of MazFec leads to the formation of persisters, allowing bacteria to regrow after antibiotic withdrawal. To further investigate the viability status of bacteria, we performed Live/Dead staining followed by flow cytometry analysis. Results revealed that without treatment, the proportion of living cells was approximately 96% (Figure 5A). However, after inducing MazFhm expression for 5 min up to 1 h, no significant changes were observed in the proportion of living cells (Figure 5C–F). These findings suggested that MazFhm does not kill cells but rather induces a bacteriostatic condition, also known as a persister state. Initial studies suggested that MazE were depleted under stress condition, activating MazF that causing programmed cell death in a (p)ppGpp-dependent manner.2 However, latter studies failed to reproduce the programmed cell death phenotype and indicated that MazF induced a bacteriostatic status which was fully reversible under antitoxin MazE expression.2, 35, 36 Based on its property, MazF was also used as counterselection marker.37 Our studies supported the bacteriostatic mode.
This study characterizes the first MazEF system in the Helicobacter genus. The MazEF system in H. macacae exhibited similar overall properties to homologs found in other species, including the organization of the toxin–antitoxin genes, the size of the encoded proteins, and the percent of amino acid similarities to E. coli homologs. However, a notable difference was observed in the isoelectric point of MazEF system in H. macacae. MazEhm and MazFhm were both higher than pH 7.0, unlike classical MazEF systems. We verified the toxicity of MazFhm in three E. coli strains, namely BW25113, MG1655, and DH5α. The toxicity of MazFhm was neutralized by MazEhm, indicating that they function as a toxin–antitoxin pair. Furthermore, our findings demonstrated that MazF from H. macacae induced a bacteriostatic state or persister formation in E. coli, as evidenced by Live/Dead staining and flow cytometry analysis. This increased persister formation subsequently led to enhanced tolerance to levofloxacin. These results highlight the significance of the toxin–antitoxin system as part of the arsenal for Helicobacter virulence and antibiotic resistance. Unexpectedly, we discovered an interplay between the two MazEF systems of H. macacae and E. coli, which may be overlooked in previous studies of MazEF from other species. This observation should be taken into consideration when evaluating the toxicity and functionality of MazF homologs from different species in future studies.
AUTHOR CONTRIBUTIONS
Guo-Qing Li and Logen Liu concepted and designed the research. Xi Zeng, Limiao Hu, Qi Ai, Cai-Juan Liu, Lu-Xi Xiong, and Wei-Wei Yang performed the experiments. Xi Zeng, Limiao Hu, Logen Liu, and Xiaotuan Zhang analyzed the data. Limiao Hu and Xi Zeng drafted the paper. Logen Liu and Guo-Qing Li revised the manuscript, and all authors approved the final version of the manuscript.
FUNDING INFORMATION
This study was supported by grants from the Natural Science Foundation of Hunan province (grant numbers 2021JJ40483 and 2022JJ30517), the Science and Technology Department of Hunan Province (grant numbers 2021SK4029, 2021QZY020, 2021ZK4259, and 2023TP1014), and the Science and Technology Bureau of Hengyang (grant number 202010041574).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The raw Illumina sequencing data of the knockout strains are stored in NCBI under BioProject number PRJNA816422.




