Global Comparative Antithrombin Field Study: Impact of Laboratory Assay Variability on the Assessment of Antithrombin Activity Measurement at Fitusiran Clinical Decision-Making Points
Funding: This study was funded by Sanofi.
ABSTRACT
Introduction
Fitusiran is a subcutaneous, investigational small interfering RNA therapeutic that lowers antithrombin (AT) to increase thrombin generation and rebalance haemostasis in people with haemophilia A or B with or without inhibitors.
Aim
To evaluate and compare the performance of commercially available in vitro diagnostic (IVD) AT activity assays.
Methods
Field study sample kits with plasma AT activity levels (100, 36, 14 and 9 IU/dL or % of normal) were created and distributed to global haemostasis laboratories. Values were assigned based on Siemens INNOVANCE AT activity assay using BCS-XP analyser. Reliability (relative accuracy estimate), intra- and inter-laboratory variability of IVDs in measuring AT activity in plasma samples using various commercially available AT assays was assessed.
Results
At normal AT activity level (i.e., 100%), all AT assays reliably measured AT activity with acceptable recovery. Accurate results were observed for all samples across sites using Siemens INNOVANCE AT assay. Increased variability was observed for all other assays at low AT levels. Siemens Berichrom and Stago STA-Stachrom assays accurately measured 100% and 36% AT activity; however, lab-to-lab variability was observed for ≤15% AT activity (CV >20%). All laboratories for the Stago STA-Stachrom assay failed to measure 9% AT activity. The HemosIL assay significantly underestimated AT activity levels ≤36%. There were no reported values for the 14% and 9% AT samples.
Conclusions
Siemens INNOVANCE AT assay can reliably measure AT activity at clinical decision points of 15–35% of normal and is most suitable for clinical management of patients taking fitusiran.
1 Introduction
The treatment landscape for individuals with haemophilia is rapidly changing, with novel therapies targeting various components of the haemostatic system, including physiologic coagulation inhibitors [1]. Haemophilia A and B are rare bleeding disorders caused by Factor VIII (FVIII) or Factor IX (FIX) deficiencies, respectively, leading to insufficient thrombin generation and an incomplete clotting process [1, 2]. Antithrombin (AT) is a physiological anticoagulant that regulates blood clotting and prevents excessive clot formation [3]. Fitusiran is a subcutaneous, investigational, small interfering RNA (siRNA) therapeutic that lowers the AT level to increase thrombin generation and rebalance haemostasis in people with haemophilia A or B (PwHA/B) with or without inhibitors [4-6]. For optimal safety and efficacy, fitusiran dosing aims to achieve and maintain AT activity levels within a range of 15%–35% of normal [7]. This AT-based dosing regimen (AT-DR) is being investigated in PwHA/B to improve the safety profile of fitusiran compared with the original 80 mg fixed-dose regimen whilst maintaining clinically meaningful bleed protection. On AT-DR, most PwHA/B usually reach steady-state AT levels between 15% and 35% within 6 months of fitusiran treatment. As plasma AT activity levels determine fitusiran dosing, it is important to measure AT levels during the initial dose adjustment phase (6 months) until target AT activity of 15–35% is achieved, and thereafter only annual AT testing throughout the treatment duration [7].
The primary function of AT is to inhibit several clotting factors, particularly thrombin (factor IIa/FIIa) and factor Xa (FXa) [3, 8]. AT activity in plasma is usually measured by chromogenic assays that measure the effectiveness of AT in inhibiting human (h) or bovine (b) FIIa or FXa [9, 10] and are routinely used to detect inherited or acquired AT deficiencies [8]. AT activity measurements compare sample AT activity to a normal reference range. The normal AT range is approximately 80%–120%, whilst in individuals with heterozygous AT deficiency, AT activity usually ranges between 40% and 60%. As fitusiran is a first-in-class AT-targeting therapy, and the AT-DR targets AT activity levels within the 15–35% range, it is necessary to accurately measure AT activity <35% of normal [3, 8-10].
Previously, multiple global field studies provided real-world data by evaluating the performance of FVIII and FIX clinical assays across a range of concentrations for different haemophilia recombinant therapies [11-15]. These studies helped in developing guidance for safe and effective use of various haemophilia therapeutics. Similar field studies have not yet been conducted to assess treatment-based AT lowering. Laboratories use a variety of approved in vitro diagnostic (IVD) AT assays; however, due to variables such as reagents, procedures, incubation times and analysers used, there may be variability in results when analysing the same sample [8, 16]. Proficiency testing data from real-world performance or comparability studies among various Food and Drug Administration (FDA) approved, commercially available IVD AT activity assays at low AT activity levels (i.e., <30%) is scarce [8, 16, 17]. As observed with factor replacement products, variability in assay performance is particularly worse when measuring low levels of analytes [9, 18]. Prior College of American Pathologists (CAP) data indicated there could be differences among various AT activity assays, particularly at low AT activity [19]. Therefore, it is necessary to understand both the performance of prevalent AT activity assays, as well as their expected variability in real-world settings within the target AT range of 15–35% of normal, relevant for guiding fitusiran dosing.
The objective of this study was to evaluate and compare the performance of commercially available IVD AT activity assays in global clinical haemostasis laboratories when measuring AT activity around medical decision points (15–35% normal) for fitusiran dosing. In addition, reliability (relative accuracy estimate), intra- and inter-laboratory variability of IVDs in measuring AT activity in plasma samples using various commercially available AT assays were assessed.
2 Materials And Methods
2.1 Field Study Sample Kits
Field study sample kits were manufactured and distributed to participating laboratories by Precision BioLogic Inc. Kits were manufactured by mixing AT-immunodepleted and normal pooled plasmas at various ratios. The AT-depleted citrated plasma was sourced from Affinity Biologicals and prepared by immunodepletion of AT (<1 IU/dL or <1% AT antigen levels [IU/dL or %] judged by enzyme-linked immunosorbent assay [ELISA]) from pooled normal citrated human plasma. Four plasma AT activity levels (100, 36, 14 and 9% of normal) were created. Initial AT levels selected were 35%, 15% and 10%, but based on quality testing results of plasma pools, final sample values were assigned to 36%, 14% and 9%. These values allow a wider range to cover the recommended 15%–35% AT activity. Every kit included 12 tubes (1 mL plasma each) of frozen plasma containing three aliquots for each AT activity level. Samples were distributed on dry ice as frozen aliquots, mimicking patient plasma samples stored in laboratories for routine testing of AT levels. Plasma samples were assigned values based on the Siemens INNOVANCE Antithrombin activity assay, used per its instruction for use (IFU) using the BCS-XP analyser. Siemens INNOVANCE AT on BCS-XP was used as the reference assay for assigning AT activity for generated plasma samples as it was used in the fitusiran clinical development programme for all Phase 1, 2 and 3 clinical trials for testing AT activity in patient plasma.
2.2 Participating Laboratories
A total of 52 haemostasis laboratories were invited to participate in the study. Of these, 48 academic, commercial and clinical/hospital haemostasis laboratories across 16 different countries were registered (Figure S1). All laboratories were instructed to test provided plasma samples using their routine AT activity procedures and calibrators. The laboratories were blinded to the sample contents in the kits. To assess intra-laboratory variability, each sample was required to be tested in triplicate over three separate days (i.e., nine reports per sample per assay in total). Thirty-nine sites performed testing using single AT activity assays, and nine sites tested more than one AT activity assay. Two participating clinical haemostasis laboratories were previously used for fitusiran clinical trial sample testing.
2.3 Data and Statistical Analysis
Each laboratory reported detailed information on their assay components and procedures, including AT assay kits, analyser employed and so forth. Most analysers and reagents used were manufacturer-recommended. Results were reported to Precision BioLogic Inc. and entries were cross-checked against source documentation for accuracy. Results were evaluated for reliability (accuracy estimate), intra- and inter-laboratory variation and potential reagent or instrument-specific assay discrepancies.
Pre-defined acceptable recovery criteria were set at ±20% of the assigned value with an intra-assay coefficient of variation (CV) <20%. Mean AT activity of three independent daily tests per plasma sample level (100, 36, 14 and 9 IU/dL) in triplicate was measured and plotted as percentage recovery of assigned values for each sample. Statistical evaluation was performed by the Analysis of Variance (ANOVA) method to assess significant differences within respective assay reagents or instrumentation.
2.4 Compliance and Quality Assurance
Data reporting and analysis were conducted in accordance with good scientific practices and were documented in accordance with good documentation practices at Precision BioLogic Inc., Canada, an ISO 13485 certified company. Most participating laboratories have a Clinical Laboratory Improvement Amendments (CLIA) or equivalent certification and participated in external proficiency programmes. It is assumed there was confidence in the performance of their existing AT assays by performing regular QC testing. However, the performance of these assays at low AT activity levels (≤35%) remains unknown. Most participants did not verify the limit of quantification (LoQ) of AT activity assays. Data were reviewed and cross-checked for accuracy and consistency before analysis. All original documentation was archived at Precision BioLogic Inc.
3 Results
3.1 Laboratory Demographic Data
Overall, 48 clinical haemostasis laboratories performed a total of 68 AT activity assays with various analysers using AT field study sample kits. Six sets of these assay results were excluded as outliers or because they were lab-developed tests; 62 results were analysed (Figure 1). The eight AT activity assays used in the field study by participating laboratories are described in Table 1.
Distribution of (A) AT activity assays and (B) analysers used in the AT field study analysis.
AT indicates antithrombin; bFIIa, bovine factor IIa; bFXa, bovine factor Xa; hFXa, human factor Xa; n, number of (A) assays and (B) analysers.
| Assay serial number | Manufacturer/vendor | Assay name | Reagent | Source | Form | Prevalence CAP (%)a | Prevalence ECAT (%)a | Assay status | LoD (%) | LoQ (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Stago | STA-Stachrom ATIII | FIIa | Bovine | Lyophilised | ∼42 | 25 | IVD/CE | 9 | NA |
| 2 | Werfen/I-L | HemosIL–AT | FXa | Bovine | Liquid | ∼33 | ∼31 | CE | 7.2 | NA |
| 3 | Siemens | INNOVANCE AT | FXa | Human | Liquid | ∼15 | ∼21 | IVD/CE | 6 | 7.6 |
| 4 | Siemens | Berichrom AT III | FIIa | Bovine | Lyophilised | ∼10 | ∼17 | CE | NA | 2.6–9.4 |
| 5 | Hyphen Biomed | BIOPHEN AT anti-(h)-Xa LRT | FXa | Human | Liquid | NA | ∼2 | IVD/CE | ∼5 | NA |
| 6 | Hyphen Biomed | BIOPHEN AT | FXa | Bovine | Lyophilised | NA | 0.7 | IVD/CE | ∼5 | NA |
| 7 | Hyphen Biomed | BIOPHEN AT (Anti-IIa) | FIIa | Bovine | Lyophilised | NA | 0.2 | RUO | <10% | NA |
| 8 | Technoclone | Technochrom ATIII | FIIa | Bovine | Lyophilised | NA | NA | IVD/CE | NA | NA |
- Abbreviations: AT, antithrombin; CAP, College of American Pathologists; CE, the Conformité Européene; ECAT, external quality control of diagnostic assays and tests; FIIa, factor IIa; FXa, factor Xa; IVD: in vitro diagnostics; LoD, limit of detection; LoQ, limit of quantification; NA, no data available; RUO, research use only.
- a Prevalence in 2022. CAP represents the use of assays in the US, whilst ECAT is mainly used in Europe. The Limit of Quantitation (LoQ) of the Berichrom assay is dependent on the instrument on which the assay was run, hence, it varies in the range of 2.6%–9.4% of norm.
3.2 AT Activity and Recovery
Field study results showed that at normal AT activity level (i.e., 100%), four most prevalent (HemosIL, Stachrom, Berichrom and INNOVANCE) and rarely (Biophen and Technochrom) used AT activity assays can reliably measure AT activity. Recovery was within acceptable range (i.e., within ±20% of the assigned value) with acceptable variability per assay (between-laboratories CV <5.2%; Table 2).
| AT assay | Assigned value (%) | Actual measured mean value (%) | N | SD (%) | CV (%) | Recovery (%) |
|---|---|---|---|---|---|---|
| INNOVANCE (hFXa) | 100 | 100.3 | 9 | 3.2 | 3.2 | 100.3 |
| 36 | 36.4 | 9 | 2.0 | 5.4 | 101.3 | |
| 14 | 14.0 | 9 | 1.4 | 9.7 | 99.6 | |
| 9 | 9.2 | 9 | 1.7 | 18.8 | 101.5 | |
| Biophen (hFXa) | 100 | 102.8 | 3 | 2.9 | 2.8 | 102.8 |
| 36 | 34.6 | 3 | 2.7 | 7.7 | 96.3 | |
| 14 | 10.9 | 3 | 2.0 | 18.6 | 77.8 | |
| 9 | 5.7 | 3 | 2.9 | 50.1 | 62.9 | |
| AT HemosIL (bFXa) | 100 | 101.7 | 22 | 3.8 | 3.7 | 101.7 |
| 36 | 24.9 | 22 | 2.8 | 11.1 | 69.1 | |
| 14 | NA | 0 | NA | NA | NA | |
| 9 | NA | 0 | NA | NA | NA | |
| Biophen (bFXa) | 100 | 101.3 | 3 | 5.2 | 5.1 | 101.3 |
| 36 | 31.8 | 3 | 2.0 | 6.2 | 88.3 | |
| 14 | 7.8 | 3 | 2.3 | 29.7 | 55.3 | |
| 9 | 4.3 | 3 | 2.5 | 57.5 | 46.9 | |
| Berichrom (bFIIa) | 100 | 98.9 | 11 | 3.2 | 3.2 | 98.9 |
| 36 | 36.6 | 11 | 2.5 | 6.9 | 101.7 | |
| 14 | 15.8 | 11 | 3.5 | 22.4 | 112.0 | |
| 9 | 11.3 | 11 | 3.7 | 33.0 | 124.4 | |
| STA-Stachrom (bFIIa) | 100 | 103.7 | 12 | 4.3 | 4.1 | 103.7 |
| 36 | 34.9 | 12 | 4.0 | 11.4 | 97.1 | |
| 14 | 12.8 | 8 | 2.1 | 16.4 | 90.9 | |
| 9 | NA | 0 | NA | NA | NA | |
| Biophen (bFIIa) | 100 | 96.5 | 1 | NA | NA | 96.5 |
| 36 | 33.6 | 1 | NA | NA | 93.3 | |
| 14 | 9.8 | 1 | NA | NA | 69.5 | |
| 9 | 5.0 | 1 | NA | NA | 55.0 | |
| Technochrom (bFIIa) | 100 | 105.9 | 1 | NA | NA | 105.9 |
| 36 | 34.2 | 1 | NA | NA | 95.1 | |
| 14 | 13.0 | 1 | NA | NA | 92.3 | |
| 9 | 7.8 | 1 | NA | NA | 85.3 |
- Note: AT activity of samples in the field study kit was assigned based on the Siemens INNOVANCE AT assay. Percentage recovery was calculated relative to the INNOVANCE AT assay as: (measured mean value/assigned value) x 100. For HemosIL (14% and 9 %) and Stachrom (9%) no values were reported due to assay limitations; 8 out of 12 laboratories reported values for the 14 IU/dL sample using STA-Stachrom thus, CV is likely not a true representation of the variability.
- Abbreviations: AT, antithrombin; bFIIa, bovine factor IIa; bFXa, bovine factor Xa; CV, coefficient of variation; hFXa, human factor Xa; LoQ, limit of quantification; n, number of laboratories; NA, no data available; SD, standard deviation.
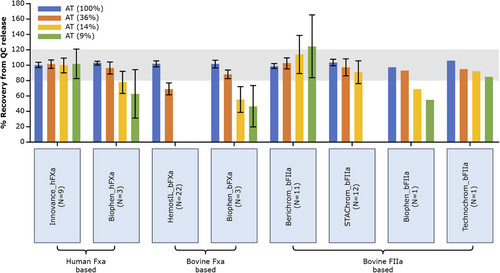
Data were grouped by the type of AT assay (human vs. bovine; FXa vs. FIIa) and percent recovery of AT activity was calculated relative to quality control (QC) release assay measurements (Siemens INNOVANCE AT/ BCS-XP assay) for each AT plasma sample at different AT activity levels (100, 36, 14 and 9 IU/dL) (Figure 2). Substantial intra- and inter-laboratory variations were observed between various AT assays at low AT levels (Table 3).
| Sample (%)a | Grand mean (%)a | N | Within-day (CV%) | Between-day (CV%) | Within-site (CV%) | Between-assays (CV%) |
|---|---|---|---|---|---|---|
| 100 | 101.4 | 62 | 1.8 | 2.3 | 2.7 | 3.9 |
| 36 | 31.6 | 62 | 3.5 | 5.2 | 5.9 | 18.8 |
| 14 | 13.4 | 36 | 7.3 | 15.3 | 15.4 | 25.3 |
| 9 | 8.9 | 28 | 10.3 | 23.5 | 23.2 | 42.2 |
- Abbreviation: CV, coefficient of variation.
- a IU/dL or %.
As shown in Figures 2, 3 and S2, decreasing AT activity levels demonstrated significant inter-assay variabilities at ≤36% AT activity: Siemens INNOVANCE AT (hFXa-based) assay consistently and accurately measured AT across all activity levels (CV <20%), with a CV of <10% for 100%, 36% and 14% AT activity and CV of ∼19% for 9% AT activity samples. Siemens Berichrom and Stago STA-Stachrom (bFIIa) assays were accurate in measuring 100% and 36% AT activity. However, higher lab-to-lab variability was observed for samples with <15% AT activity. With Siemens Berichrom assay, CV was >20% for the 14% (CV 22%) and 9% (CV 33%) AT activity samples. For Stago STA-Stachrom, only eight out of 12 laboratories reported a value for the 14% AT activity sample, thus, the reported CV of 16.4% (Table 2) is not a true representation. All laboratories using STA-Stachrom failed to measure 9% AT activity due to assay limitations. The LoQ for Siemens Berichrom was 2.5–9.5% (Berichrom's LoQ was dependent on the instrument used for the assay) and for Stago STA-Stachrom was not available (Table 1). The HemosIL (bFXa) assay significantly underestimated AT activity levels ≤36% (limit of detection [LoD] was 7.2%). For the 36% AT activity sample, recovery was 30% lower compared to the assigned value. All laboratories using HemosIL failed to report values for the 14% and 9% AT samples due to assay limitations. Three rarely used AT assays from Biophen (hFXa-, bFXa- and bFIIa-based assays) showed some underestimation, and the Technochrom (bFIIa-based assay) demonstrated accuracy within the expected range for all AT activity levels (i.e., within ±20% of assigned value for all activity levels). Due to low sample size (each n <4), the confidence in data from these four assays cannot be affirmed. No specific trend was observed with any analyser/instrument.
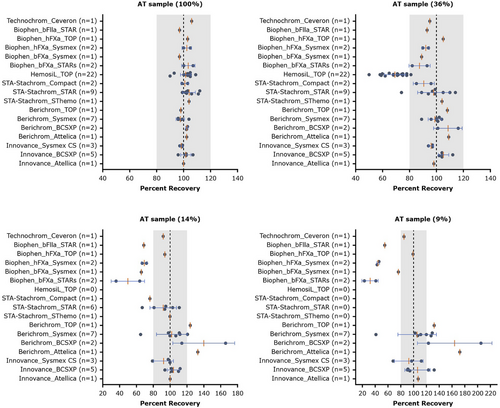
Data from the two clinical laboratories used in the fitusiran clinical development programme (for global participants’ sample testing, Siemens INNOVANCE AT activity assay was used and for participants in China, Siemens Berichrom assay) showed accurate and precise measurements at all AT activity levels, with recovery within ±20% of the expected value, except for the 9% AT sample where recovery was ∼29% higher for the laboratory in China (CV was <20% for all other AT levels).
4 Discussion
This global AT field study compared the reliability of commercially available IVD AT activity assays with the potential for fitusiran patient management. Results of this field study showed that at normal AT activity level (i.e., 100%), all assessed AT assays reliably measured AT activity, with acceptable recovery. Accurate results were also observed with respect to assigned values for all samples (100, 36, 14 and 9% of normal) across all participating sites that used the Siemens INNOVANCE AT assay. The INNOVANCE AT assay was used for the value assignment of the prepared plasma samples, which might explain the recovery results within the expected range. Increased variability was observed for all other assays at low AT levels. Regarding underperforming assays, the HemosIL (bFXa) assay significantly underestimated AT activity levels ≤36% with respect to assigned values; a similar trend of under-recovery for the ∼34% AT sample was observed during the CAP 2022 report [19]. With respect to the assays used in the fitusiran clinical development programme, these data further confirm that laboratories used in fitusiran clinical trials can measure accurately within the 15–35% range.
Fitusiran dosing is dependent upon targeting AT activity within the 15–35% range. Based on incidence rate of vascular thrombotic events during fitusiran clinical development, minimising prolonged exposure to AT activity <10% was identified as a risk mitigation measure, with a ‘buffer zone’ of AT activity at 10–15%. To optimise the benefit:risk profile of fitusiran, if a patient consistently has AT levels that are below 15% or above 35%, the dose should be adjusted so AT levels are maintained within the 15–35% range [4-6, 20-22]. Once a steady-state AT level is reached (occurring approximately 5–6 months post-dose), there are no significant fluctuations in AT activity, thus after initial testing only annual AT testing is recommended for patients on fitusiran prophylaxis.
Thus far, chromogenic assays typically have not been used for monitoring of AT lowering agents, but rather to detect AT deficiency (inherited or acquired) and to monitor treatment with AT concentrates [9, 23]. As AT is a natural inhibitor of FIIa and FXa, these factors are used (in separate assays) to saturate patient plasma samples, allowing endogenous AT to inhibit these factors. The remaining uninhibited FIIa or FXa are then measured by chromophore release when FIIa or FXa cleave the chromogenic substrate. AT is thus inversely measured by thrombin or FXa activity [9]. AT-lowering therapies decrease the production of endogenous AT, and therefore, these assays may be used for treatment monitoring in PwHA/B. However, assays accurate in detecting AT deficiency, defined as an AT level <80% (normal 80–120%), usually with a value between 40% and 60%, may not perform well with low AT levels (<35%) [23].
CAP and External Quality Control of Diagnostic Assays and Tests (ECAT) studies have shown comparable recovery rates between common AT assays, with most assays being effective within normal range (human AT values range from 112 to 140 µg/mL or 80–120%) [17, 19, 24, 25]. However, as the AT activity level decreases, variability (CV) increases. Prior CAP data indicated there could be differences among various AT activity assays, particularly when measuring abnormal AT activity; however, CAP and ECAT proficiency testing data using pre-diluted lyophilised samples do not usually examine plasma-based frozen samples (real-world comparability data) at very low AT activity levels [17, 19, 24, 25].
Variation often exists between various assays when measuring FVIII and FIX activities, with discrepancies often due to different assay components along with laboratory-based factors [11-13, 15, 23, 26]. This field study showed that there is substantial inter-laboratory and inter-assay variation when using chromogenic assays to measure AT activity levels. The potential reasons for this observed discrepancy at low levels can result from varying dilution factors, pH and diluent composition, buffer matrix used in calibration of chromogenic assays, calibration curves, plasma interference with kit components, heparin concentration used in each assay, lyophilised versus liquid components, incubation time and assay reagent components (human vs. bovine, or FXa vs. FIIa) [11-13, 15].
The two most discrepant assays used factor Xa inhibition as the intermediary, though one used hFXa (INNOVANCE) and the other bFXa (HemosIL). At lower AT levels, differences in the inhibition kinetics between bovine and human FXa potentially lead to underestimation with bFXa. Further evaluation is required to understand the underlying mechanism of this finding. The INNOVANCE AT assay was used as the benchmark for assigning reference AT activity in this study as it is used for all Fitusiran Phase 1, 2 and 3 clinical trials.
Siemens INNOVANCE AT (hFXa-based) assay can reliably measure AT activity (CV <10%) at clinical decision points of 15% and 35% and is thus recommended for AT activity measurement during fitusiran prophylaxis. This is the only AT activity assay with CV <10% in the AT target range of 15–35% and confirms that laboratories using the INNOVANCE AT assay in previous fitusiran clinical trials could accurately measure AT activity within the required 15–35% range. Siemens Berichrom and STA-Stachrom assays (bFIIa) can only be used for fitusiran monitoring after extra validation for ≤15% AT, providing CV <20%. The HemosIL (bFXa) assay significantly underestimated AT activity in samples ≤36% and is not recommended for guiding fitusiran dosing.
Previous global field studies have been conducted for recombinant factor therapies for haemophilia [14, 27-29], enabling their safe and effective use. As each product tends to have its own recommendations for assay usage [30-33], central reference laboratories may be used to measure AT activity during fitusiran prophylaxis where the recommended assay is not available at the local laboratory [30, 31, 34, 35]. Clinical guidelines regarding AT activity measurements will be established to guide the clinical use of fitusiran and allow for the design of real-world studies. This clinical tool would be useful for the management of patients with bleeding events.
In this study, various AT activity levels of plasma samples were achieved in vitro by blending pooled normal and AT-immunodepleted plasma. Ideally, AT assay performance should be further evaluated using ex vivo plasma samples from patients using fitusiran to confirm the commutability of our findings. Additionally, fitusiran works via RNA interference, lowering native AT levels without altering AT itself, thus assays used in this study should theoretically be able to measure AT levels below 10% (approved for measurement of 0%–100%). Underperforming assays may work with incorporation of a lower calibration curve to account for more accurate measurement at a lower range. However, this potentially falls outside the IFU for these assays, potentially re-classifying them as ‛lab-developed assays (LDT)’, which may be unsuitable for AT measurement during fitusiran prophylaxis. Notably, AT activity <15% is not expected to occur physiologically; these assays are developed for diagnostic purposes (i.e., AT deficiencies), which might explain why the LoQ was not verified.
5 Conclusion
This study provides important data regarding the performance of commercially available and approved chromogenic IVD AT activity assays across a range of AT activity levels. When measuring AT activity for patients treated with fitusiran, only three assays were found to be appropriate for use: Siemens INNOVANCE AT (hFXa) assay can reliably measure AT activity at clinical decision points of 15–35%, was used extensively during the fitusiran development programme and is recommended for guiding fitusiran dosing. Siemens Berichrom and STA-Stachrom (bFIIa) assays can only be used for AT measurement after extra validation for ≤15% AT activity. Additional ex vivo real-world comparability data are needed to further verify the discrepancies among the assays.
Author Contributions
All authors provided substantial contributions to the conception of the study, contributed to drafting and reviewing of the manuscript and approved the final version for submission.
Acknowledgements
Medical writing support was provided by Amy Hepple, MBBCh, of Ashfield MedComms, an Inizio Company, and was funded by Sanofi. The following laboratories participated in this study: Werfen (IL), USA; Fondazione Luigi Villa, Italy; Christian Medical College, Vellore, India; CHU de Lille, France; Hospices Civils de Lyon, France; IRCCS Humanitas Research Hospital, Italy; Azienda Ospedaliera Pugliese–Ciaccio, Italy; Sheffield Haemophilia and Thrombosis Centre, UK; K.J. Somaiya Hospital & Research Center, India; Fundación Privada Instituto de Investigación Oncológica Hospital Vall d'Hebron, Spain; North Hampshire Hospital NHS Foundation Trust, UK; University Hospital Southampton NHS Foundation Trust, UK; The Alfred Hospital, Australia; NSW Health Pathology (Royal Prince Alfred Hospital), Australia; Coagulation Profile B.V., Netherlands; Children's Hospital of Los Angeles, USA; National Coagulation Centre, Ireland; Labor Berlin, Germany; Fiona Stanley Hospital, Australia; Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, Italy; MedStar Georgetown University Hospital, USA; Special Coagulation Laboratory, University of Michigan, USA; Trustees of Columbia University in the City of New York, USA; King Faisal Specialist Hospital & Research Centre/Alfaisal University, Saudi Arabia; Hemostasis Reference Laboratory (HRL), Canada; Labcorp Coagulation (Esoterix), USA; Shaikh Shakhbout Medical City, United Arab Emirates; Vanderbilt Medical Center, USA; University Hospitals Cleveland Medical Center, USA; University of Chicago Medicine, USA; ARUP Laboratories, USA; Royal Free Hospital, Haemophilia Laboratory (HSL Analytics), UK; Tricore Reference Laboratories, USA; Tri-Service General Hospital, Taiwan; Laboratorie Hemostase, CHU de Nantes, France; Precision BioLogic, Canada; St. Joseph's Hospital, USA; Barts Health NHS Trust, UK; Machaon Diagnostics Inc, USA; Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUH), Taiwan; Chung Shan Medical University Hospital, Taiwan; Stago, France; HRLMP (McMaster), Canada; Hospital Cochin, France; University of Bonn, Germany; Klinikai Laboratóriumi Kutató Tanszék vezetője, Hungary; Shanghai Thalys Medical Laboratory Co., Ltd, China; WuXi AppTec Co., Ltd, China; Haemostasis Laboratory, University of Campinas, Brazil; Universitätsklinikum Leipzig, Germany; Düzen Biyolojik Bilimler Araştırma Geliştirmeve Üretim A.Ş, Turkey; Hyphen BioMed, France.
Ethics Statement
There were no human or animal participants in this study; only outcomes from nonclinical in vitro experiments are reported.
Conflicts of Interest
Ekta Seth Chhabra, Mingjie Liu, Cecile Le Camus, Myew-Ling Toh and Marek Demissie are current employees of Sanofi and may hold shares or stock options in the company. Ali Sadeghi-Khomami is an employee of Precision BioLogic Inc. Guy Young has received grants from Sanofi and Takeda; and speaking and consultancy fees from ASC Therapeutics, Apcintex, BioMarin, CSL Behring, Genentech/Roche, Grifols, Hema Biologics/LFB, Novo Nordisk, Pfizer, Sanofi, Spark and Takeda. Steven W. Pipe has received consultancy fees from for Apcintex, ASC Therapeutics, Bayer, BioMarin, CSL Behring, HEMA Biologics, LFB, Novo Nordisk, Pfizer, Regeneron/Intellia, Roche/Genentech, Sanofi, Takeda, Spark Therapeutics and uniQure, and research funding from Siemens and YewSavin and holds a membership on a Scientific advisory committee for GeneVentiv and Equilibra Bioscience. Margareth C. Ozelo has received consultancy fees from BioMarin, Novo Nordisk, Pfizer, Roche and Takeda; research support from BioMarin, Novo Nordisk, Pfizer, Roche, Sanofi, Spark, Takeda; honoraria from BioMarin, Novo Nordisk, Takeda; and has served on the advisory committee for BioMarin, Bayer, Novo Nordisk, Roche, Sanofi and Takeda. Flora Peyvandi has received honoraria from Roche, Sanofi, Sobi and Takeda and is a member of the advisory boards of Roche, Sanofi, Sobi and Takeda.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




