Plasma-Derived von Willebrand Factor/Factor VIII Concentrate (Haemate P) in von Willebrand Disease: A Systematic Review and Pharmacovigilance Update
Funding: This study was supported by CSL Behring.
ABSTRACT
Background
Von Willebrand disease (VWD) is an inherited bleeding disorder caused by deficient or dysfunctional von Willebrand factor (VWF). VWF replacement therapy is indicated in VWD management.
Methods
This systematic review was conducted to evaluate all available evidence of the efficacy, safety, dosing and consumption of pasteurized plasma-derived human coagulation FVIII/human VWF (pdVWF/FVIII; Haemate P/Humate-P) concentrate for on-demand (OD) treatment, surgical prophylaxis and long-term prophylaxis of patients with VWD. A systematic search was performed in MEDLINE and Cochrane Library databases to identify studies (7 June 1982–31 May 2023) reporting the use of pdVWF/FVIII in VWD according to predefined selection criteria. Pharmacovigilance data were also retrieved for the same period.
Results
Fifteen studies were identified, 12 being observational and three interventional. Efficacy and safety assessments and treatment protocols varied across the studies which hindered direct comparisons. Haemostatic efficacy of pdVWF/FVIII was rated excellent/good for OD treatment in 95%–98% of bleeds and in 94%–100% of surgeries. In two separate studies, prophylactic efficacy was rated excellent/good in 100% of treatment cycles. Where reported, median annualized bleeding rates decreased from 3–24 prior prophylaxis to 0.5–6 during prophylaxis. Analysis of pharmacovigilance safety reports showed that pdVWF/FVIII was associated with a low rate of adverse events.
Conclusions
This systematic literature review and analysis of pharmacovigilance data summarize evidence of over 40 years of clinical use of pdVWF/FVIII, supporting its safety and efficacy in VWD.
1 Introduction
Von Willebrand disease (VWD) is the most common inherited bleeding disorder [1] with a prevalence of symptomatic disease of ∼1 in 1000 individuals [2]. VWD is caused by deficient or dysfunctional von Willebrand factor (VWF), a glycoprotein that plays a pivotal and multifunctional role in haemostasis [1, 3]. Clinical manifestations of VWD mainly include mucocutaneous bleeds, such as epistaxis, menorrhagia and gastrointestinal (GI) bleeds, as well as surgical bleeds and prolonged bleeding from cuts [1].
Therapeutic strategies for VWD aim to rise VWF and FVIII levels to prevent or treat bleeds, and mainly depend on disease type, baseline FVIII and VWF levels, and the severity of bleed or surgical intervention [4-6]. Therapies are subdivided into two main categories: non-replacement, such as desmopressin (DDAVP) and antifibrinolytic agents, or clotting factor replacement therapies [3-5]. Therapeutic regimens with VWF/FVIII concentrates include on-demand (OD) treatment, long-term prophylaxis (LTP) and surgical prophylaxis (SP) [3, 5].
Pasteurized plasma-derived VWF/FVIII complex (pdVWF/FVIII; antihemophilic factor/VWF complex [Human]; Haemate P, licensed as Humate-P in the USA; CSL Behring [7]) was the first pdVWF concentrate to be approved [3]. First licensed in Germany in 1982, it now has over four decades of clinical use [8]. In 1981, a pasteurization step was introduced in the manufacturing process which was shown to inactivate both enveloped and non-enveloped viruses [7, 9]. pdVWF/FVIII is indicated for the prophylaxis and treatment of haemorrhage or surgical bleeds in patients with haemophilia A and in patients with VWD when DDAVP treatment alone is ineffective or contra-indicated [7].
This systematic review was conducted to identify and summarize the available evidence on efficacy, safety, dosing and consumption of pdVWF/FVIII in any treatment regimen in patients with inherited VWD. Furthermore, pharmacovigilance data spanning 40 years of clinical experience with pdVWF/FVIII are presented.
2 Methods
2.1 Search Strategy
An electronic search was conducted in the following databases on 31 May 2023: MEDLINE (to present) and MEDLINE In-Process Citations, through Pubmed.com interface; Cochrane Library (to present): including Central Register of Controlled Trials (CENTRAL) and Database of Systematic Reviews (CDSR) according to PRISMA. For details of the search strategy see Tables S1–S6.
2.2 Study Selection
Studies reporting efficacy, safety or consumption/dosing of pdVWF/FVIII in inherited VWD of all types, in patients of all ages who received treatment with any treatment regimen (OD, intermittent, LTP and SP) were included. More details on the inclusion/exclusion criteria are described in Table S1. Results from evidence synthesis were presented as ranges. Where haemostatic efficacy data were comparable, these were pooled across studies for OD, LTP and SP.
2.3 Pharmacovigilance Data
Post-marketing surveillance (PMS) data were collected for the period 7 June 1982–31 May 2023. Only adverse events (AEs) where a causal relationship between pdVWF/FVIII [7] and the reported AE was considered possible (adverse drug reactions [ADRs]) were included. Historically, pdVWF/FVIII was used to treat patients with haemophilia A, however, in recent years it is primarily used in patients with VWD [8]. No distinction was made between ADRs reported for either VWD or haemophilia A indications during PMS reporting, hence the pharmacovigilance data includes ADRs for both indications (for other pharmacovigilance methodology please refer to Supplemental Materials).
3 Results
3.1 Systematic Review
From a total of 164 records identified, 144 were included in the full-text screening level. Of these 144, 124 articles had exclusion criteria, resulting in the inclusion of 20 articles. Additionally, 28 records were identified in the grey literature review (Figure 1). Following eligibility screening, 18 records reporting data from 15 individual studies were reviewed (Table S7).
Characteristics of the population in these 15 studies are presented in Table 1. Most studies included both paediatric (<18 years) and adult (≥18 years) patients: one study only paediatric [10], and three only adult patients [11-13]. One study reported evidence in women with VWD admitted for childbirth [13]. For each study, a breakdown of number of patients receiving OD treatment, LTP and SP, has been included if available. For some studies, patients receiving OD or LTP treatment may have also been included in the SP population subgroup if they also underwent surgeries/invasive procedures.
| Bal et al. (2017) | Carcao et al. (2010) | Castaman et al. (2013) | Dobrkovska et al. (1998) Lillicrap et al. (2002) | Federici et al. (2007) | Franchini et al. (2003) | Gill et al. (2003)a | Gill et al. (2011) Mannucci et al. (2013) CSL-4002 | Hazendonk et al. (2018) | Lethagen et al. (2006) | Lethagen et al. (2007) Mannucci et al. (2013) CSL-4001 | Miesbach et al. (2015) | Thompson et al. (2004)a | Machin and Ragni (2020) | Auerswald et al. (2013) | Abshire et al. (2013) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number, N | 90 | 16 | 121 | 97 | 100 | 26 | 33 | 42 | 103 | 37 | 29 | 71 | 39 | 6 | 11 | 59 |
| On-demand | — | — | 75 | NR | 59 | — | 33 | — | — | — | — | 37 | — | — | — | — |
| Long-term prophylaxis | — | — | 31 | NR | 12 | — | — | — | — | 37 | — | 3 | — | — | — | 59 |
| Surgical prophylaxis | 90 | 12 | 55 | NR | 56 | 26 | — | 35 | 103 | — | 29 | 70 | 39 | 6 | 11 | — |
| Age, years | ||||||||||||||||
| Median (range) | NR | 13.3b (4.8;17.2) |
41.0 (1.0;79.0) |
20.4 (0.4;81.1) |
41.5 (2.0;87.0) |
41.5 (9.0;80.0) |
31.0 (NR) |
21.0 (1.0;75.0) |
51.0 (36.0;62.0) |
33.0 (6.0;67.0) |
c |
55.0 (25.0;90.0) |
43.0 (0.3;82.0) |
27.0 (22.7;30.5)d | e | 22.4 (2.3;77.2)f |
| Female sex, % | 80.0 | 60.0b | 56.2 | — | 56.0 | 53.8 | 55.0 | 66.7 | 67.0 | 54.1 | 69.0 | 60.6 | 56.0 | 100.0 | 17.0g | 47.5f |
| Weight, kg | ||||||||||||||||
| Median (range) | NR | NR | NR | 60.5 (11.3;113.6) |
66 (13;120) |
NR |
67.0 (NR) |
NR |
77 (65;85) |
NR | NR | NR |
79.5 (7.0;134.4) |
NR | e | NR |
| VWD type, n (%) | NR | NR | ||||||||||||||
| 1 |
85 (94.4) |
0 | 56 (46.3) | 32 (33.0) | 23 (23.0) | 19 (73.0) | 9 (27.0) | 17 (40.5) | 54 (52.4) | 10 (34.5) | 40 (60.6) | 16 (41.0) | 6 (100.0) | 5 (8.5)f | ||
| 2A | 0 | 8 (6.6) | 5 (5.2) | 7 (7.0) | 0 | 4 (12.0) | 2 (4.8) | 24 (23.3) | 10 (34.5) | 8 (11.3) | 4 (10.2) | 0 | 10 (17.0)f | |||
| 2B | 0 | 22 (18.2) | 18 (18.6) | 11 (11.0) | 7 (27.0) | 4 (12.0) | 4 (9.5) | 7 (6.8) | 0 | 1 (1.4) | 5 (12.8) | 0 | 8 (13.6)f | |||
| 2M | 0 | 1 (0.8) | 0 | 9 (9.0) | 0 | 0 | 6 (14.3) | 9 (8.7) | 1 (3.0) | 3 (4.2) | 0 | 0 | 2 (3.4)f | |||
| 2N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (2.9) | 0 | 3 (4.2) | 0 | 0 | 0 (0.0)f | |||
| 3 | 16 (100) | 31 (25.6) | 28 (28.9) | 37 (37.0) | 0 | 12 (36.0) | 13 (31.0) |
6 (5.8) |
8 (28.0) | 9 (12.7) | 8 (20.5) | 0 | 34 (57.6)f | |||
| Others | — | — | 14 (14.4)h | 13 (13.0)i | — | — | — | — | — | 7 (9.9)j | 6 (15.4)k | — | — | |||
| NA | 5 (5.6) | — | 3 (2.5) | — | — | — | 4 (12.0) | — | — | — | — | — | — | — | ||
| Diagnostic tests, median (range), IU/dL | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||
| VWF:RCo |
8 (<6;50) |
3 (<3;47) |
13 (6;124) |
1.4 (1.0;2.5) |
m | |||||||||||
| FVIII:C | 20.4 (3.0;110.0) |
24.0 (1.0;118.0) |
39.0 (0.5;96.0) |
4.4 (2.8;6.0) |
m | |||||||||||
| VWF:Ag | 12.2 (1.0;116.0) |
7.0 (<3;74.0) |
— |
2.8 (2.1;3.8) |
m | |||||||||||
| Severe VWD, n (%) | NR | 16 (100) | 52 (43.0)l | NR | 71 (71.0)l | NR | NR | 18 (42.9) | NR | NR | NR | 20 (28.2) | NR | NR | NR | NR |
- Abbreviations: BMI, body mass index; dL, deciliter; FVIII:C, factor VIII coagulation activity; IU, international unit; kg, kilogram; kg/m2, kilogram per square meter; NA, not available; pdVWF/FVIII, pasteurized plasma-derived von Willebrand factor/Factor VIII concentrate; VWD, von Willebrand disease; VWF:Ag, von Willebrand factor antigen; VWF:RCo, von Willebrand factor ristocetin cofactor activity.
- a The publications by Gill et al. (2003) [17] and Thompson et al. (2004) [24] reported results from different subgroups of the same study.
- b Estimated according to individual patient data of subpopulation of interest.
- c Reported as age range: 5–16 years (n = 2; 7%); 17–64 years (n = 22; 76%); ≥65 years (n = 5; 17%).
- d Interquartile range reported.
- e Reported as age range (4.5–48.3 years), weight range (12.8–94.0 kg) and BMI range (17.9–30.7 kg/m2).
- f Data reported for the overall population with 77.1% of patients treated with Humate-P, 16.5% with Fandhi, 4.5% with Alphanate and 1.9% NR.
- g Data reported for the overall population (n = 46) which also included patients with haemophilia A and B.
- h Included four patients with acquired VWD and the rest with undiagnosed VWD type.
- i Included type 2M Vicenza type.
- j Included uncategorized type 2 (n = 1), type 1 VWD and haemophilia A (n = 2), acquired VWD (n = 1), haemophilia A (n = 3).
- k Included patients with type 2M, 2N or unknown subtype.
- l Severe disease defined as baseline VWF:RCo <10 IU/dL.
- m Reported as median (interquartile range): VWF:RCo at baseline 5.5 (3.2;6.2) and at 8 months 12.9 (9.4;15.9); FVIII:C at baseline 9.2 (6.0;10.9) and at 8 months 19.5 (14.6;26.3); VWF:Ag at baseline 7.4 (4.8;8.1) and at 8 months 19.2 (14.1;21.0).
Treatment protocols differed between studies; in the interventional studies, protocols ranged from conventional dosing regimens (per product labelling) [7] to tailored pharmacokinetic (PK)-guided dosing based on VWF levels. Data are reported as median or mean with corresponding ranges in the tables.
3.2 OD Treatment With pdVWF/FVIII
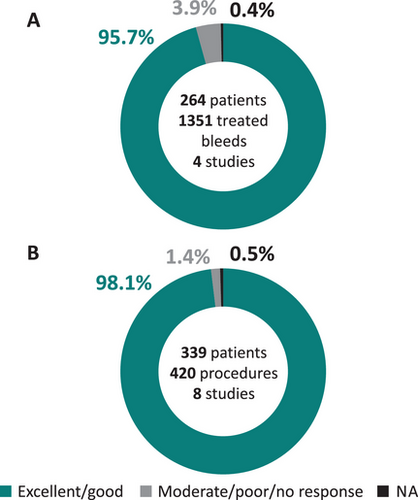
Five studies evaluated the efficacy of pdVWF/FVIII for OD treatment of bleeding events (Table 2) [11, 14–18]. Four studies reported bleeding pattern data, with epistaxis and GI bleeds as the main causes for OD treatment [11, 14, 15, 17]. Overall, the control of bleeding events with pasteurized pdVWF/FVIII was rated excellent/good in 95%–98% of treated bleeds, with only a few as a moderate/poor response [14, 15, 17, 18]. The latter cases tended to be patients with type 3 and type 2B VWD (Supplemental Material). Pooled haemostatic efficacy data for 1351 treated bleeds from 264 patients across four studies (Figure 2A), showed that 95.7% of bleeds (N = 1293) resolved with excellent/good efficacy and 3.9% (N = 53) with moderate/poor efficacy or no response [14, 15, 17, 18].
| On-demand treatment | |||||
|---|---|---|---|---|---|
| Castaman et al. (2013) | Dobrkovska et al. (1998) Lillicrap et al. (2002) |
Federici et al. (2007) | Gill et al. (2003) | Miesbach et al. (2015) | |
| Patient number, N | 75a | 97b | 59 | 33 | 37 |
| VWD type, n (%) | b | ||||
| 1 | 29 (38.7) | 32 (33.0) | 7 (11.9) | 9 (27.0) | 13 (35.1) |
| 2A | 5 (6.7) | 5 (5.2) | 3 (5.1) | 4 (12.0) | 6 (16.2) |
| 2B | 14 (18.7) | 18 (18.6) | 6 (10.2) | 4 (12.0) | 0 |
| 2M | 1 (1.3) | 0 | 6 (10.2) | 0 | 3 (8.1) |
| 2N | 0 | 0 | 0 | 0 | 1 (2.7) |
| 3 | 25 (33.3) | 28 (28.9) | 32 (54.2) | 12 (36.0) | 8 (21.6) |
| Others | — | 14 (14.4)c | 5 (8.5)d | — | 6 (16.2)e |
| NA | — | — | — | 4 (12.0) | — |
| Treated bleeds, n | 677 | 344 | 280 | 53 | 371 |
| Median per patient (range) | 4 (1;55) | NR | 3 (1;43) | NR | NR |
| Bleeding event type, n (%) | |||||
| Epistaxis | 203 (30.0) | h | 70 (25.0) | 10 (18.9) | 189 (50.9) |
| Oral cavity | 126 (18.6)f | 5 (1.7)f | 6 (11.3) | 12 (3.2)f | |
| Menorrhagia | 104 (15.4) | 17 (6.1) | g | 6 (1.6) | |
| Joint | 119 (17.6) | 48 (17.1) | 9 (17.0) | 11 (3.0)i | |
| GI bleeding | 64 (95.0) | 81 (28.9) | 14 (26.4) | 84 (22.6) | |
| Intracranial | — | — | 1 (1.9) | — | |
| Soft tissue | — | — | 2 (3.8) | — | |
| Other | — | — | 11 (20.7)c | — | |
| Bleeding event severity, n (%) | NR | NR | NR | NR | |
| Severe | 16 (30.1) | ||||
| Moderate | 34 (64.2) | ||||
| Mild | 3 (5.7) | ||||
| Bleeding event cause, n (%) | NR | NR | |||
| Spontaneous | 677 (100.0) | 258 (92.1) | NR | ||
| Traumatic | 0 | 22 (7.9) | 60 (16.2) | ||
| Hemostatic efficacyj, n (%) | N = 674k | ||||
| Overall | l | ||||
| Excellent | 316 (46.9) | ||||
| Excellent/good | 332 (96.5) | 266 (95.0) | 52 (98.0) | ||
| Good | 327 (48.5) | ||||
| Moderate/poor | 26 (3.8) | — | 14 (5.0) | 1 (2.0) | |
| Poor/no response | — | 12 (3.4) | — | — | |
| NA | 5 (0.7) | — | — | — | |
| Long-term prophylaxis | |||||
|---|---|---|---|---|---|
Dobrkovska et al. (1998) Lillicrap et al. (2002) |
Federici et al. (2007) | Lethagen et al. (2006) | Abshire et al. (2013) | ||
| Patient number, N | b | 12m | 37n | 55o | |
| Reasons for prophylaxis, n (%) | NR | ||||
| GI bleeding | 5 (41.7) | 4 (11.0) | 13 (23.6) | ||
| Joint bleeding | 4 (33.3) | 8 (22.0) | 12 (21.8) | ||
| Menorrhagia | — | 3 (8.0) | 4 (7.3) | ||
| Nose and joint bleeding | — | 10 (27.0) | — | ||
| Nose and mouth bleeding | — | 11 (29.0) | 13 (23.6) | ||
| Otherq | 3 (25.0) | 1 (3.0) | 8 (14.6) | ||
| Mixedr | — | — | 5 (9.1) | ||
| Observation period, days | NR | 201 (30;730)s | NRt | NRu | |
| Prophylactic efficacy outcome | |||||
| ABR, median (range) Prior to prophylaxis / after the start of prophylaxis | NR | NR | 11 (2;100) / 1 (0;5) | Overallp | 12.0 (6.0;24.0) / 3.6 (0.96;9.4) |
| Epistaxis | 24.0 (12.0;48.0) / 6.0 (2.9;12.0) | ||||
| GI bleeding | 8.4 (6.0;12.0) / 6.0 (3.0;6.0) | ||||
| Joint bleeding | 15.6 (5.0;21.5) / 1.3 (0.3;3.2) | ||||
| Menorrhagia | 12.0 (12.0;12.0) / 4.0 (1.0;9.0) | ||||
| Otherq | 3.0 (1.0;10.5) / 0.5 (0.2;2.8) | ||||
| Mixedr | 12.0 (6.0;18.0) / 6.0 (1.2;12.0) | ||||
| Prophylactic efficacy as rated by the treating physician, n (%) | Per prophylactic regimen n = 20 cycles | Per prophylactic regimen n = 17 cycles | NR | NR | |
| Excellent/good | 20 (100) | 17 (100) | |||
| Moderate | |||||
| Poor | |||||
| NA | |||||
- Efficacy outcomes varied across the identified studies and therefore the table was populated according to the different variables reported.
- Abbreviations: ABR, annualized bleeding rate; GI, gastrointestinal; NA, not available; NR, not reported; pdVWF/FVIII, pasteurized plasma-derived von Willebrand factor/Factor VIII concentrate; VWD, von Willebrand disease.
- a 49 patients received exclusively on-demand treatment, while 26 also received long-term prophylaxis.
- b VWD type and efficacy data for on-demand treatment reported for overall patient population (n = 97), without specifying the number of patients treated on-demand; authors indicated that all long-term prophylaxis patients were type 3 VWD.
- c Included four patients with acquired VWD and the rest with undiagnosed VWD type.
- d Included five patients with type 2M Vicenza variant VWD.
- e Included one acquired VWD, 1 type 2 VWD uncategorized and four patients with haemophilia A.
- f Reported only as gingival bleeds.
- g Other bleeding events included gynaecological bleeding and postoperative haemorrhage in the setting of alternate treatment failures.
- h Reported per organ system: mouth, nose and pharynx (n = 130); musculoskeletal system (n = 108); digestive system (n = 49); integument (n = 22); urinary system (n = 49); female genital system (n = 9); male genital system (n = 1); and other (n = 19), including pregnancy, delivery, and post-partum period, cystoscopy, gastroscopy, and other invasive diagnostic techniques.
- i Reported as joint/muscle bleeds.
- j Efficacy rated by the investigator.
- k Total number of treated bleeding events was 677, but clinical response only reported for 674 events.
- l Successful resolution of a bleeding episode was extracted from the medical records as per the original judgment made by the treating clinician and due to the retrospective nature of the study, no specific criteria were defined; treatment of a bleeding episode was considered successful if bleeding did not recur after the planned course of treatment and if the patient did not require increased or additional doses of pdVWF/FVIII; effective haemostasis was achieved in >95% of bleeding events.
- m VWD type reported as: type 1, 1 (8%); type 2B, 1 (8%); type 2M Vicenza variant, 1 (8%); type 3, 9 (76%).
- n Authors reported that most patients were type 3 VWD with severe joint bleeds.
- o Data by bleeding type are reported as published in the original publication for n = 55 while VWD type breakdown were reported for the overall population of 59 patients as listed in Table 1; overall, 77.1% of patients were treated with Humate-P, 16.5% with Fandhi, 4.5% with Alphanate and 1.9% NR.
- p Data reported overall and stratified per bleeding type, reported as median (interquartile range).
- q Other: the primary indication occurred with low frequency. This category includes intracranial haemorrhage, haematomas in soft tissue, oral, dental extraction, scraped knees, ovarian cysts, or bleeding.
- r Mixed: bleeding pattern was mixed with no primary indication.
- s Reported as median (range).
- t Data were collected retrospectively; authors reported a median (range) of 11 (2;45) years on prophylaxis.
- u The median period of time on prophylaxis was 2.2 years. Data were collected between 2008 and 2011; bleeding history was derived from centre records or registries, diaries and logs. Records were available for every bleeding episode during the period of study for nine (15%) participants. For all others, the investigator assessed available documentation to determine the average number of bleeding episodes that occurred each month.
Five studies reported dosing and consumption data for pdVWF/FVIII for OD treatment (Table S8) [11, 14–17]. In the four observational studies, pdVWF/FVIII treatment posology was at the discretion of the treating physician and varied greatly, making comparison difficult [11, 14–16].
Five studies assessed the safety of pdVWF/FVIII for OD treatment, although the outcomes of interest varied between studies (Table 3) [11, 14–17]. The methodologies varied in terms of the definition of AE and ADR and duration of follow-up [16]. Two of the studies reported no AEs [11, 15], whereas Gill et al. reported a single possibly related AE of a mild allergic reaction, and two serious adverse events (SAEs) considered unrelated to study treatment; one menorrhagia and anaemia, and one haemorrhage [17].
| On-demand treatment | |||||
|---|---|---|---|---|---|
| Castaman et al. (2013) | Lillicrap et al. (2002) | Federici et al. (2007) | Gill et al. (2003) | Miesbach et al. (2015) | |
| Patient number, N | 75 | a | 59 | 33 | 37 |
| Treatment exposure, days | NRb | NR | NRc | NR | d |
| Patients with any AE, n (%) | NR | NR | 0 | 10 (30.3) | 0 |
| Treatment-related AEs | 0 | NR | NR | 1 (3.0) | NR |
| Patients with any SAE, n (%) | NR | NR | NR | 2 (6.0) | NR |
| Treatment discontinuations due to AE, n (%) | NR | NR | NR | 0 | NR |
| Severe hypersensitivity reactions, n (%) | NR | NR | NR | NR | 0 |
| Patients with TEs, n (%) | 0 | 0 | 0 | 0 | 0 |
| Patients with inhibitor antibodies, n (%) | 0 | NR | NR | NR | 0 |
| Long-term prophylaxis | ||||||
|---|---|---|---|---|---|---|
| Castaman et al. (2013) | Lillicrap et al. (2002) | Federici et al. (2007) | Lethagen et al. (2006) | Miesbach et al. (2015) | Abshire et al. (2013) | |
| Patient number, N | 31 | a | 12 | 37 | 3 | 59e |
| Time of exposure, days | NRb | NR | 4358 | NRf | NRd | NRg |
| Patients with any AE, n (%) | NR | NR | 0 | NR | 0 | NR |
| Treatment-related AEs | 0 | NR | NR | NR | NR | NR |
| Patients with any SAE, n (%) | NR | NR | NR | NR | NR | NR |
| Treatment discontinuations due to AE, n (%) | NR | NR | NR | NR | NR | 1 (1.7)h |
| Severe hypersensitivity reactions, n (%) | NR | NR | NR | NR | 0 | NR |
| Patients with TEs, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Patients with inhibitor antibodies, n (%) | 0 | NR | NR | 1 (2.7) | 0 | 1 (1.7) |
| Surgical prophylaxis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bal et al. (2017) | Castaman et al. (2013) | Federici et al. (2007) | Franchini et al. (2003) | Dobrkovska et al. (1998) Lillicrap et al. (2002) |
Gill et al. (2011) Mannucci et al. (2013) CSL-4002 |
Lethagen et al. (2007) Mannucci et al. (2013) CSL-4001 |
Hazendonk et al. (2018) | Miesbach et al. (2015) | Thompson et al. (2004) | Machin and Ragni (2020) | Auerswald et al. (2013) | |
| Patient number, N | 90 | 55 | 56 | 26 | i | 35j | 28k | 103 | 70 | 39 | 6 | 11 |
| Treatment exposure, days | NR | NRl | NRm | NR | NR | NR | NR | NR | NRn | NR | 3 | 4–11 |
| Patients with any AE, n (%) | NR | NR | 0 | NR | NR | 30 (86.0) | 21 (75.0) | NR | 0 | 24 (57.1) | NR | 0 |
| Treatment-related AEs | NR | 0 | NR | 0 | NR | 3 (8.6) | 5 (18.5) | NR | NR | 7 (16.7) | NR | NR |
| Patients with any SAE, n (%) | NR | NR | NR | NR | NR | 3 (8.6) | NR | NR | NR | 3 (7.1) | NR | NR |
| Treatment-related SAEs | NR | NR | NR | NR | NR | 0 | 1 (3.7) | NR | NR | 0 | NR | NR |
| Treatment discontinuations due to AE, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1 (2.4) | NR | NR |
| Severe hypersensitivity reactions, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | 0 | NR | NR | NR |
| Patients with TEs, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7.1) | 0 | 1 (1.4) | 0 | 0 | 0 |
| Patients with inhibitor antibodies, n (%) | NR | 0 | NR | NR | NR | NR | 0 | NR | 0 | NR | NR | 0 |
- Abbreviations: AE, adverse event; NR, not reported; pdVWF/FVIII, pasteurized plasma-derived von Willebrand factor/Factor VIII concentrate; SAE, serious adverse event; TE, thromboembolic event.
- a Data reported for overall patient population (n = 97), without specifying the number of patients treated on-demand or in long-term prophylaxis.
- b 24-month observation period.
- c 36-month observation period.
- d Observation period ranged from 5 to 25 years.
- e Data reported for the overall population with 77.1% of patients treated with Humate-P, 16.5% with Fandhi, 4.5% with Alphanate and 1.9% NR.
- f Authors only reported a median (range) of 11 (2;45) years on prophylaxis.
- g Authors report a median of 2.2 years on prophylaxis.
- h One patient who developed inhibitor antibodies discontinued prophylaxis.
- i Data reported for overall patient population (n = 97), without specifying the number of patients treated in surgical prophylaxis.
- j Safety data reported only for patients submitted to surgery (n = 35).
- k 28 patients received loading dose and as such were considered in the safety population analysis.
- l 24-month observation period.
- m 36-month observation period.
- n Observation period ranged from 5 to 25 years.
Thromboembolic event (TE) incidence was the safety outcome most frequently assessed, with all five studies reporting the absence of any TEs following pdVWF/FVIII treatment [11, 14-17]. FVIII or VWF inhibitor formation was only investigated in two studies [11, 14] with no cases being reported [11, 14].
3.3 LTP With pdVWF/FVIII
For efficacy of pdVWF/FVIII in LTP treatment, only studies reporting efficacy outcomes as changes in annualized bleeding rate (ABR) or efficacy rated by the treating physician, were evaluated in the analysis (Table 2) [15, 16, 18–20]. In particular, Abshire et al. reported outcome data for patients who were treated prophylactically with a variety of VWF/FVIII concentrates, with pdVWF/FVIII being the most frequently used (77% of patients) [20]. Therefore, data interpretation/comparison may warrant caution.
The most frequent indications for prophylactic treatment initiation were joint bleeds, GI bleeds and epistaxis [15, 19, 20].
Two studies reported haemostatic efficacy for LTP rated per cycle by the treating physician [15, 16], with various definitions of rating categories and units of analysis. In the study by Federici et al., although the duration of a cycle was not defined, patients were treated with multiple LTP sessions (17 cycles per 12 patients; n = 9 type 3 VWD) [15]. Overall rating of excellent/good was reported in 100% (37/37) of prophylactic cycles as assessed by Federici et al. [15] and Lillicrap et al. [16]. Efficacy of pdVWF/FVIII was reported in Lethagen et al. as reduced ABRs, with a median of 11 and one bleeding event(s) before and after prophylaxis initiation, respectively [19]. ABR data were stratified per bleeding type in the study by Abshire et al. with the most pronounced reduction in ABR for joint bleedings (AjBR); AjBR was 15.6 before and 1.3 during prophylaxis [20].
Four studies included dosing and consumption data of pdVWF/FVIII during LTP (Table S8) [14–16, 20]. Data were not homogeneously reported, with only two studies providing a median treatment duration per patient of 201 and 803 days [15, 20]. An LTP regimen of 2–3 weekly infusions was reported in three studies [14, 15, 20], with one also specifying dosing of 20 IU/kg FVIII in about 90% of cases [14]. In one of the studies, the median number of weekly infusions was reported per prophylactic indication, with epistaxis, GI bleeds and menorrhagia as indications with the highest median of three infusions [20].
Six studies evaluated the safety of pdVWF/FVIII in LTP (Table 3), with limited data on outcomes of interest and varying exposure time and safety assessment methodology. Two studies [11, 15] observed no AEs during follow-up, and another reported no treatment-related AEs [14]. SAEs or TEs did not occur during patient follow-up in any of the studies. Lethagen et al. reported one case of antibodies to VWF in a patient with type 3 VWD during LTP treatment with pdVWF/FVIII [19]. In another study, antibodies were detected in one patient who then discontinued treatment [20]. No cases of VWF or FVIII inhibitors were reported in the studies by Castaman et al. [14] and Miesbach et al. [11].
3.4 SP With pdVWF/FVIII
Thirteen studies evaluated the efficacy of pdVWF/FVIII in SP (Table 4) with various definitions of major surgery. All but one study reported the types of procedure [16, 18]. The study by Carcao et al. [10] included only dental procedures, whilst the remaining studies included major (6%–74% of all procedures), minor (9%–57%), minimally invasive (12%–26%) and dental procedures (2%–52%). The proportion of procedures for which the overall haemostatic efficacy was rated excellent/good, ranged from 94% to 100%. Pooled haemostatic efficacy rating for 420 procedures in 339 patients across eight studies showed that 98.1% of surgical bleeds (N = 412) were resolved with excellent/good efficacy and 1.4% (N = 6) had moderate/poor efficacy or no response (Figure 2C) [14, 15, 21–26]. One study [16, 18] could not be included in the pooled analysis as the number of patients receiving specific treatment regimens were not reported. One study reported the expected intra-operative blood loss, and all procedures had less than the expected volume according to the surgeon [25].
| Bal et al. (2017) | Carcao et al. (2010) | Castaman et al. (2013) | Dobrkovska et al. (1998) Lillicrap et al. (2002) | Federici et al. (2007) | Franchini et al. (2003) | Gill et al. (2011) Mannucci et al. (2013) CSL-4002 |
Lethagen et al. (2007) Mannucci et al. (2013) CSL-4001 |
Hazendonk et al. (2018) | Miesbach et al. (2015) | Thompson et al. (2004) | Machin and Ragni (2020) | Auerswald et al. (2013) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number, N | 90 | 10 | 55 | a | 56 | 26 | 35b | 27b | 103 | 70 | 39 | 6 | 11 |
| Number of procedures, n | 90 | NRc | 126 | 73 | 73 | 43 | 35 | 27 | 148 | 292 | 42 | 6 | 11 |
| Procedure type, n (%) | NR | d | e | f | |||||||||
| Any surgery | — | — | 28 (22.2)g | — | — | — | — | — | — | — | — | — | — |
| Major surgery | 29 (32.2) | — | — | — | 17 (23.3) | 14 (32.6) | 25 (71.4) | 16 (59.3) | 110 (74.0) | 18 (6.2) | 25 (64.1) | 2 (33.3)h | 8 (72.7) |
| Minor surgery | 51 (56.6) | — | — | — | 28 (38.3) | 11 (25.6) | 7 (20.0) | 11 (40.7) | 38 (26.0) | 26 (8.9) | 17 (35.9) | — | 3 (27.3) |
| Minimally invasive procedures | — | — | 32 (25.6) | — | 9 (12.3) | 7 (16.3) | — | — | — | 55 (18.8) | — | — | — |
| Dental procedures | 2 (2.0) | NR (100.0)c | 66 (52.1) | — | 19 (26.0) | 11 (25.6) | 3 (8.6) | — | — | 64 (21.9) | — | — | — |
| Other classification | — | — | — | — | — | — | — | — | — | 129 (44.2)i | — | 4 (66.7)j | — |
| Unknown | 8 (8.9) | — | — | — | — | — | — | — | — | — | — | — | — |
| Patients with adjuvant treatment, n (%) | NR | NR | NR | NR | |||||||||
| Anti-fibrinolytick | 10 (100.0) | 18 (32.1) | 0 | 19 (54.3) | 20 (74.1) | l | — | 0 | — | ||||
| DDAVP | — | 1 (1.8) | 0 | — | — | — | — | — | — | ||||
| Thromboprophylaxis | — | 2 (3.6) | — | — | — | 63 (61.2) | m | — | n | ||||
| Prophylactic efficacy, n (%) | NRo | NRs | NRv | ||||||||||
| Overall | n = 102p | q | r | t | n = 39u | ||||||||
| Excellent | 84 (93.3) | 86 (84.3) | — | — | — | — | 27 (100.0) | — | — | ||||
| Excellent/good | — | — | 72 (98.6) | 71 (97.2) | 42 (97.7) | 33 (94.3) | — | 39 (100.0) | 11 (100.0) | ||||
| Good | 6 (6.6) | 13 (12.7) | — | — | — | — | — | — | — | ||||
| Moderate | — | 1 (0.9) | — | — | — | — | — | — | — | ||||
| Poor | — | — | — | — | 1 (2.3) | 2 (5.7) | — | — | — | ||||
| Poor/No response | — | — | 1 (1.3) | 2 (2.7) | — | — | — | — | — | ||||
| NA | — | 2 (1.9) | — | — | — | — | — | — | — |
- Abbreviations: DDAVP, desmopressin; NA, not available; NR, not reported; pdVWF/FVIII, pasteurized plasma-derived von Willebrand factor/Factor VIII concentrate.
- a Data reported for overall patient population (n = 97), without specifying the number of patients submitted to surgical prophylaxis.
- b Patients submitted to surgery and included in the efficacy analysis set.
- c Reported number of teeth with procedure, but not clear if one tooth accounted for more than one procedure.
- d Surgery procedures stratified according to potential of bleeding risk: major procedures included thoracic/abdominal surgery, obstetric/gynaecological surgery, orthopaedic surgery, endocrine surgery, and vascular surgery; minor procedures included minor surgical interventions, ophthalmic, skin, and ear, nose and throat surgery; dental procedures included oral surgery; and invasive procedures.
- e Surgical procedures classified as: major surgery defined as procedures involving considerable hazard or risk to life or limb, frequently involving general anaesthesia; minor surgery defined as simple operations not considered a risk to life that could be performed in an outpatient setting with or without sedation; oral surgery defined as simple tooth extraction.
- f Definition according to risk categories proposed by Koshy et al. (1995) [47].
- g Data not stratified for major and minor surgeries.
- h Patients who had a caesarean section.
- i Procedures not classified in the above categories included: gastro-/colonoscopies with biopsy removal (n = 58; 19.9%); orthopaedic procedures (n = 44; 15.1%); gynaecological procedures (n = 27; 9.2%); dermatological operations (n = 18; 6.2%); urological procedures (n = 9; 3.1%); otolaryngological procedures (n = 5; 1.7%); cardiac operations (n = 4; 1.4%); neurosurgical operations (n = 4; 1.4%); and abdominal procedures (n = 4; 1.4%).
- j Patients who had a vaginal delivery.
- k Includes tranexamic or aminocaproic acid.
- l Number of procedures with use of adjuvant treatment (n = 51; 34.5%).
- m Author reported limitations with available data, with 84.6% of procedures without thromboprophylaxis information.
- n n = 5 patients received heparin although data are reported for the overall population that also included patients with haemophilia A and B.
- o The authors reported that for only three patients more factor concentrate was needed because of concerns of post-surgery bleeding.
- p Only 102 procedures with clinical evaluation of prophylactic efficacy.
- q Reported haemostatic efficacy assessment in the immediate postoperative period, defined as investigator assessment of efficacy 24 h after the last factor infusion or on day 14.
- r Reported haemostatic efficacy assessment at the final evaluation by investigator on day 14.
- s Clinically relevant bleeding complications were reported in only 3.4% of surgeries.
- t The authors reported that effective haemostasis, without further complication, was achieved in >90% of surgical procedures.
- u Based on only 39 procedures with clinical evaluation of prophylactic efficacy.
- v Median (interquartile range) of the estimated blood loss was 462 (362–806) mL although no clinical assessment has been reported.
Twelve studies reported dosing and consumption data for pdVWF/FVIII in SP (Table S9). Treatment protocols and variables of interest varied across the studies. Three studies reported the median number of infusions, ranging from 3 to 6 administrations per procedure [14, 15, 24]. Stratified data per procedure type were reported in Federici et al. [15], Lethagen et al. [26], Franchini et al. [23] and the CSL-4002 study [25], with a median of 7–10 infusions and 3–9.5 infusions in major and minor procedures, respectively. Median treatment duration ranged between 2.0 and 6.4 days [16, 21, 24, 26], varying according to procedure type (see more details in Supplemental Materials) [15].
Twelve studies evaluated the safety of pdVWF/FVIII for SP (Table 3). Both CSL-4001 and -4002 trials reported similar safety evaluations, including AE monitoring during the surgery phase, from loading dose to post-operative day 14 or, until the end of treatment [25, 26]. In the CSL-4001 and -4002 trials, 75% (n = 21) and 86% (n = 30) of patients reported at least one AE, of which 19% (n = 5) and 9% (n = 3) were considered treatment-related, respectively [25-27]. These included headache, nausea, itching, dizziness, thrombophlebitis of the leg, pulmonary embolism, vomiting, rash and an increase in alanine aminotransferase [25-27]. Thompson et al. reported safety data per procedure, with AEs reported in 57% (n = 24) of patients, of which 17% (n = 7) were classified as treatment-related [24]. Gill et al. reported SAEs in 9% (n = 3) of patients [25] and Thompson et al. in 7% (n = 3) of patients, all considered unrelated to treatment [24]. Lethagen et al. reported a single SAE, a pulmonary embolism, possibly related to treatment (details in Supplemental Results) [26]. In another study, one patient with type 2B VWD discontinued due to a pseudo-thrombocytopenia [24]; the patient was not withdrawn from the study and treatment efficacy was rated good to excellent [24].
All studies reported the incidence of TEs ranging from 0% to 7% of patients; three cases of TEs of interest are discussed in supplemental results. No cases of inhibitor development occurred in the four studies that reported this safety outcome [11, 14, 21, 26].
3.5 Pharmacovigilance Data of pdVWF/FVIII
From 7 June 1982 to 31 May 2023 a total of 3227 ADRs in 1396 case reports were identified from post-marketing sources pertaining specifically to pdVWF/FVIII; 1201 of these were spontaneous and 195 were solicited (Table 5).
| Pharmacovigilance data reported in post-marketing surveillance | |
|---|---|
| IU VWF of pdVWF/FVIII distributeda | 18228112015 |
| Number of reported cases | 1479b |
| Case reports specific to Haemate P | 1396 |
| Number of ADRs specific to Haemate P | 3227 |
| Anti-FVIII/VWF inhibitors | |
| Reported cases (N)c | 134 |
| Anti-FVIII/VWF inhibitors ADRs (N) | 148 |
| Non-serious ADRs (n) | 2 |
| Serious ADRs (n) | 146 |
| Anti-FVIII inhibitors (number of events) | 83 |
| Anti-VWF inhibitors (number of events) | 47 |
| Thromboembolic events | |
| Reported cases (N) | 64 |
| Thromboembolic event ADRs (N) | 76 |
| Non-serious ADRs (n) | 0 |
| Serious ADRs (n) | 76 |
| Most common events | |
| Pulmonary embolism (number of events) | 19 |
| Deep vein thrombosis (number of events) | 14 |
| Hypersensitivity reactions | |
| Reported cases (N) | 214 |
| Hypersensitivity ADRs (N) | 321 |
| Non-serious ADRs (n) | 136 |
| Serious ADRs (n) | 185 |
| Most common events | |
| Hypersensitivity (number of events)d | 92 |
| Anaphylactic reactions (number of events)e | 57 |
| Suspected transmission of infectious agents | |
| Reported cases (N)f | 142 |
| Transmission of infectious agents ADRs (N) | 184 |
| Number of cases confirmed as associated with pdVWF/FVIII | 0 |
- Abbreviations: ADRs, adverse drug reactions; COVID-19, coronavirus disease 2019; pdVWF/FVIII, pasteurized plasma-derived von Willebrand factor/Factor VIII concentrate; VWD, von Willebrand disease; VWF, von Willebrand factor.
- a For the period 1990–31 May 2023.
- b Includes remaining 83 cases pertaining to human factor VIII VWF (generic) that were excluded.
- c Haemophilia A and VWD indication was not available.
- d Including preferred terms hypersensitivity (n = 85) and drug hypersensitivity (n = 7).
- e Including preferred terms anaphylactic reaction (n = 42), anaphylactoid reaction (n = 7) and anaphylactic shock (n = 8).
- f Including case reports with events describing the laboratory identification of virus antibodies without acute disease and cases of other infectious agents not known to be transmitted via blood products such as influenza or COVID-19.
Over 18 billion IU VWF of pdVWF/FVIII were distributed worldwide from the year 1990 to 31 May 2023 as indicated from available CSL commercial records. Key ADRs associated with the product included the development of FVIII/VWF inhibitors, TEs, hypersensitivity reactions (including anaphylaxis) and suspected transmission of infectious agents.
3.6 Inhibitor Development
The number of case reports associated with the development of neutralizing FVIII/VWF inhibitors was 134 (9.6% of all cases) and described a total of 148 ADRs (146 serious, two non-serious), including inhibitors (both low- and high-titers) against FVIII (n = 83) and VWF (n = 47). Due to the nature of PMS reporting, we could not discern whether the inhibitors were from patients with haemophilia A or VWD.
3.7 Thromboembolic Events
A total of 64 cases (4.6% of all ADR cases) reporting 76 TEs, all considered serious ADRs, were retrieved. Most commonly reported TEs were pulmonary embolism (n = 19) and deep vein thrombosis (n = 14).
3.8 Hypersensitivity Reactions
Hypersensitivity reactions (including anaphylaxis) were identified from 214 cases (15.3% of all ADR cases) describing a total of 321 ADRs (185 serious, 136 non-serious). Among ADRs relating to hypersensitivity reactions, hypersensitivity (n = 92) and anaphylactic reactions (n = 57) were most common.
3.9 Transmission of Infectious Agents
Suspected transmission of infectious agents was identified from 142 cases (10.2% of all ADR cases) reporting 184 ADRs (168 serious, 16 non-serious). These also included reports describing the laboratory identification of virus antibodies without acute disease and cases of other infectious agents not known to be transmitted via blood products such as influenza or COVID-19. None of the suspected virus transmission cases were confirmed to have been associated with pdVWF/FVIII.
4 Discussion
This systematic review summarizes evidence from 18 publications describing 15 studies where pdVWF/FVIII was used for OD treatment, LTP and SP treatment in paediatric and adult patients with VWD. In addition, pharmacovigilance data representing 40 years of PMS are reported for the first time. pdVWF/FVIII was the first virus-inactivated pdVWF concentrate introduced in the European market and has been used since 1982 for the treatment of VWD [8]. The amount of data confirming the efficacy and safety of pdVWF/FVIII, far exceeds that of any other VWF concentrate, supporting it as the standard of care for VWF replacement therapy in patients with VWD [3].
The results of this systematic literature review update previously published reports [3, 8, 9] and highlight that additional efficacy and safety data more recently reported are still in line with the early publications on pdVWF/FVIII. The majority (n = 12) of the studies included in this review were observational. Three interventional post-marketing studies assessed the safety and efficacy of pdVWF/FVIII in SP, reflecting the need for evidence-based guidelines for pdVWF/FVIII use in a surgical setting at that time [17, 25, 26]. The sample size varied (6–121 patients), with interventional studies including fewer patients than multicentric observational studies, which is expected considering restrictive clinical trial eligibility criteria.
OD treatment data revealed mucosal bleeding as the most common type of bleed, mostly spontaneous, consistent with the VWD bleeding phenotype [1]. Haemostatic efficacy was rated excellent/good in 95%–98% of treated bleeds.
Although populations varied across the studies, in line with current guidelines, prophylactic treatment was reserved for patients with severe forms of VWD to prevent recurrent mucosal and joint bleeds [28]. Although ABR is considered an important efficacy outcome for LTP, ABR data were reported in only two of the four studies, showing a reduction in ABRs from a median of 3–24 prior prophylaxis to 0.5–6 during prophylaxis [19, 20]. In the other two studies, prophylactic efficacy was rated by the treating physician as excellent/good in 100% (37/37) of treatment cycles [15, 16, 18].
SP data were reported in 13 publications, where variations in procedure classification, response definition and evaluation methodology affected study comparisons. The main efficacy outcome was prophylactic efficacy rated excellent/good in 94%–100% of procedures by the investigator.
Safety outcomes were consistently assessed in the CSL-4001 and -4002 trials but rarely reported among observational studies. A single study discontinuation was reported due to thrombocytopenia occurring in a subject with type 2B VWD [24]. It is known that patients with type 2B VWD have increased risk for developing thrombocytopenia [28].
In all studies except the reports by Auerswald et al. [21] and Abshire et al. [20], women represented >50% of the study population. In fact, women are more likely to come to medical attention than men because of gynaecologic and obstetric bleeding, despite VWD being equally prevalent across genders [1]. One retrospective observational study reported the efficacy of pdVWF/FVIII and another recombinant VWF concentrate in preventing postpartum haemorrhage (PPH) in women with VWD [13]. In this study, 3/12 women experienced PPH despite receiving higher than recommended doses (80 IU/kg) of VWF concentrate [13]. Compared with women without VWD, women with VWD carry a 1.5-fold higher risk of developing PPH, a condition associated with an increased risk of morbidity and mortality [13, 29]. The recommended treatment for low VWF levels in pregnancy is DDAVP or VWF concentrates [5, 30], however, only one publication on pdVWF/FVIII in pregnancy was identified in this search, highlighting a gap in the literature [28].
TEs are a concern associated with the administration of VWF/FVIII concentrates [31, 32]. In the general population, the incidence of symptomatic venous thromboembolism (VTE) across a spectrum of different surgeries has been estimated between 0.80% and 0.96% [33, 34], with higher rates in major orthopaedic surgeries at 1%–1.8% upon thromboprophylaxis [35-37]. Across all studies, incidence of TEs was the only safety outcome consistently reported, with the majority reporting no TEs. Only three cases of TE were reported out of the 966 (0.3%) surgical procedures across all studies and could be attributed to several risk factors (advanced age, recent major orthopaedic surgery, thrombocytosis and absence of thromboprophylaxis). Additionally, in one case the post-operative dosage was not adjusted according to FVIII levels during treatment course [26]. Although TE rates in the general population reported above do not account for individual predisposing factors, data identified in this review suggest that the appropriate use of pdVWF/FVIII in patients with VWD does not increase the risk of TEs. Persistently elevated levels of FVIII (>150 IU/dL) are a known risk factor for TEs and repeated VWF/FVIII concentrate infusions may lead to such elevations [31, 32]. Therefore, regular monitoring of FVIII levels during VWF/FVIII treatment is recommended in VWD patients undergoing surgeries [38, 39]. Although specific guidelines on the use of antithrombotic therapy in patients with VWD are lacking, thromboprophylaxis remains recommended in the highest risk settings such as orthopaedic surgery and in presence of known pro-thrombotic risk factors [4, 31, 38, 39].
Since consumption outcomes and data stratification were not uniformly reported, comparisons between studies were not feasible. Dosing largely varied across the studies reflecting the lack of specific guidelines at the time of study conduction. As expected, treatment duration varied according to procedure type, with a longer median duration in major procedures (7 days) than in minor procedures (3–4 days). Only one study reported continuous infusion of pdVWF/FVIII during elective surgery as opposed to the typical intermittent bolus infusion regimen [21].
PMS data collected over 40 years of clinical pdVWF/FVIII use indicates that the rate of ADRs is low, reaffirming the product's positive safety profile. PMS revealed more common inhibitors to FVIII than to VWF. FVIII inhibitor incidence is relatively common, ∼30% in haemophilia A in previously untreated patients [40]. Antibodies against VWF represent a serious but rare complication of type 3 VWD developing in ∼5%–10% of patients [41]. It typically affects patients with partial or complete VWF gene deletions and manifests as lack or loss of haemostatic efficacy to VWF concentrates [41] but, in rare cases, also as anaphylactic reactions, further complicating VWD management [41-43].
Transmission of infectious agents is a known risk of plasma-derived products, however, the manufacturing process of pdVWF/FVIII includes highly effective virus removal and inactivation steps involving pasteurization at 60°C for 10 h [3]. Additional steps to prevent transmission of blood-borne diseases include the selection of donors and screening of plasma pools for transfusion-relevant viruses [3]. PMS data revealed that despite 184 ADRs of suspected virus transmissions reported, none were confirmed to be associated with the use of pdVWF/FVIII to date.
This review provides an overview of the use of a specific VWF product, and its findings agree with two recent systematic literature reviews which summarized studies investigating surgical procedures and LTP in VWD patients, and were conducted to support the development of the 2020 ASH ISTH NHF WFH 2021 VWD management guidelines [28, 44, 45]. This present study, compiling the efficacy and safety of a specific VWF product, brings additional value to those recent publications since treatment optimization in VWD should consider the different FVIII/VWF ratios and characteristics across VWF concentrates as well as the high variability of the disease itself and between patients. As such, a product-specific review might aid treatment optimization in patients with VWD across the various treatment regimens. Furthermore, this study reports real-world safety data which has not been published before.
Limitations of this systematic review are that only observational studies and non-randomized, single-arm trials met the inclusion criteria, and variations in protocols, safety evaluations, procedure definitions and outcomes across the studies hindered comparability. As previously highlighted [46], there is urgent need for a standardization in outcomes reporting and methodologies across VWD studies, especially in light of the already relatively scarce evidence across the various treatment regimens and (bleeding pheno-) types of VWD. A further limiting factor related to the PMS, was that the indication was not always specified and some ADRs likely occurred in patients with haemophilia A.
In conclusion, this systematic literature review summarizes evidence on the efficacy, safety, dosing and consumption of pdVWF/FVIII for the treatment of patients with inherited VWD. Data published over four decades support the long-term efficacy and safety of pdVWF/FVIII in patients of all ages and VWD types, and in all treatment regimens.
Author Contributions
C.E.E., R.L., G.E., C.M. and E.B. contributed to the concept and design of the systematic literature review and provided critical appraisal and interpretation of the data presented. L.H. and K.S. performed analysis and interpretation of the pharmacovigilance data. All authors provided critical appraisal and revisions of this document during its creation. All authors reviewed and approved the final version of this manuscript prior to submission.
Acknowledgements
The authors would like to thank Meridian HealthComms, Macclesfield UK, for providing medical writing support in accordance with Good Publication practice (GPP2022), which was funded by CSL Behring GmbH, Hattersheim am Main, Germany. Medical writing assistance was provided by Elisa Venturi and Lucy Craggs of Meridian HealthComms, Macclesfield UK, under the guidance of all authors.
Ethics Statement
This is a systematic literature review of existing publications and does not present any new data on humans, thus ethics committee approval was not required.
Conflicts of Interest
C.E.E. has acted as a consultant and received speaker's fees and/or research funding from Bayer, Biomarin, Biotest, CSL Behring, Grifols, Kedrion, LFB, Octapharma, Novo Nordisk, Pfizer, Roche/Chugai, SOBI, Sanofi and Takeda. R.L. has been a member of advisory boards for Biomarin, CSL Behring, Sanofi, SOBI and Takeda and is CSO of Aplagon Ltd. G.E. has received honoraria/consultant fees from Bayer, BMS, Boehringer Ingelheim, CSL Behring, Grifols and Terumo (BCT). C.M. has received grants/research support from Bayer, Takeda, Biotest, CSL Behring, Novo Nordisk, SOBI; personal honoraria from Bayer, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Pfizer, Roche, SOBI, Takeda; travel support from Bayer, Biotest, CSL Behring, Novo Nordisk and SOBI. K.S. and L.H. are CSL Behring employees. E.B. has acted as a consultant for Bayer and CSL Behring.
Open Research
Data Availability Statement
CSL Behring will only consider requests to share data that are received from systematic review groups or bona-fide researchers. CSL will not process or act on data requests until 12 months after article publication on a public website. A data request will not be considered by CSL unless the proposed research question seeks to answer a significant and unknown medical science or patient care question. Applicable country specific privacy and other laws and regulations will be considered and may prevent sharing of data.
Requests for use of the data will be reviewed by an internal CSL review committee. If the request is approved, and the researcher agrees to the applicable terms and conditions in a data sharing agreement, data that has been appropriately anonymized will be made available. Supporting documents including study protocol and Statistical Analysis Plan will also be provided.
For information on the process and requirements for submitting a voluntary data sharing request for data, please contact CSL at [email protected].




