Dilemmas on emicizumab in children with haemophilia A: A survey of strategies from PedNet centres
Susanna Ranta, Jayashree Motwani, Nadine G. Andersson Christoph Königs contributed equally to this article.
Abstract
Introduction
Haemophilia A care has changed with the introduction of emicizumab. Experience on the youngest children is still scarce and clinical practice varies between haemophilia treatment centres.
Aim
We aimed to assess the current clinical practice on emicizumab prophylaxis within PedNet, a collaborative research platform for paediatricians treating children with haemophilia.
Methods
An electronic survey was sent to all PedNet members (n = 32) between October 2022 and February 2023. The survey included questions on the availability of emicizumab, on the practice of initiating prophylaxis in previously untreated or minimally treated patients (PUPs or MTPs) and emicizumab use in patients with or without inhibitors.
Results
All but four centres (28/32; 88%) responded. Emicizumab was available in clinical practice in 25/28 centres (89%), and in 3/28 for selected patients only (e.g. with inhibitors). Emicizumab was the preferred choice for prophylaxis in PUPs or MTPs in 20/25 centres; most (85%) started emicizumab prophylaxis before 1 year of age (30% before 6 months of age) and without concomitant FVIII (16/20; 80%). After the loading dose, 13/28 centres administered the recommended dosing, while the others adjusted the interval of injections to give whole vials. In inhibitor patients, the use of emicizumab during ITI was common, with low-dose ITI being the preferred protocol.
Conclusion
Most centres choose to initiate prophylaxis with emicizumab before 12 months of age and without concomitant FVIII. In inhibitor patients, ITI is mostly given in addition to emicizumab, but there was no common practice on how to proceed after successful ITI.
1 INTRODUCTION
Haemophilia care is rapidly evolving with emerging treatment options including extended half-life (EHL) factor concentrates, non-factor replacement therapies and gene therapy. Emicizumab, a recombinant bispecific monoclonal antibody that mimics the function of factor VIII (FVIII), is the first licensed and now widely available non-factor replacement therapy.1 In most countries, emicizumab was first used in patients with persisting inhibitors as prophylaxis with good protection from bleeds.2
The data on emicizumab in previously untreated patients (PUPs) and minimally treated patients (MTPs) is scarce. A case series on four infants on emicizumab was published3 and, very recently and, very recently, the interim analysis of HAVEN 7 (NCT04431726), were published as an abstract and demonstrated the safety and efficacy of emicizumab in a relatively small number of children under the age of 12 months with severe haemophilia A.4
Several issues remain unanswered in the use of emicizumab in PUPs and MTPs. First, when to start emicizumab prophylaxis. Early start during the first 3 months of life has been motivated by the risk of intracranial bleeding, but there has been concern that the physiologically low FIX in the first months of life may limit the effectiveness of emicizumab. Moreover, not all patients are diagnosed early, and the highest risk of intracranial bleeding is at the time of delivery when emicizumab will not yet have been commenced.5, 6 Also, the issue of vial sizes and whether to use whole vials in neonates and very young children is not resolved.
Second, should PUPs and MTPs receive concomitant FVIII to achieve tolerance and avoid inhibitor development or will this prove disadvantageous. In severe haemophilia A, inhibitors develop in approximately 30% of patients within the first 50 exposure days (ED) to FVIII.7 Several studies investigating the concomitant use of emicizumab and FVIII have been initiated but no data are showing if parallel administration of FVIII for example, the first 20−50 EDs and stopping thereafter is protective against inhibitor development. Such a practice carries significant burden to very young children due to the need for regular intravenous injections and frequent inhibitor testing.
Third, in case of inhibitor development PUPs or MTPs, a decision needs to be made if it is still warranted to attempt inhibitor eradication through immune tolerance induction (ITI). The potential advantages of inhibitor eradication include being able to use FVIII in the future for treating bleeds and undertaking surgery, to provide greater level of protection during sport activities, and to allow future access to gene therapy. Consequently, most haemophilia treaters today still advocate for offering ITI, yet many patients/caregivers are declining to undertake it due to the burdens of ITI. Furthermore, it is unclear how to proceed if ITI is successful and tolerance is achieved; will patients need to remain on regular FVIII exposure long-term negating many of the benefits of being on subcutaneously administered emicizumab. We face the same dilemma in patients with previously eradicated inhibitors after initiating emicizumab. With the cessation of regular FVIII injections, when FVIII is administered intermittently for acute bleeds, there are concerns regarding loss of tolerance and inhibitor recurrence.
To assess the current views and practices in implementing emicizumab prophylaxis in children with haemophilia A with and without inhibitors, we conducted a survey in paediatric haemophilia treatment centres participating in the PedNet (Pediatric Network on hemophilia management, see appendix).
2 METHODS
The PedNet Registry (NCT02979119) is a collaborative research group for pediatricians treating children with haemophilia with members across Europe, Canada and Israel.8 The Registry includes > 2800 children with haemophilia. At the time of data collection, 32 haemophilia treatment centres in 18 countries were actively participating in the PedNet Registry. An electronic survey was sent to members of all PedNet centers in October 2022 with the possibility of reply until February 2023. The survey included questions on availability of emicizumab as well as the local practice of initiating prophylaxis in PUPs, including age at start of prophylaxis, emicizumab regimen and concomitant use of FVIII as well as use of emicizumab in patients with current or previous inhibitors. The complete survey is provided in Supplementary file 1.
3 RESULTS
In total, 28 of 32 (88%) PedNet centres from 18 countries responded.
3.1 Availability
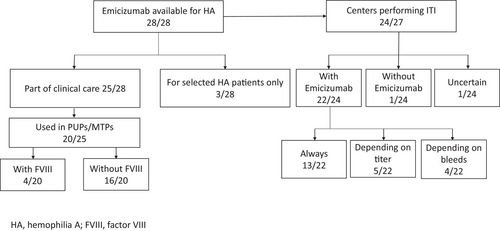
Emicizumab was available as part of routine clinical care in non-inhibitor and inhibitor patients in 25/28 centers (89%); in 1/28 centres for patients with difficult venous access or inhibitors or in a clinical trial; in 2/28 centres for patients in clinical trials or with inhibitors only (Figure 1). Most centres (16/27; 59%, missing n = 1) used emicizumab for severe haemophilia A only, eight (30%) were able to use emicizumab on patients with severe or moderate haemophilia A and three (11%) centres could use emicizumab in haemophilia A patients of all severities.
3.2 Initiating treatment for PUPs or MTP
Of the 25 centres able to use emicizumab for prophylaxis in patients without inhibitors, 20 (80%) used emicizumab to initiate prophylaxis in PUPs or MTPs. The age of start of prophylaxis with emicizumab varied, but 17/20 (85%) of centres started before the age of 12 months: eleven (55%) centres started between 6 and 12 months; four (20%) centres between 3 and 6 months, and two centres (10%) started before the age of 3 months. Three centres (15%) waited until after 12 months to start emicizumab prophylaxis. All but two centers used the loading dose according to the drug label, one started with once monthly and another without a loading dose. At the initiation of emicizumab in PUPs or MTPs, only 4 of the 20 (20%) centres gave concomitant FVIII to all patients with the goal of achieving tolerance: two centres gave FVIII once a week up to 75 EDs, whilst two centres gave it every other week up to 20−50 EDs. An additional centre gave concomitant FVIII every other week up to 20−50 EDs to selected patients considered to be at high risk for inhibitor development and one centre to patients with good venous access. All but two centres tested patients for inhibitor development to FVIII, either regularly or after exposure to FVIII.
3.3 Emicizumab prophylaxis after the loading period
The regimen for prophylaxis after the loading phase varied, with 15/28 centres administering entire vials, while 13/28 administered the exact recommended dose. The preferred interval for most centres (13/28) was every other week; seven centres mainly used once-a-week administration, and eight centres adjusted the interval depending on the vial size and weight of the patients. One centre continued concomitant FVIII to selected non-inhibitor patients playing high level of sports. Emicizumab concentration levels were measured regularly in 16 centres, in two of these only after the loading period. Strategies in case of FVIII inhibitor development under emicizumab are presented in Figure 2.

3.4 Emicizumab prophylaxis in patients during ITI
Twenty-four or 28 centres responded that they offered ITI to patients with inhibitors. Twenty-three of 24 (96%) centres used emicizumab during ITI for bleed prophylaxis: 13 centres used concomitant emicizumab during ITI for all patients, five for some patients depending on their inhibitor titre, four for some patients depending on their bleeding phenotype and one centre was undecided as the situation had not occurred yet. The approach to ITI varied depending on the inhibitor titre, but most centres favored low-dose ITI for all but the very high inhibitor titres (> 200 BU) in whom daily high-dose ITI was still favoured (Table 1). It should be noted that most centers would advocate for ITI particularly in children with inhibitor titers of 5−40 and 40−200 BU/mL with a much lower proportion of centres advocating for ITI in children with inhibitor titres < 5 or > 200 BU/mL. Of centres using emicizumab prophylaxis during ITI, most would use the FVIII concentrate that caused the inhibitor (n = 12/23) for ITI, while six would choose extended half-life FVIII, four favoured plasma derived FVIII and one standard half-life recombinant FVIII. Reasons for considering ITI unsuccessful resulting in cessation of ITI (with continuation of emicizumab) varied: no success after 6–12 months (n = 4), no success after 3 years (n = 3), difficulties with venous access (n = 4) or family's wishes (n = 4). In case of successful ITI, 19 centres would continue treatment with emicizumab, 14 of them initially with regular injections of FVIII and five with emicizumab prophylaxis only. Only one centre would stop prophylaxis with emicizumab and continue FVIII only, while another centre would either continue emicizumab or switch to FVIII prophylaxis (unsure n = 2; missing n = 1). Seven of the 19 centres continuing FVIII were planning to administer it initially once a week, two centres would continue it every other week and two centres indicated that they would continue FVIII two to three times a week.
| ≥100 IU/kg once daily | ≥100 IU/kg twice daily | 30-99 IU/kg once daily | >30-99 IU/kg 2-3 times/ wk | Total no of centresa | |
|---|---|---|---|---|---|
| Which ITI protocol do you use with emicizumab | |||||
| in children with low titer inhibitors < 5 BU/mL | 2 | 1 | 1 | 18 | 18 |
| in children with high inhibitor titre ≥5–40 BU/mL | 8 | 0 | 3 | 15 | 20 |
| in children with high inhibitor titre ≥40–200 BU/mL | 10 | 2 | 2 | 10 | 20 |
| in children with high inhibitor titre > 200 BU/mL | 9 | 5 | 0 | 7 | 17 |
| TOTAL | 19 | 8 | 6 | 31 | |
- a It was possible to choose more than one option.
4 DISCUSSION
In this PedNet survey, conducted at the end of 2022/start of 2023 involving 28 centres from the PedNet Registry across Europe, Canada and Israel, we found that emicizumab in children—both PUPs, PTPs and children with inhibitors—is broadly used. The main treatment decisions regarding emicizumab reached a majority: to start emicizumab as prophylaxis in PUPs/MTPs, mainly between 6 and 12 months, with loading doses as per the drug label and regular checks for FVIII inhibitor development and emicizumab levels. Concomitant administration of FVIII in PUPs was rarely used. For inhibitor patients, ITI is still offered for most patients together with emicizumab as bleed prophylaxis. Most centres preferred low-dose ITI. After successful ITI, most centres would continue FVIII, but in variable dose regimens.
Introduction of emicizumab has transformed the lives of children with FVIII inhibitors offering effective protection against bleeds. Further, emicizumab treatment also offers significant benefits for severe haemophilia A patients without inhibitors, such as stable protection against bleeds corresponding to the level of protection seen in patients with mild haemophilia, easy subcutaneous administration and infrequent dosing both of which lead to a reduced treatment burden. This is reflected in the survey by most centres using emicizumab as first line prophylaxis treatment with the opportunity to start significantly earlier than in traditional intravenous FVIII prophylaxis. Over the years, prophylaxis with FVIII concentrates has started earlier; in the PedNet centres, the median age for start of prophylaxis with coagulation factor concentrates in severe haemophilia A has decreased from 17.3 to 13.1 months over the last decades.9-11 However, an even earlier start of FVIII prophylaxis is hampered by the need for intravenous injections, which is extremely challenging in very young children. Although the use of central venous access devices (CVADs) may permit even younger start of prophylaxis with factor concentrates the insertion of such devices necessitates general anesthesia, leaves scars and such devices are prone to thrombosis and/or infections. Starting emicizumab prophylaxis avoids the need for CVAD and the issue of poor venous access in young children. Almost all (85%) PedNet centres are choosing emicizumab prophylaxis prior to 12 months of age; many are starting before 6 months of age. However, long-term data on the effectiveness and safety of early start of prophylaxis with emicizumab are lacking, and FVIII might have functions beyond haemostasis, such as preserving bone mineral density.12 On the other hand, better protection of bleeds with emicizumab could potentially influence joint health and might even influence the risk for osteoporosis. In a case-series, Mason and Young have summarized the pros and cons of emicizumab prophylaxis including a reduced need for venipuncture and less parental anxiety, but they stress the unknown efficacy in infants and unknown risk of inhibitor development of this approach.3
Traditionally, haemophilia treaters have given whole vials of coagulation factor concentrates to avoid medication waste and optimize costs. Current dosing recommendations allow the use of emicizumab once a week, every other week or every fourth week. In all cases, this involves administering 6 mg/kg/over a 4-week (28-day) period. Yet, some studies suggest that lower doses might be effective.13 Furthermore, slightly higher than recommended doses are not thought to lead to safety issues. A previous study simulating pharmacokinetics suggested that using whole vials with emicizumab is feasible.14 Also, it has been published that adjusting the interval of administering emicizumab between 1 and 4 weeks while keeping the recommended weekly dose leads to therapeutic plasma concentrations.15, 16
Recognizing this some PedNet centres indicated that they are using whole vials, which might result in patients slightly more or slightly less than 6 mg/kg/over a 4-week period. However, data on pharmacokinetics in neonates and very young infants is lacking; therefore, no guidelines for very young children exist and the pros and cons of using whole vials or discarding emicizumab should be discussed with families.
After initiation of emicizumab in PUPs/MTPs, it is unclear if children with severe haemophilia are at higher/lower/equal risk of inhibitor development as they are not being regularly exposed to FVIII but rather only in the setting of bleeds or surgery. However, the corollary is that emicizumab prophylaxis commenced early will almost certainly lead to a significant reduction in exposure to FVIII leading to children only reaching 20−50 EDs to FVIII at much older ages—hence, emicizumab may delay or modify the development of FVIII inhibitors. Several clinical trials (Emicizumab PUPs and Nuwiq ITI Study, NCT04030052 and NAVIGATE trial; NCT05802836) aim to assess these questions.
Despite clinicians considering that routine concomitant FVIII treatment along with emicizumab may be beneficial to prevent inhibitor development, this approach is only practiced in a minority (4/20; 20%) of PedNet centres. This is not surprising, given how difficult it is to give regular intravenous injections of FVIII to very young children and the additional cost of treatment on top of the emicizumab. Furthermore, data on the efficacy of concomitant FVIII concentrates along with emicizumab to achieve tolerance (i.e. avoid/decrease inhibitor incidence) against FVIII is missing.
In case of inhibitor development, the Future of Immunotolerance Treatment (FIT) group recommended several years ago to attempt ITI at least once to eradicate inhibitors.17 In our survey, most PedNet centres offered ITI and used emicizumab during ITI to prevent bleeds. Evidence on ITI with concomitant emicizumab is emerging, with MOTIVATE (NCT04023019), NAVIGATE (NCT05802836) and the Emicizumab PUPs and Nuwiq ITI Study (NCT04030052) addressing this topic.18
Also, the question about maintenance of tolerance after successful ITI without regular exposure to FVIII currently remains unanswered. It is reasonable to assume that the risk of recurrence of inhibitors in previously tolerized patients when stopping regular FVIII exposure varies depending on the historical inhibitor titers and time since ITI. In our survey, most centres planned to maintain FVIII exposure with regular FVIII injections after successful ITI, but practical experience is lacking, for example regarding dose and frequency of FVIII injections. While some centres stated that continued FVIII exposure was planned for a limited time only, no set time was generally defined.
5 CONCLUSION
In the absence of evidence-based guidelines for prophylaxis with emicizumab in PUPs and use of emicizumab during and after ITI clinical practices vary, but opinions from PedNet centers show a rather concordant approach and may help decision making in the meantime. While ongoing clinical trials will hopefully shed some light on the open questions, there is still a need for large cohort studies and real-life data, which can be obtained from large international registries.
ACKNOWLEDGEMENTS
All the authors have contributed to planning of the survey, summarizing the results and writing the manuscript. They thank all the PedNet members who shared their current views on treatment with emicizumab.
This study is supported by the PedNet Hemophilia Research Foundation. Unrestricted sponsorship for the PedNet Hemophilia Research Foundation is currently received from Bayer AG, Takeda, Novo Nordisk, CSL Behring, Pfizer Inc., Swedish Orphan Biovitrium AB, Hoffmann-La Roche, LFB Biotechnologies. Supported by MH CZ—DRO (FNBr, 65269705).
CONFLICT OF INTEREST STATEMENT
M. Carcao received research support from Bayer, Bioverativ/Sanofi, Novartis, Novo Nordisk, Pfizer, Roche and Shire/Takeda, honoraria for speaking/participating in advisory boards from Bayer, Bioverativ/Sanofi, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche and Shire/Takeda. G. Kenet received research grant support from BSF, Pfizer, Roche, Tel Aviv University, Consultant ASC therapeutics, BPL, Bayer, Novonordisk, Pfizer, Roche, Sanofi- Genzyme, Takeda, has been in advisory boards and received honoraria for lectures Bayer, BioMarin, CSL, Pfizer, Sanofi- Genzyme, Spark, Takeda, Uniquore. S. Ranta is an investigator in clinical trials sponsored by Novo Nordisk, Roche, Sobi, Boehringer Ingelheim (payment to institution, not to author), member in a study steering committee Roche; has received grants for research from the Childhood Cancer Foundation and Stockholm County Council; and is a member of a study steering committee for Roche. M Olivieri received grants/research support from Bayer, Takeda, CSL Behring, Pfizer and Swedish Orphan Biovitrium, consultancy, and speaker fees from Bayer, Biomarin, Biotest, Novo Nordisk, Takeda, CSL Behring, Pfizer, Roche, Stago and Swedish Orphan Biovitrium. K. Fischer has received speaker's fees from CSL Behring, NovoNordisk; consultancy fees from Biogen, CSL-Behring, Freeline, NovoNordisk, Roche and SOBI; and research support from Bayer, Pfizer and Novo Nordisk. K Fischer is the epidemiologist for the EUHASS and the PedNet Registry. M. Kartal-Kaess: consulting or advisory role: Bayer, SOBI, Takeda; research funding: NovoNordisk, Roche; travel, accommodations, expenses: Bayer, NovoNordisk, SOBI, Takeda, Jazz. M Bührlen received honoraria from NovoNordisk for speaking. B. Nolan is an investigator in clinical trials sponsored by NovoNordisk, CSL Behring, Roche, Bayer, SOBI, Sanofi, Bioverativ and Alnylam and has received speaker fees from SOBI. C. Escuriola Ettingshausen has acted as a consultant, received speaker's fees and/or research funding from the following companies: Bayer Healthcare, Biomarin, Biotest, CSL Behring, Grifols, Kedrion, LFB, Octapharma, NovoNordisk, Pfizer, Takeda, Sanofi; Swedish Orphan Biovitrium, Roche/Chugai. NG Andersson served as a speaker and/or on advisory boards for Bayer, CSL Behring, Octapharma and Sobi/Sanofi. J Blathny received speakers and/or consultation fees from NovoNordisk, Roche, CSL Behring, Octapharma, Takeda, Sobi. H. Chambost received fees or honoraria for attending advisory boards, or speaking at symposia, or research funding from: BioMarin, CSL Behring, LFB, NovoNordisk, Pfizer, Roche/Chugaï and Sobi/Sanofi, and has no financial interest in pharma companies. R. d'Oiron received fees or honoraria for attending advisory boards, or speaking at symposia, or research funding from: BioMarin, CSL Behring, LFB, NovoNordisk, Octapharma, Roche, Shire/Takeda, Sobi/Sanofi and UniQure, and has no financial interest in pharma companies. E Zapotocka consulting or advisory role for Roche, Novo Nordisk, Sobi, travel support from Roche, Sobi, Takeda. C. Male received research support/grants to institution from Bayer, Biotest, CSL Behring, Novo Nordisk, SOBI, Takeda, personal honoraria from Bayer, Biomarin, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Pfizer, Roche, Takeda, travel support from Bayer, Biotest, CSL Behring, Novo Nordisk. C. König har received research support to Goethe University by Bayer, Biotest, CLS Behring, Intersero, Novo Nordisk, Pfizer, Roche/Chugai, Sobi/Sanofi, Takeda, DFG, EU H2020 ITN and has received fees for presentations or advice by BFSH, Bayer, CLS Behring, MSD, Novo Nordisk, Roche/Chugai, Sobi/Sanofi, Takeda.
The authors declare no conflicts of interest. (if no conflicts for an article with multiple authors)
ETHICS STATEMENT
Ethical approval and informed consent were obtained according to the regulations in each participating center for the Pednet registry ; no patient data from the registry was used for this survey.
APPENDIX
The PedNet Study group members and centres
Europe
- - MT Alvarèz Román, Unidad de Coagulopatías, Hopital Universitario La Paz, Madrid, Spain
- - O Benitez Hidalgo, Unitat Hemofilia, Hospital Vall d'Hebron, Barcelona, Spain
- - J Blatny, Department of Paediatric Haematology, Children's University Hospital, Brno, Czech Republic
- - M Bührlen, Gesundheit Nord, Klinikum Bremen Mitte, Prof.-Hess-Kinderklinik, Bremen, Germany
- - M Carvalho, Immunohemotherapy Department, Congenital Coagulopathies Reference, Centro Hospitalar e Universitário São João, E.P.E., Porto, Portugal
- - G Castaman, Department of Oncology, Careggi University Hospital, Florence Italy
- - H Chambost, APHM, La Timone Children's Hospital, Center for Bleeding Disorders & Aix Marseille Univ, INSERM, INRA, C2VN, Marseille, France
- - A Rosa Cid & S Bonanad, Unidad de Hemostasia y Trombosis, Hospital Universitario y Politécnico La Fe, Valencia, Spain
- - C Escuriola Ettingshausen, HZRM Hämophilie Zentrum Rhein Main GmbH, Mörfelden-Walldorf, Germany
- - K Fischer, Van Creveld Kliniek, University Medical Center Utrecht, Utrecht, The Netherlands
- - C Van Geet & V Labarque, Catholic University of Leuven, Campus Gasthuisberg, Service of Pediatric Haematology, Leuven, Belgium
- - H Glosli & H Knudsen, Oslo University Hospital HF, Oslo, Norway
- - N Gretenkort Andersson, Department of Clinical Sciences, Lund University, Lund; Department of Pediatrics and Malmö Centre for Thrombosis and Haemostasis, Skåne University Hospital, Malmö, Sweden
- - C Königs, University Hospital Frankfurt, Department of Paediatrics and Adolescent Medicine, Frankfurt, Germany
- - M Koskenvuo, New Children's Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
- - C Male, Department of Paediatrics, Medical University Hospital of Vienna, Vienna, Austria
- - T Stamm Mikkelsen, Department of Pediatrics, University Hospital of Aarhus at Skejby, Aarhus, Denmark
- - A Molinari, Dipartimento di Ematologia ed Oncologia, Unità Trombosi ed Emostasi, Ospedale Pediatrico Giannina Gaslini, Genova, Italy
- - J Motwani, Department of Haematology, The Children's Hospital, Birmingham, UK
- - B Nolan, Department of Paediatric Haematology, Our Lady's Children's Hospital for Sick Children, Crumlin, Dublin, Ireland
- - R d'Oiron, Centre de Référence de l'Hémophilie et des Maladies Hémorragiques Constitutionnelles, et HITh UMR_S1176 INSERM, Hopital Bicêtre, APHP Université Paris Saclay, Le Kremlin Bicêtre, France
- - J Oldenburg & K Herbst, Institut für Experimentelle Hämatologie und Transfusionsmedizin, Universitätsklinikum Bonn, Germany
- - M Olivieri, Dr. V. Hauner Children's Hospital, University of Munich, Munich, Germany
- - C Oudot, Centre Regional d'Hemophilie, Centre Hospitalo Universitaire, Toulouse, France
- - H Pergantou, Haemophilia Centre/Haemostasis and Thrombosis Unit, Aghia Sophia Children's Hospital, Athens, Greece
- - F Pinto, Department of Haematology, Royal Hospital for Sick Children, Yorkhill, Glasgow, UK
- - S Ranta, Pediatric Coagulation Unit, Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden
- - M Kartal-Kaess, Division of Pediatric Hematology & Oncology, Department of Pediatrics, Inselspital, University Hospital, University of Bern, Bern, Switzerland
- - E Zápotocká, Department of Pediatric Hematology and Oncology, Prague, Czech Republic
- - Royal Hospital for Sick Children, Edinburgh, UK*
- - Universitäts-Klinik für Kinder- und Jugendheilkunde, Graz, Austria*
- - Hemophilia Comprehensive Care Centre, Great Ormond Street Hospital for Children, London, UK*
- - Maggiore Hospital Policlinico, A. Bianchi Bonomi Hemophilia and Thrombosis Centre, Milan, ITALY*
- - Hospital General Unidad de Hemofilia, Hospitales Universitarios Virgen del Rocio, Sevilla, Spain*
* No longer participating as PedNet center
Israel
- - G Kenet, National Hemophilia Center Sheba Medical center, Tel Hashomer & Amalia Biron Research Institute of Thrombosis & Hemostasis, Tel Aviv University, Israel
Canada
- - M Carcao, Division of Haematology/Oncology, Hospital for Sick Children, Toronto, Canada
- - G Rivard, Division of Hematology/Oncology, Hôpital St Justine, Montréal, Canada
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the registry of the PedNet Hemophilia Research Foundation. Data are available from the authors with the permission of the PedNet Hemophilia Research Foundation upon reasonable request. (www.pednet.eu).




