IDEAL study: A real-world assessment of pattern of use and clinical outcomes with recombinant coagulation factor IX albumin fusion protein (rIX-FP) in patients with haemophilia B in Italy
Abstract
Introduction
Factor IX replacement therapy is used for treatment and prophylaxis of bleeding in haemophilia B. rIX-FP is an extended half-life albumin-fusion protein, which, in clinical studies, has demonstrated prolonged dosing intervals up to 21 days for routine prophylaxis, providing therapeutic benefit.
Aims
To describe dosing frequency and consumption (primary endpoint), efficacy and safety of rIX-FP treatment during routine clinical practice in Italy.
Methods
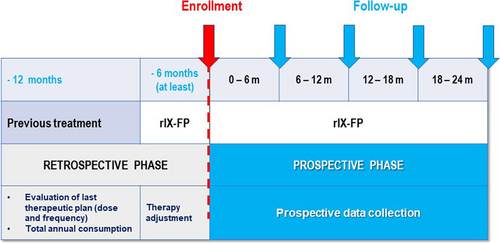
Patients with moderate/severe haemophilia B on prophylaxis with rIX-FP for ≥6 months, were enrolled in this observational study from October 2017 to February 2019 and followed-up for 2 years. Descriptive analysis included prospective and retrospective data (12 months prior to switching to rIX-FP).
Results
Data were collected from 59 male patients (median age 30.1 years) enrolled by 23 Italian centres. Of them, 50 were on prophylaxis during the entire observation period and completed the study. The infusion frequency changed from 2–3 times/week in 86.0% of patients with previous treatment, to less than once a week in 84.0% of patients treated with rIX-FP at the 2nd-year follow-up. The annual number of infusions decreased by about 70%, whereas the mean FIX activity trough level increased from 3.8% to 14.4% (mean > 10% in all the infusion regimens). Median Annualised Bleeding Rate of .0 was achieved across all prophylaxis regimens. Subjects with zero bleedings increased from 66.0% to 78.0% with rIX-FP.
Conclusion
Treatment with rIX-FP reduced infusion frequency, while providing higher FIX trough levels with substantial benefit in terms of annualised bleeding rate and a good safety profile.
1 INTRODUCTION
Haemophilia B (HB) is a rare X-linked congenital bleeding disorder characterised by a deficiency of coagulation factor IX (FIX), accounting for 15%–20% of all haemophilia cases.1
Replacement therapy with FIX concentrates provides a temporary correction of the deficiency and the bleeding tendency. This can effectively reduce joint damage and disabilities thereby improving the patients’ quality of life.2
Regular, long-term prophylaxis with clotting factor concentrates (CFCs) started early in life (before the age of 3) is the standard care for patients with severe HB of all ages.1
The objective of standard prophylaxis is to convert a person with severe haemophilia (baseline FIX level < 1 IU/dl [1%]) to a bleeding phenotype typical of moderate or mild haemophilia by maintaining factor levels above 1 IU/dl (1%) at all times. However, there has been increasing recognition and evidence that factor trough levels of 1%–3% are insufficient to totally prevent bleeds, resulting in gradual progression of joint disease over a lifespan.1, 3, 4
Moreover, due to the relatively short half-life of the available standard half-life (SHL) plasma-derived or recombinant FIX products (rFIX), prophylactic infusions are required 2–3 times weekly in order to achieve a significant reduction of bleeding episodes and a clinical outcome.5 Repeated regular intravenous infusions mean a substantial burden for patients and caregivers. Treatment in younger children often requires the insertion of a venous access device with its potential associated complications.6, 7
rIX-FP (albutrepenonacog alfa, IDELVION®, CSL Behring), is a recombinant fusion protein developed to extend the half-life of recombinant factor IX through genetic fusion with recombinant albumin.5, 8, 9
In clinical studies, rIX-FP has shown great flexibility in dosing with intervals between prophylactic infusions from 7 up to 21 days, resulting in a median of zero annualised spontaneous bleeding rate (AsBR). Furthermore, nearly all (96.2%) steady-state through FIX measurements were above 5%, across all doses and dose intervals, consistent with a mild haemophilia phenotype.10 In particular, in the PROLONG-9FP clinical trial programme, rIX-FP prophylaxis achieved median AsBR of .00 with 7-, 10- or 14-day dosing intervals in adults (≥12 years) and 7-day dosing interval in children (< 12 years): patients maintained a mean trough of 20.0 and 12.4 IU/dl FIX activity on prophylaxis with rIX-FP 40 IU/kg weekly and 75 IU/kg every 2 weeks, respectively.11 The extension study12 confirmed the results in patients who participated in the pivotal phase III, adding a dosing interval of 21 days for the first time.
Whereas pre-approval clinical trials demonstrated robust efficacy and safety data, when evaluating FIX products in clinical practice, real-world utilisation and outcomes also need to be considered.
Limited real-world data on replacement therapy in haemophilia B are available in Italy. No observational study has been conducted on rIX-FP so far; the only data available from clinical practice refer to a retrospective review of clinical charts13, 14 and to the assessment of patient-reported adherence.15
In this study, subjects switching from their previous SHL FIX product to prophylaxis with rIX-FP have been evaluated in a real-world setting. Dosage and infusion frequency adjustments as per physicians’ decisions and their impact on clinical outcome, tolerability and immunogenicity of rIX-FP have been investigated.
2 METHODS
2.1 Study conduct
The study was approved by the ethics committee at each participating centre and performed in accordance with good clinical practice and local regulatory requirements. Written informed consent was obtained from all patients or their legal guardians and consent could be withdrawn at any time.
2.2 Study design and patient population
This is a multicentre, non-interventional, retrospective-prospective study conducted at 23 sites across Italy (Supplementary Appendix 1). Male patients of any age, with moderate to severe haemophilia B (residual FIX activity ≤5%), who had been using rIX-FP for long-term prophylaxis, intermittent prophylaxis and on-demand treatment for at least 6 months, were eligible for enrolment and were followed-up for two years. Patients with other inherited bleeding disorders and/or participating in any clinical trial (including non–interventional studies) were excluded. In the retrospective part of the study, patients with missing data on previous treatment (with rIX-FP, at least 6 months, and with the FIX concentrate used over the previous 12 months), annual factor consumption, ABR, bleeding events (total, joint, life threatening – e.g., internal hemorrhage and ICH) were excluded. According to the common clinical practice at Italian haemophilia centres, the study aimed to collect data from visits planned every 6 months (±2), but only 1-year and 2-year visits were considered mandatory in order to keep the patients in the study. The retrospective phase was designed to collect data related to the 12-month period before the patients’ switch to rIX-FP (information about the previous SHL FIX products) and to the following period from the first infusion with rIX-FP until enrolment in the study (at least 6 months).
Patients were assigned to treatment regimens and doses according to current local practice. In case of a switch to another treatment they were withdrawn from the study (Figure 1).
Patients were asked to fill in an infusion diary throughout the observational period including information on date, duration of each infusion, IU infused, reasons for infusion and site of bleeding, if any. Labels of the used vials were also required to be stuck onto the diary to assess patient's adherence to prescription.
2.3 Trial objectives and outcome measures
The primary objective of this study was the assessment of dosage patterns and annual consumption of rIX-FP in real life. Endpoints included annual consumption of rIX-FP versus previous treatment, number of infusions, and dosage per infusion required to prevent or resolve bleeding episodes in prophylaxis or on-demand regimens (rIX-FP vs. previous treatment).
The secondary objectives were the assessment of annualised bleeding rate (ABR) and site of bleeds, the assessment of joint status (joint damage and arthropathy), of haemostatic efficacy of rIX-FP in the treatment of non-surgical bleeding episodes and during prevention and treatment of perioperative bleeding episodes. Moreover, the tolerability/immunogenicity of rIX-FP was assessed by collecting incidence of inhibitors in the prospective observational period and type/incidence of severe adverse events, adverse drug reactions and special situations.
Data regarding frequency, Annualised Bleeding Rate-ABR (calculated as the number of reported bleeding events divided by the number of months of observation and multiplied by 12), severity, patterns and sites of bleeds were collected, as well as the number of infusions, doses and total dose of rIX-FP required to prevent or treat episodes of surgical bleeding and blood product consumption during treatment of perioperative bleeding episodes. Factor IX trough levels were measured at site according to the clinical practice through one-stage or chromogenic assay.
2.4 Data analysis
Due to the observational nature of the study, no hypothesis testing was performed in relation to the primary objective and therefore, no formal sample size calculation was computed a priori.
As specified in the study protocol, data were presented only in a descriptive manner, with mean and standard deviation or median with range for continuous variables and count and percentage for categorical data. On an exploratory basis, the statistical significance of changes over time within groups or any relationship between variables was assessed at univariate level (parametric or non-parametric test, as appropriate).
3 RESULTS
3.1 Patients’ characteristics
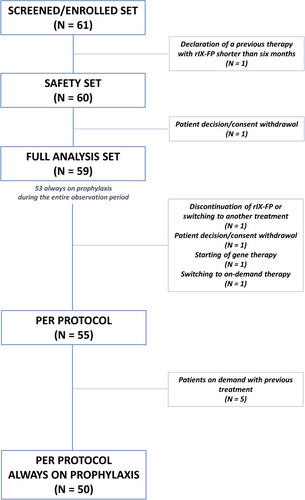
Overall, 61 male patients with HB (FIX activity ≤5%) were enrolled between October 2017 and February 2019. Sixty (60) patients received at least one infusion of treatment after enrolment, therefore were included in the Safety Set (SS). Fifty-nine (59) subjects were included in the Full Analysis Set (FAS) and fifty-five (55) of them completed the two-year observation period (per protocol population). Patients' disposition is presented in Figure 2.
Patients’ characteristics of FAS at baseline are shown in Table 1. Age ranged from 2 to 68 years (median 31.0); 25% of patients were < 18 years. The patient population was geographically distributed throughout Italy, 95% of them were Caucasian. About 75% of the subjects reported moderate or intense physical activity (96.4% agreement in performance between subject's and investigator's perception).
The median age at diagnosis was 1 year old (range: 0–21). Severe haemophilia was recorded in 83.1% of the cases; in the remaining “moderate” group (16.9%) the median FIX residual activity was 2.0% (range: 1.0%-5.0%).
Six (10.2%) patients were previously treated only on demand, 53 (89.8%) had a previous prophylaxis regimen with rFIX (nonacog alfa, n = 43) or plasma-derived FIX concentrates (pd-FIX, n = 10).
3.2 Dosage regimen and annualised total dose
Dosage regimen and frequency were reported for 50 patients who were always on prophylaxis during the entire observation period (retrospective and prospective phase) and completed the study. The frequency of infusion, mainly 2–3 times/week (86.0%) with previous treatment, in the second year of follow-up with rIX-FP was less than once a week in 84% of the cases. In particular, 54% of the patients received the treatment every 8–10 days, 24.0% every 11–14 days and 6% every 15–21 days (Figure 3B).
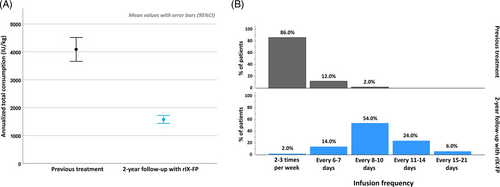
The annual number of prescribed infusions decreased by about 70%, with a mean of 116.5 ± 31.85 with the previous rFIX, and 37.6 ± 14.79 with rIX-FP (Wilcoxon signed rank test: p < .001).
The annualised total dose decreased from 4098.2 ± 1340.10 IU/kg with previous treatment, to 1562.6 ± 433.23 IU/kg of rIX-FP after enrolment (Wilcoxon signed rank test: p < .001) (Figure 3A).
The prescribed dose of rIX-FP remained quite stable during the entire 2-year follow-up, mean value ranging from 43.4 IU/kg to 46.0 IU/kg, irrespective of the infusion frequency. In particular, at the end of second-year follow-up, for each frequency, the following mean (± SD) doses were reported: 2–3 times/week (n = 1, one patient underwent a percutaneous coronary intervention): 43.5 IU/kg; Every 6–7 days (n = 7): 47.4 ± 7.33; Every 8–10 days (n = 27): 41.8 ± 8.83; Every 11–14 days (n = 12): 45.7 ± 8.59; every 15–21 days (n = 3): 45.4 ± 9.54 (Kruskal–Wallis test, p = .54).
The posology was changed in seven patients (14.3%) after one year of treatment and in 10 patients (20%) after 2 years. The reasons for change are shown in Supplementary Appendix 2.
3.3 FIX trough levels
FIX trough level (%) increased from 3.8 ± 2.6 (min-max: .3–11.0) with previous treatment to 14.4 ± 7.2 (7.0–33.8) after a 2-year treatment with rIX-FP (Paired samples t-test: p < .001). (Table 2 and Supplementary Appendix 3)
| Characteristic | Overall, N = 59 |
|---|---|
| Age, years (mean, SD) | 30.1 (16.20) |
| BMI, kg/m2 (mean, SD) | 23.6 (4.30) |
| Perception of the patient's own physical performance (N = 56) (n, %) | |
| Inactive/Sedentary | 14 (25.0%) |
| Moderate | 34 (60.7%) |
| Vigorous | 8 (14.3%) |
| Agreement with investigator's perception (N = 56) (n, %) | 54 (96.4%) |
| Disease severity (n, %) | |
| Severe | 49 (83.1%) |
| Moderate | 10 (16.9%) |
| If moderate, FIX activity (mean, SD) | 2.5 (1.18) |
| Genetic mutations (n, %) | |
| Not tested | 19 (32.2%) |
| Missense | 25 (42.4%) |
| Nonsense | 6 (10.2%) |
| Deletion | 4 (6.8%) |
| Inversion | 3 (5.1%) |
| Other | 2 (3.4%) |
| Family history of haemophilia (n, %) | 21 (35.6%) |
| Presence of target joints (n, %) | 15 (25.4%) |
| Presence of chronic synovitis (n, %) | 11 (18.6%) |
| Presence of joint pain (n, %) | 15 (25.4%) |
| Treatment used in the previous year | |
| On demand (N = 6) (n, %) | |
| rFIX | 5 (83.3%) |
| pdFIX | 1 (16.7%) |
| Prophylaxis (N = 53) (n, %) | |
| rFIX | 43 (81.1%) |
| pdFIX | 10 (18.9%) |
- Abbreviations: BMI, body mass index; FIX, factor IX; pdFIX, plasma derived factor IX; rFIX, recombinant Factor IX; SD, standard deviation.
| With previous treatment | After two years of treatment | p* | |
|---|---|---|---|
| Prescribed dose (IU/kg), mean (SD) | 41.4 (14.39) | 43.8 (8.57) | .06 |
| Annual number of infusions, n (SD) | 116.5 (31.85) | 37.6 (14.79) | <.001 |
| Annualized total consumption (IU/kg), mean (SD) | 4098.2 (1340.10) | 1562.6 (433.23) | <.001 |
| Annualized bleeding rate, median [min-max] | .0 [.0 -12.0] | .0 [.0 – 7.8] | .86 |
| Zero bleedings, n (%) | 33 (66.0%) | 39 (78.0%) | .18 |
| FIX activity trough level, % (SD) | 3.8 (2.56) | 14.4 (7.23) | <.001 |
- *Comparison within group between ‘previous treatment’ and ‘two years of treatment’.
Analysis of trough level by frequency of prescription at second-year follow-up showed that a mean trough level of 23.6 ± 8.5 % was reached when an infusion frequency every 6–7 days was adopted.
Notably, all the infusion regimens achieved mean trough level > 10%, in particular: every 8–10 days: 12.6 ± 5.5; every 11–14 days: 10.8 ± 3.8; every 15–21 days: 11.9.
3.4 ABR
No change in the median Annualised Bleeding Rate was observed at the second year of follow-up with rIX-FP treatment: ABR remained .0 compared to the previous treatment (Table 2 and Figure 4A).
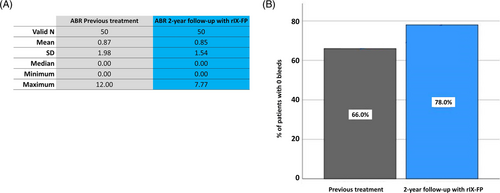
However, the rate of subjects with zero bleedings increased with rIX-FP (78.0%), compared to the previous treatment (66.0%) (Figure 4B).
3.5 On demand patients switched to prophylaxis
Six patients from FAS switched to prophylaxis with rIX-FP from a previous on-demand regimen. The factor concentrate used for the longest period prior to rIX-FP were nonacog-alfa (n = 5) or pdFIX (n = 1). With the previous treatment, the mean ABR was 4.7 ± 4.13 and the mean annualised total dose was 53666.7 ± 52121.65.
After switching to prophylaxis treatment, 5 out of 6 patients maintained adherence to the prescribed treatment throughout the 2-year observation period with a mean ABR value of .9 ± 1.46.
3.6 Safety
None of the assessments performed as per clinical practice detected the presence of inhibitors.
Out of 60 treated patients (Safety Set), 9 (15.0%) reported 14 Treatment Emergent Adverse Events (TEAEs). The most frequently reported were “vascular disorders” (4 events) and “injury, poisoning and procedural complications” (4 events), followed by “gastrointestinal disorders” (3 events).
Two events led to discontinuation of the study drug: one case of haematuria and one case of the drug being ineffective. The latter, together with one case of “left arm superficial thrombophlebitis in the site of drug administration” (defined as serious) were considered related to treatment.
A total of six Serious AEs (SAEs) were observed in 5 (8.5%) patients: none except the superficial thrombophlebitis was judged related to rIX-FP (Supplementary Appendix 4).
3.7 Discussions/conclusions
The results of this real-world study of rIX-FP in Italian patients with severe/moderate haemophilia B confirm the data from pre-approval clinical trials11, 12, 16 in terms of safety and efficacy, demonstrating that rIX-FP prophylaxis provides an excellent bleed control, in parallel with a relevant reduction of factor consumption and treatment burden due to reduced frequency of infusions, and potentially improved adherence.
Indeed, for 84% of patients on rIX-FP prophylaxis it was possible to lengthen the dosing interval up to > 7 days (30% up to > 10 days). The efficacy of rIX-FP over longer treatment intervals translates into a reduced number of annual injections for patients compared with other available products (about 70%).
Clinical studies on severe haemophilia B have clearly demonstrated the efficacy of prophylaxis.17 However, patients’ adherence is a key factor to achieve and maintain good outcomes, and the need for frequent intravenous infusions is a significant burden for patients that results in reduced patient adherence.17, 18
Consequently, infusion schedules should be simple to implement and acceptable to the patients, taking into consideration their lifestyle and activities:19 extended half-life (EHL) products have the potential to improve adherence and patient acceptance of prophylaxis by reducing the infusion schedule burden.15
In our real-world study a high adherence to treatment, also in patients who switched from on-demand regimen to rIX-FP prophylaxis, was demonstrated.
With longer intervals and single infusion dosing similar to the previous standard treatments, rIX-FP allowed us to achieve much higher mean trough levels (14.4% at the end of the study vs. 3.8% with previous treatment) and resulted highly effective in bleeding prevention, maintaining a median ABRs of .00 across all prophylaxis regimens at the end of the study. It is worth noting that the proportion of patients with 0 bleeds increased on rIX-FP prophylaxis compared to previous SHL (78% vs. 66%), while the maximum ABR value decreased from 12 to 7.7.
The goal of zero bleeding events is currently considered the target of prophylaxis,1 it being particularly important for the preservation of joint function and avoiding/delaying permanent joint damage.2, 17, 20-22 The FIX activity level plays a key role in preventing bleeding: in general, the higher the factor levels at all times, the less the bleeding1 and some experts recommend a FIX level higher than 3%–5% to guarantee joint protection.4 However, optimal trough levels are currently unknown and should be individualised, according to specific patients’ characteristics, including bleeding phenotype, joint status, lifestyle and physical activity. In this respect, a recent study depicts the factor levels thought to be needed to ensure adequate protection from bleedings, in keeping with the type of physical activity, in patients with or without joint morbidity. The authors report that, the higher the haemorrhagic risk associated to physical activity is, the higher the minimum factor level to be achieved is, which further increases in the presence of articular damage.23
Den Uijl et al.,24 showed that joint bleeding occurs in a relevant proportion of HA patients with factor levels up to 12 IU/dl. Although not including HB patients, this study suggests that the factor levels required to prevent bleeding may be higher than currently targeted with standard prophylaxis regimens.
Analysis on FIX trough level vs infusion frequency at second-year follow-up shows that mean levels > 10% are reached with all the dose regimens, very high levels (mean 23.3%) being maintained with weekly infusions.
These data confirm that rIX-FP can allow individualised dosing for patients, with longer dosing intervals and higher trough levels compared with SHL FIX products. The optimal trough level for the single patient can be chosen on the basis of the patient's characteristics and needs thereby driving the choice of the dosage regimen, with ample opportunities for personalisation.
In our population, the preferred choice of infusion interval resulted 8–10 days (54%), coherent with a population primarily young, with intense physical activity, therefore needing high protection (mean FIX levels obtained: 12.6 ± 5.47).
Economic evaluation was beyond the scope of this work; nevertheless, it is remarkable that the consistent reduction in the number of infusions translates not only into patients’ benefit but also into the reduced consumption (mean annualised total dose decreased 2.6-fold compared to previous treatment). Longer-term studies are needed to assess the impact of prophylactic regimens with EHL concentrates on joint status and to elucidate such further potential benefits for patients’ health, quality of life and for costs of haemophilia management.
The results of our study are consistent with other real-world observations, limited to retrospective data collection. An analysis conducted on patients with haemophilia B who switched to prophylaxis with rIX-FP from prior FIX product in Italy, Belgium and the United Kingdom, showed a similar factor consumption reduction, on average by 2.9.13 Our results are also consistent with a retrospective analysis of patients treated with rIX-FP in Germany,14 with a mean factor consumption of 44.2 IU/kg/week, mean ABR of .3 ± .6 on rIX-FP and 81% of patients having zero bleeds.
A retrospective chart review conducted in 69 patients in the USA with ≥6 months rFIXFc prophylaxis 25 reported higher frequency of infusion and dosages: the infusion frequency was once a week; mean dosage was equal to 55 UI/kg in severe and 57 UI/kg in moderate patients. Overall, ABRs were 1.2 for patients with severe disease (n = 34) and 3.2 for patients with moderate disease (n = 10).
In our study, the median ABR level was equal to zero at the second year of follow-up, using a mean dose ranging from 43.4 to 46.0 IU/kg, and an infusion frequency of less than once a week in 84% of the subjects. Finally, as regards safety findings, no patient developed an inhibitor and no safety concerns were identified.
The main strength of this work is that this is the first real-world prospective observational study conducted on rIX-FP, which combines a long-term prospective observation (2-year follow-up) with retrospective data on rIX-FP treatment (6-month therapy at least) and previous 12 months of other standard replacement therapies.
A possible limitation of the study could be the relatively low number of patients: nevertheless, considering the prevalence of this rare disease and the distribution of sites throughout Italy, our sample, including all ages, is quite representative of the Italian population. In addition, information on reasons for switching products were not reported. Moreover, for the retrospective part, recall bias could affect data reliability.
Encouraged by the results of the IDEAL Study and following the latest update of the SmPC of IDELVION®, which introduced the possibility of extending the dosing interval up to 21 days for adult patients (> 18 years old), a study amendment led to prolong the prospective phase of the study in order to collect real-world data in adult patients on prophylaxis regimen every 14 days or more. Moreover, data related to outcomes not described in this paper, such as assessment of joint status (joint damage and arthropathy) and haemostatic efficacy of rIX-FP in the treatment of bleeding episodes, will be presented and detailed in a new manuscript.
4 CONCLUSIONS
In conclusion, this real-world study demonstrates that patients receiving rIX-FP had a notably reduced treatment burden and drug consumption vs previous treatment, while showing increased protection, with higher factor levels, ABR equal to zero and no bleeds in 78% of patients.
ACKNOWLEDGEMENTS
The authors thank all the patients and their families who contributed to this study, all the participating clinicians, research nurses and data coordinators. This study was funded by CSL Behring. Trial operation and data analysis were conducted by Hippocrates Research S.r.l. Medical writing assistance was provided by Patrizia Mascagni in accordance with good publication practice (GPP3) and funded by CSL Behring.
CONFLICTS OF INTEREST
A.T. has acted as a member of the advisory board for Bayer. F.P. received honoraria as a member of the advisory board of Sanofi, SOBI, Takeda, Biomarin, Roche and speaker at educational meeting of Roche. A.C. received fees as a consultant or advisory board member or invited speaker from Bayer, Kedrion, Novo Nordisk, Roche, Sobi, Takeda and Werfen. C.B. acted as a consultant for Bayer, Novo Nordisk, Roche and CSL Behring. D.C. acted as a consultant for Bayer, Novo Nordisk, CSL Behring. R.D.C received fees for research activities from SOBI and for participation on the advisory board for Bayer, Takeda, Roche, SOBI and Pfizer. F.D. has been a member of the speaker bureau for CSL Behring, Novo Nordisk and Takeda. P.G. received fees for speaking from Bayer, Roche and CSL Behring. C.S. has acted as a consultant for SOBI, Novo Nordisk, Bayer, Takeda, Roche and CSL Behring. R.C.S. has acted as a consultant for SOBI, Takeda, Roche, Novo Nordisk and CSL Behring. S.S. has acted as a consultant for CSL Behring, Amgen, Novartis, Novo Nordisk, SOBI, Bayer. G.S. has acted as a paid speaker for CSL Behring, Novo Nordisk, Takeda/Shire and he received funding as a Study Investigator from Shire and Pfizer. M.R.V. has received fees for speaking from AbbVie and Novo Nordisk. E.Z. has acted as member of the advisory board for Bayer and Biomarin. G.C. has received speaker fees/advisory board from Alexion, Bayer, Sanofi, Grifols, Kedrion, CSL Behring, SOBI, LFB, Uniqure, Roche, Novo Nordisk, Pfizer, Biomarin. A.F. is a CSL Behring Employee. I.S. received consulting fees from Eye Pharma and D.M.G Italia. D.V. is a Hippocrates Research employee (CRO delegated for study conduction). A.C.M., A.B., E.M., M.M., R.M., B.P., P.R., A.T., L.P. have no conflicting interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




